Abstract
Sialic acids are family of extraordinary monosaccharides with nine-carbon backbone that are thoroughly expressed on all cell surfaces of vertebrates and higher invertebrates and also on specific bacteria that interact with vertebrates. In liver diseases, alterations in the carbohydrate content of plasma glycoproteins have been described either due to tissue destruction, tissue proliferation, depolymerization, or inflammation. Accordingly, sialic acid is being studied in liver diseases since the last two decades. Sialyltransferase group of enzymes catalyzes the transfer of the activated form of sialic acid onto the acceptor sugar. Sialidases (EC 3.2.1.18, also called neuraminidases) are glycosidases that catalyze the removal of sialic acid residues linked α-glycosidically from carbohydrate groups of glycoproteins and glycolipids. The sialyltransferases, sialidases, and sialic acids have been evaluated in liver diseases, and significant alterations have been found. Detailed understanding of the role of sialic acid, sialidases, and sialyltransferases and their interactions in liver diseases may significantly contribute to open new exciting frontiers of basic and therapeutic exploration.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
Key Facts of Sialyltransferases in Liver Disease
-
1.
Sialyltransferases catalyze the transfer of the activated form of sialic acid onto the acceptor sugar and catalyze the formation of different linkages and differ in their acceptor specificities.
-
2.
Sialyltransferases have the similar architecture, and vertebrate sialyltransferases are membrane-bound proteins with the catalytic domain facing into the lumen.
-
3.
More than 20 different sialyltransferases are involved in the biosynthesis of sialylated glycoproteins and glycolipids, and they are encoded by the human genome.
-
4.
Sialyltransferase enzymes differ in their substrate specificity, tissue distribution, and various biochemical parameters.
-
5.
In early years, it has been reported that serum from a patient with α-1-antitrypsin deficiency and hepatic cirrhosis was substantially deficient in sialyltransferase.
-
6.
The serum sialyltransferase deficiency in patients arose after chronic and extensive liver disease involving hepatic accumulation of α-1-antitrypsin rather than the enzyme deficiency as the major cause of the hepatic cirrhosis and α-1-antitrypsin deficiency.
-
7.
Plasma sialyltransferase has been suggested to be a useful marker enzyme for monitoring effectiveness of therapeutic programs for disseminated neoplasms.
-
8.
Altered sialylation has been associated with metastatic cell behaviors like invasion and enhanced cell survival and the sialic acid linkage to other sugars, existing in three main configurations, α2,3, α2,6, and α2,8, catalyzed by sialyltransferases.
Key Facts of Sialidases in Liver Disease
-
1.
There are four types of mammalian sialidases that have been identified and characterized as NEU1, NEU2, NEU3, and NEU4.
-
2.
In humans, the identity of NEU1 to other sialidases is low, but NEU2, NEU3, and NEU4 show higher homology to each other, and their substrate specificities are also different.
-
3.
Sialidases have been suggested to be candidate therapeutic agents for some diseases such as spinal cord injury and for future approaches and therapeutic purposes.
-
4.
It has been reported that a major fraction of sialidase with activity toward ganglioside substrate was localized in the plasma membrane of the liver cell.
-
5.
Decreased NEU1 expression has been shown in various cancers, and there is an inverse relationship between NEU1 expression level and metastatic ability.
-
6.
Liver NEU3 overexpression has been shown to positively improve insulin sensitivity and glucose tolerance in C57BL/6 and insulin-resistant mice by increased deposition of glycogen and triglycerides.
-
7.
Neuraminidase inhibitors are another important subject related to sialidases with some adverse effects of their use have been reported.
Definitions of Words and Terms
- Acute phase response:
-
The acute phase response is a nonspecific response to tissue injury or infection which affects several organs and tissues.
- Bioavailability:
-
It is the proportion of the administered substance available for use or storage and capable of being absorbed.
- Cirrhosis:
-
Cirrhosis is a late stage of scarring or fibrosis of the liver caused by many forms of liver diseases and conditions like hepatitis and chronic alcohol abuse.
- CRP:
-
C-reactive protein is a major component of the acute phase response. It is synthesized in the liver, and it is believed to mediate binding of foreign polysaccharides and phospholipids and also activate complement via the classic pathway.
- Fibrinogen:
-
It is a globulin of the blood plasma, converted into fibrin by the action of thrombin in the presence of calcium to produce coagulation.
- Glomerular filtration:
-
Glomerular filtration is the process by which the kidneys filter the blood in order to remove excess wastes and fluids.
- Glycolipid:
-
A lipid containing carbohydrate groups.
- Glycoprotein:
-
A class of conjugated proteins consisting of a compound of protein with a carbohydrate group.
- Half-life:
-
Half-life is the amount of time required for the amount of something to fall to half its initial value.
- Inflammation:
-
A localized protective response emerged by injury or destruction of tissues, serving to destroy both the injurious agent and the injured tissue.
- Orosomucoid:
-
Orosomucoid (ORM) or alpha-1-acid glycoprotein is an acute phase and is synthesized primarily in hepatocytes.
- Pegylation:
-
Pegylation is the term used to improve the pharmacokinetic properties of many biopharmaceutical proteins aiming to increase the elimination half-life and stability of the protein in question.
- Sialic:
-
Pertaining to the saliva.
- Sialidases:
-
They are glycosidases that catalyze the removal of sialic acid residues linked α-glycosidically from carbohydrate groups of glycoproteins and glycolipids.
- Sialylation:
-
Sialylation is the introduction of additional sialic acid residues to a protein.
- Sialyltransferase:
-
They are group of enzymes that catalyze the transfer of the activated form of sialic acid onto the acceptor sugar.
Introduction
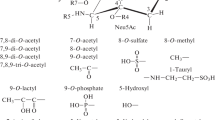
Sialic acids are a different family of extraordinary monosaccharides that are thoroughly expressed on all cell surfaces of vertebrates and so-called “higher” invertebrates and also on specific bacteria that interact with vertebrates (Varki and Gagneux 2012). There are over 50 natural analogues of sialic acid resulting from modifications occurring at the sialic acid backbone, such as the introduction of lactoyl, sulfate, methyl, and phosphate groups at the hydroxyl groups at C-4, C-7, C-8, and C-9. The most common sialic acid derivatives, N-acetylneuraminic acid (Neu5Ac), N-glycolylneuraminic acid (Neu5Gc), 2-keto-3-deoxy-d-glycero-d-galacturononic acid, and neuraminic acid (Neu), are formed by the solitary substitution at C-5. Polysialic acids (PSA) are produced by the interaction of the residues of Neu5Ac with each other in a conjugation process (Fig. 1). These homopolymers may be referred to as colominic acids and are added posttranslationally to a glycoprotein on the surface of glial cells and the skeletal muscle called neuronal cell adhesion molecule. Sialic acids may participate in several neurological processes, including the maintenance of plasticity during neuron development, fasciculation, and axonal branching by regulating homophilic interactions through this association. The attraction and repulsion of charged molecules and other entities are maintained by the negative charge at C-1 (Traving and Schauer 1998; Mühlenhoff 1998; Gruszewska and Chrostek 2016). Major functions of sialic acids are given in Fig. 2 (Varki 2008), and the main enzyme groups and their functions in sialic acid metabolism are given in Tables 1, 2, and 3 (Traving and Schauer 1998).
The term sialylation is the introduction of additional sialic acid residues to a protein. Sialylation has been defined as an effective alternative to pegylation and being not as challenging technically as pegylation. Pegylation is used to improve the pharmacokinetic properties of many biopharmaceutical proteins aiming to increase the elimination half-life and stability of the protein in question. Increasing the overall size of the circulating protein is one modification as molecules that are not associated with plasma-based proteins and with molecular weights <5 kDa tend to be excreted rapidly via the kidneys (Werle and Bernkop-Schnürch 2006). As the rate of glomerular filtration is reduced due to this enlargement, the protein has more time to interact with the target tissue or antigen. Pegylation is used for such increases in size involving the covalent attachment of either linear or branched chains of polyethylene glycol via a chemically reactive side chain, including a hydroxyl succinimidyl ester or an aldehyde group, to link to either the alpha or the epsilon amino groups on the protein (Byrne et al. 2007). On the other hand, oversialylation can increase the malignancy and metastatic potential of tumor cells by protecting cells from humoral or cellular defense systems as often observed in tumor cells or on placental syncytioblasts (Varki 2008). Microorganisms that coat themselves by colominic acid, a PSA, apply a similar antirecognition strategy that allows better survival in the host and thus enhances virulence. Group B Streptococcus is an example presenting terminal Sia α2,3-Gal b1,4 GlcNAc units, similar to human neutrophils that interact in the cell membrane with siglec-9 in cis. The binding of bacterial oligosaccharide in trans to the same neutrophil siglec-9 in a Sia-dependent manner has been shown to result in the weakening of the neutrophil immune response, thus demonstrating a new mechanism of bacterial immune evasion (Carlin et al. 2009).
Sialic Acid in Liver Diseases
Changes in the carbohydrate content of plasma glycoproteins have been described in patients with different liver diseases. Sialic acid in the human serum has been shown to be higher in a number of pathological states where the pathology is either due to tissue destruction, tissue proliferation, depolymerization, or inflammation. Accordingly, sialic acid is being studied in liver diseases since the last two decades (O’Kennedy et al. 1991; Okude et al. 1993, 1995; Lindberg et al. 1997; Arif 2005; Alturfan et al. 2014). In 1978, Martinez et al. reported abnormal sialic acid content of the dysfibrinogenemia associated with liver diseases. They reported that sialic acid content of the purified fibrinogen was 12.7–71.4% higher in patients when compared to controls (Martinez et al. 1978). Variation in serum sialic acid level in a variety of inflammatory liver diseases is an important diagnostic and prognostic tool. In liver cirrhosis, liver cancer, viral hepatitis, fatty liver, and hepatoma, abnormal sialic acid levels have been reported. On the other hand, sialic acid levels were much higher than the upper range in metastatic liver cancer (Matsuzaki et al. 1981).
Arif et al. (2005) carried out a study to evaluate levels of sialic acid in the patients with liver cirrhosis, and they reported a marked increase of sialic acid level in the blood. They reported increased serum sialic acid in advanced and terminal stages of liver cirrhosis in patients who had developed complications of the disease. On the other hand, the level was normal in the early stage of disease in the patients who had no complications. In previous studies conducted in hepatobiliary diseases, it has been indicated that an increase in sialic acid level may occur in the patients suffering from viral hepatitis, liver cirrhosis, inflammation of biliary tract, and malignant neoplasms of the liver (Carlson 1980; Narvaiza et al. 1986; Gruszewska et al. 2014).
The elevation of sialic acid content has been suggested to be a consequence of liver damage resulting in abnormal carbohydrate composition of the fibrinogen since fibrinogen contains 0.6% sialic acid and fibrinogen and sialic acid are both acute phase reactants (Arif 2005). In another study in liver cirrhosis and viral hepatitis, a decrease in sialoprotein has been reported that varied with the course of disease (Kaniak et al. 1980).
Sialic acid or N-acetylneuraminic is a component of the terminal part of some acute phase proteins like α-1-antichemotrypisin, α-1-antitrypsin, haptoglobin, and orosomucoid. Seventy percent of plasma sialic acid concentration might be explained by these glucoproteins. The production of sialic acid in hepatocytes is stimulated in inflammation and metabolic/oxidative stress situations. Therefore, sialic acid might be considered as a biomarker of serum concentration of many acute phase proteins and could be considered a systemic inflammatory biomarker since it could predict the risk for type 2 diabetes and cardiovascular diseases. Sialic acid and α-1-antitrypsin and the C-terminal fragment of α-1-antitrypsin, ceruloplasmin, fibrinogen, haptoglobin, homocystein, and plasminogen activator inhibitor-1 are among the hepatic biomarkers of inflammation related to the atherosclerotic process (Pinheiro et al. 2015).
Increased sialic acid levels have been suggested to reflect generalized endothelial cell dysfunction or macrovascular disease either through loss of sialic acid containing glycoproteins from vascular cells into the bloodstream or through an acute phase response. In liver destruction, sialic acid level rises proportional to hepatic damage since most of the circulating sialic acid is covalently attached to glycoproteins and more than 50% of total sialic acid comes from acute phase proteins such as α-acid glycoproteins, α-antitrypsin and fibrinogen, factor VII antigen, and activation markers of coagulation (Arif et al. 2005).
Authors aimed to investigate the sialic acid concentrations as total sialic acid (TSA), lipid-bound SA (LSA), and free SA (FSA) levels in the sera in liver cirrhosis in relation with the severity of liver disease. They found that the sialylation of serum proteins and lipids changes in liver cirrhosis, but only the serum concentrations of FSA are stage related and reflect the severity of liver disease (Chrostek et al. 2014; Gruszewska et al. 2014).
Other researchers carried out a study to investigate oxidant-antioxidant status and serum total sialic acid levels as alternative markers complementary to routine laboratory tests in an experimental obstructive jaundice model (OJM). Accordingly, rats were divided into three groups: sham-operated control (SOC), OJM monitored for 7 days (OJM-7), and OJM monitored for 14 days (OJM-14). We found that in both OJM groups, sialic acid and C-reactive protein (CRP) levels were significantly increased when compared with the SOC group. Moreover, sialic acid and CRP levels were significantly correlated in both groups. Therefore, we concluded that serum sialic acid may serve as an adjunct when combined with other markers in disease screening and progression as a marker of inflammation and oxidative stress in obstructive jaundice (Alturfan et al. 2014).
General Information on Sialyltransferases
Sialyltransferase group of enzymes catalyzes the transfer of the activated form of sialic acid, CMP-NeuNAc, onto the acceptor sugar. They catalyze the formation of different linkages (α2,3, α2,6, and α2,8) and differ in their acceptor specificities. Having the similar architecture, all vertebrate sialyltransferases are type II transmembrane glycoproteins that predominantly reside in the trans-golgi compartment. They are membrane-bound proteins with the catalytic domain facing into the lumen. Sialyltransferases have a short N-terminal cytoplasmic tail, a unique transmembrane domain, and a stem region of variable length from 20 to 200 amino acids followed by a large C-terminal catalytic domain. A specific enzyme catalyzes each transfer reaction, and for that reason, nearly 20 individual sialyltransferase enzymes have been proposed. Although the reactions catalyzed are very similar, all of the sialyltransferase enzymes cloned to date exhibit very little homology except for a short consensus sequence that is called the sialyl motif, to which the activated sugar donor is suggested to bind (Breen et al. 1998; Harduin-Lepers et al. 2001, 2005).
More than 20 different sialyltransferases involved in the biosynthesis of sialylated glycoproteins and glycolipids have been reported to be encoded by the human genome; however, only 15 different human sialyltransferase cDNAs have been cloned and characterized. Sialyltransferase genes are differentially expressed in a tissue-, cell type-, and stage-specific manner in order to regulate the sialylation pattern of cells. Accordingly, sialyltransferase enzymes differ in their substrate specificity, tissue distribution, and various biochemical parameters. On the other hand, one linkage has been suggested to be synthesized by multiple enzymes as conducted by enzymatic analysis (Harduin-Lepers et al. 2001).
Sialyltransferases in Liver Disease
In early years, it has been reported that serum from a patient with α-1-antitrypsin deficiency and hepatic cirrhosis was substantially deficient in sialyltransferase (EC 2.4.99.1), which is described as an enzyme transferring sialic acid from cytidine 5′-monophosphate-N-acetylneuraminic acid to a variety of asialoglycoprotein acceptors. The same authors extended their studies and included serum from five additional patients with α-1-antitrypsin deficiency and juvenile hepatic cirrhosis as well as a liver specimen obtained at an autopsy of one of these patients. They reported the sialyltransferase activity in serum from six patients with α-1-antitrypsin deficiency and hepatic cirrhosis to be 50% of healthy pediatric control values and 30% of pediatric patients with liver disease. On the other hand, interestingly the serum of family members homozygous for α-1-antitrypsin deficiency but without hepatic cirrhosis, and serum of patients with a variety of other kinds of liver disease, failed to exhibit the marked sialyltransferase deficiency. They carried out their study with similar assays on a liver sample homogenate obtained from a patient with α-1-antitrypsin deficiency and hepatic cirrhosis and found out that the deficiency of sialyltransferase activity was not demonstrable in the liver. The authors suggested that the serum sialyltransferase deficiency in such patients probably arose after chronic and extensive liver disease involving hepatic accumulation of α-1-antitrypsin rather than the enzyme deficiency as the major cause of the hepatic cirrhosis and α-1-antitrypsin deficiency (Kuhlenschmidt et al. 1976).
In another early study, Henderson and Kessel measured the levels of sialyltransferase activity in the plasma of patients with neoplastic disease and found elevated above-normal control values in 85% of patients that were examined. Moreover, there was a correlation between enzyme levels and course of disease in 46 of 57 patients studied serially during therapy. The authors concluded that plasma sialyltransferase could be a useful marker enzyme for monitoring effectiveness of therapeutic programs for disseminated neoplasms (Henderson and Kessel 1977).
Long-term ethanol has been shown to downregulate Gal β-1, 4GlcNAcα2, and 6-sialyltransferase (ST6Gal1), leading to defective glycosylation of a number of proteins including apolipoprotein E and apo J and the appearance of asialoconjugates in the blood of continuously alcohol-fed animals also in human alcoholics. The same authors explored the possibility of whether ethanol-induced downregulation of ST6Gal1 could contribute toward alcoholic steatosis in human alcoholics because of impaired lipid and lipoprotein transport caused by this downregulation. They suggested that alcohol-mediated downregulation of hepatic ST6Gal1 gene led to defective glycosylation of lipid-carrying apolipoproteins such as apo E and apo J, resulting in defective intracellular lipid and lipoprotein transport, which in turn may contribute to alcoholic steatosis (Gong et al. 2008).
Cao et al. suggested that sialyltransferases by sialylating plasma glycoproteins in hepatocytes may constitute markers for liver diseases. In their study, they examined the expression of the prevalent α2,6 sialyltransferase and sialoglycans in normal liver, cirrhotic liver, and hepatocellular carcinoma (HCC). They reported in normal and cirrhotic liver ST6Gal1 and sialoglycans were localized in the golgi region of hepatocytes surrounding the bile canaliculi and along the bile canaliculi, respectively. They showed the expression patterns of ST6Gal1 and sialoglycans in various liver tissues and demonstrated an altered expression of these structures between benign and malignant hepatocellular lesions (Cao et al. 2002).
Changes in glycosylation are accepted as a common feature of cancer cells. Altered sialylation has been associated with metastatic cell behaviors like invasion and enhanced cell survival, and the sialic acid linkage to other sugars, existing in three main configurations, α2,3, α2,6, and α2,8, catalyzed by sialyltransferases, has also suggested to be altered. All three configurations have been shown to be aberrantly expressed in cancer progression, with the increased α2,6 sialylation catalyzed by β-galactoside α2,6-sialyltranferase 1 (ST6Gal1), being frequent in many types of the cancers (Lu and Gu 2015).
General Information on Sialidases
Sialidases (EC 3.2.1.18, also called neuraminidases) are glycosidases that catalyze the removal of sialic acid residues linked α-glycosidically from carbohydrate groups of glycoproteins and glycolipids. Based on their substrate specificity and catalytic mechanism, sialidases can be separated into three different classes. Hydrolytic sialidases cleave the glycosidic bond of terminal sialic acids and release free sialic acid. Trans-sialidases’ role is to transfer the cleaved sialic acid to other glycoconjugates. Both classes belong to exo-α-sialidases (EC 3.2.1.18). Hydrolytic sialidases usually have a wide substrate specificity and cleave α2,3-, α2,6-, and α2,8-linked terminal sialic acids; however, trans-sialidases prefer α2,3-linked substrates. IT-sialidase (EC 4.2.2.15) is the third class that is strictly specific for α2,3-linked sialic acids and produces 2,7-anhydro-Neu5Ac (Li et al. 1990; Tailford et al. 2015). Sialidases are distributed in vertebrates widely, and they can also be found in microorganisms including viruses, bacteria, fungi, mycoplasma, and protozoa. Sialidase activity has been shown in higher organisms and in a wide variety of mammalian cells and tissues for a long time. In early years, it was not clear whether the activities originated from the same or different types of sialidase due to the molecular instability and low levels of expression. After biochemical isolation and characterization of sialidases from rat tissues, evidence was provided, and four types of sialidases were shown that differ in their subcellular localization and enzymatic properties.
To date, there are four types of mammalian sialidases that have been identified and characterized as NEU1, NEU2, NEU3, and NEU4 (Taeko 2010). Today, it is known that NEU1, NEU2, and NEU3 are localized predominantly in the lysosomes, cytosol, and plasma membranes, respectively, whereas NEU4 is found in lysosomes or in mitochondria and endoplasmic reticulum (Miyagi and Yamaguchi 2012). Major functions of the four groups of sialidases are given in Fig. 3 (Miyagi and Yamaguchi 2012). The presence of NEU1 has recently been reported in the outer and NEU3 in the inner membrane of the nuclear envelope (Wang et al. 2009). NEU2 has been detected in cytosol and in the nucleoplasm of rat muscle fibers (Akita et al. 1997).
In humans, the identity of NEU1 to other sialidases is low, but NEU2, NEU3, and NEU4 show higher homology to each other, and their substrate specificities are also different. For example, NEU1 hardly hydrolyzes gangliosides, and NEU3 acts more on gangliosides but not on glycoproteins, whereas NEU4 acts on mucin (Seyrantepe et al. 2003; Yamaguchi et al. 2005). They also have different glycosidic linkage specificity, like oligosaccharide substrates possessing the α2,3 sialyl linkage that are hydrolyzed faster than those with α2,6 and α2,8 linkages by NEU1 (Miyagi and Tsuiki 1984).
In recent years, sialidases have been suggested to be candidate therapeutic agents for some diseases such as spinal cord injury, and for future approaches and therapeutic purposes, modulation of sialidase expression can be maintained by the appropriate use of recombinant sialidases technology (Miyagi and Yamaguchi 2012).
Sialidases in Liver Disease
It was reported that a major fraction of sialidase with activity toward ganglioside substrate was localized in the plasma membrane of the liver cell and that ganglioside substrate was also localized in this cellular structure (Schengrund et al. 1972).
In 1981, intracellular α-l-fucosidase and hexosaminidase have been reported to show similar isoelectric focusing patterns in control, cystic fibrosis, and neuraminidase (sialidase)-deficient fibroblasts. They were unaffected by neuraminidase treatment. Extracellular hexosaminidases A and B were found to be sensitive to neuraminidase for the cell types where cystic fibrosis extracellular α-l-fucosidase and hexosaminidase acted as for control fibroblasts (Butterworth and Priestman 1981). Sialidase activity of peripheral mononuclear cells, which are mostly lymphocytes prepared from patients with alcoholic liver disease, was found to be decreased or not increased in 50% of the cases (Matsuzaki et al. 1987).
Decreased Neu1 expression has been shown in various cancers, and there is an inverse relationship between NEU1 expression level and metastatic ability, also evident with the NEU1 reduction which occurs in rat 3Y1 fibroblasts after src-transformation. Moreover, a more severe decrease in sialidase activity and acquired higher lung metastatic potential was evident after v-fos transfer to these transformed cells (Taeko 2010). The in vivo liver metastatic potential significantly reduced after the injection of NEU1-overexpressing cells transsplenically into mice. Integrin β4 has been found to be one of the target molecules for NEU1 which underwent desialylation and decreased phosphorylation (Uemura et al. 2009).
NEU3 which was originally described as a plasma membrane ganglioside sialidase is a peripheral or extrinsic membrane-associated enzyme with the ability to act on gangliosides located on the same membrane or on the membrane of adjacent cells that also play a major role in cell-cell interactions (Fanzani et al. 2012). Liver NEU3 overexpression has been shown to positively improve insulin sensitivity and glucose tolerance in C57BL/6 and insulin-resistant mice by increased deposition of glycogen and triglycerides (Yoshizumi et al. 2007). This suggested that the effects of NEU3 on insulin responsiveness may be different between the skeletal muscle and liver depending on the tissue-specific pattern of gangliosides (Fanzani et al. 2012).
Neuraminidase inhibitors are another important subject related to sialidases. They have been widely used in Japan since 2001 with some adverse effects of their use being reported. A case of generalized rash and erythema toxicum after treatment with the neuraminidase inhibitors administered prophylactically to prevent influenza infection in two patients with hepatoma associated with liver cirrhosis has been reported (Kaji et al. 2005).
The Interaction of Sialyltransferases, Sialidases, and Sialic Acids in Liver Diseases
Sialic acid has been shown to be increased in many pathological states where the pathology is due to tissue destruction, tissue proliferation, depolymerization, or inflammation. Accordingly, in liver cirrhosis, liver cancer, viral hepatitis, fatty liver, and hepatoma, abnormal sialic acid levels and alterations in sialyltransferases and sialidases enzymes have been reported as mentioned in previous sections. Variations in serum levels of sialyltransferases , sialidases, and sialic acids in these diseases are important diagnostic and prognostic tools. A large number of glycoproteins are secreted into the circulation by the liver, and they are sialylated on the termini of their glycans. The addition of these sialic acids are necessary for the survival of the serum proteins, and their removal can result in rapid clearance mediated by hepatic receptors that recognize the underlying sugar chain. Recently, the classic hepatic asialoglycoprotein “Ashwell receptor” has been shown to serve to reduce the level of coagulation determinants, like platelets and Von Willebrand factor (vWF), that have been desialylated by a sialidase released during sepsis with organisms like pneumococcus. Ashwell receptor rapidly clears glycoproteins bearing glycan ligands including galactose and N-acetylgalactosamine from circulation. This asialoglycoprotein receptor activity is suggested to be a key factor in the development and use of glycoprotein pharmaceuticals. The Ashwell receptor regulates vWF homeostasis and is responsible for thrombocytopenia during systemic Streptococcus pneumoniae infection through elimination of platelets desialylated by the bacterium’s sialidase enzyme. Therefore, this clearance process might serve to avoid excessive intravascular coagulation and death and protect the organism (Grewal et al. 2008; Varki 2008).
Applications of Sialyltransferases, Sialidases, and Sialic Acids to Biomarker Discovery in Liver Diseases
The changes in glycosylation and sialylation of proteins and lipids play an important role in the pathogenesis and progression of liver diseases. Altered sialic acid expression is reported in many pathological states which can be detected in histological sections by using plant lectins or antibodies to detect specific sialylated glycans (Wearne et al. 2006). Sialic acid measurements of body fluids are used to predict disease risk, and in many studies, total sialic acid levels were measured to predict the risk of various diseases although the logic by which such measurements are of prognostic value is largely unknown. Their secretion might be increased as an indication of an “acute phase response” (Varki 2008). The linking of sialic acid levels and sialylation of lipids and proteins in liver diseases can be detected in the serum as markers of liver disease progression. For instance, loss of sialylation on serum transferrin is used as a screening test both for chronic alcohol consumption and for congenital disorders of glycosylation (Varki 2008). On the other hand, sialidases can affect a number of different signaling pathways by modifying the cell content of gangliosides and sialylated receptorial and non-receptorial proteins although there are many things that need to be evaluated about sialidases in various physiological and pathological conditions of the liver. Proteomic techniques will produce a more complete characterization of sialidase substrates in order to fully understand their role. As a conclusion, a more detailed understanding of the role of sialic acid, sialidases, and sialyltransferases in liver diseases may significantly contribute to open new exciting frontiers of basic and therapeutic exploration.
Potential Applications to Prognosis, Other Diseases, or Conditions
Alterations in sialic acid levels are related to cancer, and analysis of the enzymes involved in addition of terminal sugars can give useful information to the understanding of the mechanism of elevations in sialic acid values during malignancy. Accordingly, increased circulatory sialyltransferase activities have been reported in various types of cancer. Therefore, determination of sialyltransferase in tumor tissue and sera of cancer patients may be useful for tumor detection and monitoring (Raval et al. 2004). On the other hand, sialidases play important roles in the cell by regulating the content of cellular sialic acid through the removal of sialic acid residues from glycoproteins and glycolipids. The altered and aberrant sialylation is related to malignant properties like invasiveness and metastatic potential. Moreover, in some cancers, in order to discriminate cancerous from noncancerous tissues and to determine the pathological stage, estimation of the mRNA levels of sialidases has been suggested. Additionally, immunohistochemical evaluation of cancer tissues using the antibody against the plasma membrane sialidase has been shown to be useful for clinical diagnosis (Miyagi et al. 2004, 2008). Therefore sialidases are considered as potential targets for cancer diagnosis and therapy.
Summary Points
-
This chapter focuses on the interaction of sialyltransferases, sialidases, and sialic acids in liver diseases.
-
Sialic acids are a different family of extraordinary monosaccharides sharing nine-carbon backbone that are thoroughly expressed on all cell surfaces of vertebrates and “higher” invertebrates and also on specific bacteria that interact with vertebrates.
-
Sialic acid is being studied in liver diseases since the last two decades, and changes in the carbohydrate content of plasma glycoproteins have been shown in patients with different liver diseases.
-
Sialic acid in the human serum has been shown to be higher in a number of pathological states where the pathology is either due to tissue destruction, tissue proliferation, depolymerization, or inflammation.
-
Sialyltransferase group of enzymes catalyzes the transfer of the activated form of sialic acid onto the acceptor sugar. They catalyze the formation of different linkages (α2,3, α2,6, and α2,8), and they differ in their acceptor specificities.
-
Sialidases (EC 3.2.1.18, also called neuraminidases) are glycosidases that catalyze the removal of sialic acid residues linked α-glycosidically from carbohydrate groups of glycoproteins and glycolipids. Based on their substrate specificity and catalytic mechanism, they are separated into three different classes as hydrolytic sialidases, trans-sialidases, and IT-sialidases.
-
Altered sialic acid levels, sialyltransferase, and sialidase activities and expressions have been reported in many pathological states related to the liver.
-
The interaction of sialic acid, sialyltransferase, and sialidase contributes to open new exciting frontiers of basic and therapeutic exploration in liver diseases.
Abbreviations
- Apo:
-
Apolipoprotein
- CMAH:
-
Cytidine monophosphate acetylneuraminic acid hydroxylase
- CMP:
-
Cytidine monophosphate
- CRP:
-
C reactive protein
- FSA:
-
Free sialic acid
- HCC:
-
Hepatocellular carcinoma
- LSA:
-
Lipid-bound sialic acid
- NEU1:
-
Neurominidase 1
- NEU2:
-
Neurominidase 2
- NEU3:
-
Neurominidase 3
- NEU4:
-
Neurominidase 4
- Neu5Ac:
-
N-acetylneuraminic acid
- OJM:
-
Obstructive jaundice model
- PEG:
-
Polyethylene glycol
- PSA:
-
Polysialic acids
- SOC:
-
Sham operated control
- ST6Gal I:
-
Galactoside α2,6 sialyltranferase 1
- TSA:
-
Total sialic acid
- vWF:
-
Von Willebrand factor
References
Akita H, Miyagi T, Hata K, Kagayama M. Immunohistochemical evidence for the existence of rat cytosolic sialidase in rat skeletal muscles. Histochem Cell Biol. 1997;107:495–503.
Alturfan AA, Aytaç E, Emekli-Alturfan E, Yarat A, Sarıbeyoğlu K, Pekmezci S, Seymen O. Serum total sialic acid as a novel complementary candidate marker of hepatic damage in obstructive jaundice. Ann Clin Lab Sci. 2014;44:56–61.
Arif S, Haq N, Hanif R, Khan AS, Rehman J, Mufti TA. Variations of serum sialic acid level in liver cirrhosis. J Ayub Med Coll Abbottabad. 2005;17:54–57.
Breen KC, Potratz A, Georgopoulou N, Sandhoff K. The generation and characterization of a rat neural cell line overexpressing the a2,6(N) sialyltransferase. Glycoconj J. 1998;15:199–202.
Butterworth J, Priestman D. Susceptibility to neuraminidase of alpha-l-fucosidase and N-acetyl-beta-d-glucosaminidase of cystic fibrosis, I-cell and neuraminidase-deficient fibroblasts. Clin Chim Acta. 1981;5:319–26.
Byrne B, Donohoe GG, O’Kennedy R. Sialic acids: carbohydrate moieties that influence the biological and physical properties of biopharmaceutical proteins and living cells. Drug Discov Today Ther Strateg. 2007;12:319–26.
Cao Y, Merling A, Crocker PR, Keller R, Schwartz-Albiez R. Differential expression of beta-galactoside alpha 2,6 sialyltransferase and sialoglycans in normal and cirrhotic liver and hepatocellular carcinoma. Lab Invest. 2002;82:1515–24.
Carlin AF, Uchiyama S, Chang YC, Lewis AL, Nizet V, Varki A. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil siglec-9 and dampen the innate immune response. Blood. 2009;113:3333–6.
Carlson J. α-antitrypsin and other acute phase reactants in liver-disease. Acta Med Scand. 1980;207:79–80.
Chrostek L, Supronowicz L, Panasiuk A, Cylwik B, Gruszewska E, Szmitkowski M. Serum sialic acids levels according to the severity of liver cirrhosis. J Clin Lab Anal. 2014;28:465–8.
Fanzani A, Zanola A, Faggi F, Papini N, Venerando B, Tettamanti G, Sampaolesi M, Monti E.Implications for the mammalian sialidases in the physiopathology of skeletal muscle. Skelet Muscle. 2012;1:23–4.
Gong M, Castillo L, Redman RS, Garige M, Hirsch K, Azuine M, Amdur RL, Seth D, Haber PS, Lakshman MR. Down-regulation of liver Galbeta1, 4GlcNAc alpha2, 6-sialyltransferase gene by ethanol significantly correlates with alcoholic steatosis in humans. Metab Clin Exp. 2008;57:1663–8.
Grewal PK, Uchiyama S, Ditto D, Varki N, Le DT, Nizet V, Marth JD. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat Med. 2008;14:648–55.
Gruszewska E, Cylwik B, Panasiuk A, Szmitkowski M, Flisiak R, Chrostek L. Total and free serum sialic acid concentration in liver diseases. Biomed Res Int. 2014. 10.1155/2014/876096. Accessed 18 May 2014.
Gruszewska E, Chrostek L. Biomarkers in Liver Disease, Biomarkers in Disease: Methods, Discoveries and Applications. In: Preedy VR, Patel VB, editors. Serum sialic acid as a biomarker in liver disease Serum sialic acid as a biomarker in liver disease. 1st ed. Springer; 2016. in press. DOI 10.1007/978-94-007-7742-2_19-1
Harduin-Lepers A, Vallejo-Ruiz V, Krzewinski-Recchi MA, Samyn-Petit B, Julien S, Delannoy P.The human sialyltransferase family. Biochimie. 2001;83:727–37.
Harduin-Lepers A, Mollicone R, Delannoy P, Oriol R. The animal sialyltransferases and sialyltransferase-related genes: a phylogenetic approach. Glycobiology. 2005;15:805–17.
Henderson M, Kessel D. Alterations in plasma sialyltransferase levels in patients with neoplastic disease. Cancer. 1977;39:1129–34.
Kaji M, Fukuda T, Tanaka M, Aizawa H. A side effect of neuraminidase inhibitor in a patient with liver cirrhosis. J Infect Chemother. 2005;11:41–3.
Kaniak J, Mejbaum KBW, Jelewska KZ, Kudreweiz HZ, Kowal GZ. Sialic acid contents of glycoproteins and seromucoid in liver diseases. Pol Med J. 1980;1:1076–81.
Kuhlenschmidt MS, Peters SP, Pinkard OD, Glew RH, Sharp H. Asialoglycoprotein sialic acid transferase activity in liver and serum of patients with juvenile hepatic cirrhosis and alpha-1-antitrypsin deficiency. Biochim Biophys Acta. 1976;8:359–73.
Li YT, Nakagawa H, Ross SA, Hansson GC, Li SC. A novel sialidase which releases 2,7-anhydro-alpha-N-acetylneuraminic acid from sialoglycoconjugates. J Biol Chem. 1990;265:21629–33.
Lindberg G, Iso H, Rastam L, Lundblad A, Folsom AR. Serum sialic acid and its correlates in community samples from Akita, Japan and Minneapolis. Int J Epidemiol. 1997;26:58–63.
Lu J, Gu J. Significance of β-galactoside α2,6 sialyltransferase 1 in cancers. Molecules. 2015;20:7509–27.
Martinez J, Palascak JE, Kwasniak D. Abnormal sialic acid content of dysfibrinogenemia associated with liver disease. J Clin Invest. 1978;61:535–8.
Matsuzaki S, Itakura M, Iwamura K, Kamiguchi H. Serum sialic acid levels in liver cirrhosis and liver cancer. Nippon Shonika Gakkai Zasshi. 1981;78:2395–401.
Matsuzaki S, Itakura M, Kadosaka T, Kamiguchi H, Yamamura M, Katsunuma T. Effect of ethanol on sialidase activity of peripheral lymphocytes. Alcohol Alcohol Suppl. 1987;1:509–11.
Miyagi T, Tsuiki S. Rat-liver lysosomal sialidase. Solubilization, substrate specificity and comparison with the cytosolic sialidase. Eur J Biochem. 1984;141:75–81.
Miyagi T, Yamaguchi K. Mammalian sialidases: physiological and pathological roles in cellular functions. Glycobiology. 2012;22:880–96.
Miyagi T, Wada T, Yamuguchi K, Hata K. Sialidase and malignancy. Glycoconj J. 2004;20:189–98.
Miyagi T, Wada T, Yamaguchi K, Shiozaki K, Sato I, Kakugawa Y, Yamanami H, Fujiya T. Human sialidase as a cancer marker. Proteomics. 2008;8:3303–11.
Mühlenhoff M. Polysialic acid: three-dimensional structure, biosynthesis and function. Curr Opin Struct Biol. 1998;8:558–64.
Narvaiza MJ, Fernandez J, Cuesta B, Paramo JA, Rocha E. Role of sialic acid in acquired dysfibrinogenemia associated with liver cirrhosis. Ric Clin Lab. 1986;16:563–8.
O’Kennedy R, Berns G, Moran E, Smyth H, Carroll K, Thornes RD. A critical analysis of the use of sialic acid determination in the diagnosis of malignancy. Cancer Lett. 1991;58:91–100.
Okude M, Yamanka A, Moriomoto Y, Akihama S. Sialic acid in fibrinogen: effects of sialic acid on fibrinogen-fibrin conversion by thrombin and properties of asialofibrin clot. Biol Pharm Bull. 1993;16:448–52.
Okude M, Yamanaka A, Akihama S. The effects of pH on the generation of turbidity and elasticity associated with fibrinogen fibrin conversion by thrombin are remarkably influenced by sialic acid in fibrinogen. Biol Pharm Bull. 1995;18:203–7.
Pinheiro VAC, Santos SFC, Bressan J. Hepatic inflammatory biomarkers and its link with obesity and chronic diseases. Nutr Hosp. 2015;1:1947–56.
Raval GN, Parekh LJ, Patel DD, Jha FP, Sainger RN, Patel PS. Clinical usefulness of alterations in sialic acid, sialyltransferase and sialoproteins in breast cancer. Indian J Clin Biochem. 2004;19:60–71.
Schengrund CL, Jensen DS, Rosenberg A. Localization of sialidase in the plasma membrane of rat liver cells. J Biol Chem. 1972;10:2742–6.
Seyrantepe V, Poupetova H, Froissart R, Zabot MT, Maire I, Pshezhetsky AV. Molecular pathology of NEU1 gene in sialidosis. Hum Mutat. 2003;22:343–52.
Taeko M. Mammalian sialidases and their functions. Trends Glycosci Glcy. 2010;22:162–72.
Tailford LE, Owen CD, Walshaw J, Crost EH, Hardy-Goddard J, Gall GL, de Vos WM, Taylor GL, Juge N. Discovery of intramolecular trans-sialidases in human gut microbiota suggests novel mechanisms of mucosal adaptation. Nat Commun. 2015;6:7624–5.
Traving C, Schauer R. Structure, function and metabolism of sialic acids. Cell Mol Life Sci. 1998;54:1330–49.
Uemura T, Shiozaki K, Yamaguchi K, Miyazaki S, Satomi S, Kato K, Sakuraba H, Miyagi T. Contribution of sialidase NEU1 to suppression of metastasis of human colon cancer cells through desialylation of integrin beta4. Oncogene. 2009;28:1218–29.
Varki A. Sialic acids in human health and disease. Trends Mol Med. 2008;14:351–60.
Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Ann NY Acad Sci. 2012;1253:16–36.
Wang J, Wu G, Miyagi T, Lu ZH, Ledeen RW. Sialidase occurs in both membranes of the nuclear envelope and hydrolyzes endogenous GD1a. J Neurochem. 2009;111:547–54.
Wearne KA, Winter HC, O’Shea K, Goldstein IJ. Use of lectins for probing differentiated human embryonic stem cells for carbohydrates. Glycobiology. 2006;16:981–90.
Werle M, Bernkop-Schnürch A. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids. 2006;30:351–67.
Yamaguchi K, Hata K, Koseki K, Shiozaki K, Akita H, Wada T, Moriya S, Miyagi T. Evidence for mitochondrial localization of a novel human sialidase (NEU4). Biochem J. 2005;390:85–93.
Yoshizumi S, Suzuki S, Hirai M, Hinokio Y, Yamada T, Tsunoda U, Aburatani H, Yamaguchi K, Miyagi T, Oka Y. Increased hepatic expression of ganglioside-specific sialidase, NEU3, improves insulin sensitivity and glucose tolerance in mice. Metabolism. 2007;56:420–9.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media Dordrecht
About this entry
Cite this entry
Alturfan, A.A., Emekli-Alturfan, E. (2017). Interaction of Sialyltransferases, Sialidases, and Sialic Acids in Liver Diseases and Applications to Biomarker Discovery. In: Patel, V., Preedy, V. (eds) Biomarkers in Liver Disease. Biomarkers in Disease: Methods, Discoveries and Applications. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7675-3_19
Download citation
DOI: https://doi.org/10.1007/978-94-007-7675-3_19
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7674-6
Online ISBN: 978-94-007-7675-3
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences