Abstract
Type 1 diabetes is an autoimmune disorder that destroys the insulin-producing cells of the pancreas. The mainstay of treatment is replacement of insulin through injectable exogenous insulin. Improvements in islet isolation techniques and immunosuppression regimens have made islet transplants a treatment option for select patients. Islet transplants have improved graft function over the years; however, graft function beyond year two is rare and notably these patients require immunosuppression to prevent rejection. Cell encapsulation has been proposed for numerous cell types, but it has found increasing enthusiasm for islets. Since islet transplants have experienced a myriad of success, the next step is to improve graft function and avoid systemically toxic immunosuppressive regimens. Cell encapsulation hopes to accomplish this goal. Encapsulation involves placing cells in a semipermeable biocompatible hydrogel that allows the passage of nutrients and oxygen and however blocks immune regulators from destroying the cell, thus avoiding systemic drugs. Several advances in encapsulation engineering and cell viability promise to make this a revolutionary discovery. This paper provides a comprehensive review of cell encapsulation of islets for the treatment of type 1 diabetes including a historical outlook, current research, and future studies.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
Introduction
Islet transplantation to treat type 1 diabetes has achieved vast improvements over the years, with more recipients able to achieve insulin independence for a longer period of time (Barton et al. 2012). Unfortunately, the lack of available donor organs and the use of antirejection medications continue to impede further progress. Encapsulation of islets for transplantation into diabetic recipients aims to provide a solution to these problems. Cell encapsulation is a revolutionary method of enveloping cells in a biocompatible matrix that provides a gradient for the diffusion of oxygen and nutrients but prevents large immune molecules from reaching the cell, thus avoiding rejection. This idea has been described since the 1930s but notable achievements have occurred over the last decade. This chapter aims to provide a review including a historical perspective, current research, and future applications of cell encapsulation of islets for the treatment of type 1 diabetes.
History
Over 25 million people in the United States (US) suffer from diabetes with approximately 5 % characterized as type 1. Diabetes is ranked as the seventh leading cause of death in the United States (CDC 2012). Type 1 diabetes (T1DM) is characterized as an autoimmune destruction of the β-cells of the pancreas with resultant insulin deficiency (ADA 2004). Currently, the mainstay of treatment for T1DM is glycemic control through injectable insulin. However, improvements in islet transplantation continue to occur, hopefully changing the treatment paradigm into cell replacement rather than supportive care. According to the Collaborative Islet Transplant Registry (CITR), there have been a total of 677 islet transplant recipients from 1999 to 2010. The percentage of recipients that achieve insulin independence for 3 years is 44 % between 2007 and 2010 compared to 27 % from 1999 to 2002 (Barton et al. 2012). Various immunosuppressive regimens have been implemented in islet transplant centers to maintain graft function. However, like other solid organ transplants, immune rejection medications are implicated in numerous adverse effects to the patient, as well as toxicity to the graft (Hafiz et al. 2005; Niclauss et al. 2011). To circumvent these issues, cell encapsulation has been proposed as the next treatment option for islet transplants with the goal of elimination of immunosuppression. Although cell encapsulation has been tested in other disease processes, such as neurodegenerative diseases, pain, and epilepsy to name a few, by far the greatest achievement using this technology has been in the encapsulation of islets for the treatment of T1DM (Bachoud-Lévi et al. 2000; Jeon 2011; Eriksdotter-Jönhagen et al. 2012; Fernández et al. 2012; Huber et al. 2012). It is clear that insulin independence can be achieved through the infusion of isolate islets, but improvements in graft viability and avoidance of systemically toxic drugs can be accomplished through encapsulation (Hearing and Ricordi 1999; Shapiro et al. 2003). The following paragraph will discuss advances in encapsulated islet technology.
Current Research
Animal and Human Trials
The first researcher to be accredited with transplantation of encapsulated tissue was Biscegeli in 1933. He placed mouse tumor cells in a polymer matrix into the abdomen of a guinea pig and was able to achieve survival without rejection (Bisceglie 1933). This idea was not replicated until approximately 50 years later when Lim and Sun were the first to use encapsulation for islet transplants in diabetic animals. They placed 2,000–3,000 islet equivalent (IEQ) in an alginate hydrogel for transplantation into the peritoneum of diabetic rats to achieve normoglycemia for up to 3 weeks compared to only 8 days in unencapsulated islets (Lim and Sun 1980). Currently, there are a number of achievements in encapsulating islets seen in small and large animal studies, as well as in early-phase clinical trials. In studies performed by Kobayashi et al. in 2003, NOD mice were used as both donor and recipient. The authors used a 5 % agarose microcapsule encasing 1,500–2,000 islet equivalents (IEQ) per mouse for intraperitoneal implantation as well as omental pouch transplants and witnessed prolonged euglycemia for a period of 100 days compared to 8 days for unencapsulated islet transplants (Kobayashi et al. 2003). The same authors repeated the study in 2006 and observed the same period of normoglycemia in the recipients; however, they also retrieved the devices after a period of 400 days and noted viable islets were recovered with a small percentage of necrotic cells (Kobayashi et al. 2006). In a more recent study, Dong et al. performed syngeneic transplants into streptozotocin (STZ)-induced BALB/c mice using polyethylene glycol-polylactic-co-glycolic acid (PEG-PLGA) nanoparticles with only 500–600 IEQ and revealed that over half of the mice achieved normal glucose levels for up to 100 days (Dong et al. 2012).
Less consistent but still notable results were accomplished with large animal models. Shoon-Shiong performed several encapsulated islet transplants into diabetic canines. In one publication in 1993, islets at a dose of 15,000–20,000 islets/kg were encapsulated in a microcapsule made of alginate into the intraperitoneal cavity and witnessed independence from exogenous insulin for a period of 110 days, as well as the presence of C-peptide in the blood for an average of 483 days (Soon-Shiong et al. 1993). In 2010, Dufrane used nonhuman primates as donor and recipients for subcutaneous and kidney capsule transplants of alginate micro- and macroencapsulated islets using 30,000 IEQ/kg. The authors noted correction of diabetes for a period of up to 28 weeks (Dufrane et al. 2010). In another study using cynomolgus monkeys as recipients by Elliott et al., neonatal pig islets were isolated (10,000 IEQ/Kg) and encapsulated in alginate microcapsules resulting in a more than 40 % reduction in injectable insulin dose compared to preimplantation (Elliott et al. 2005). Based on some noteworthy achievements in large animal studies, several researchers have been granted approval for stage one and two human clinical trials. Due to the previous success by Shoon-Shiong using a canine model, the authors were authorized for the first human clinical trial in 1994. A 38-year-old male, with type 1 diabetes and end-stage renal disease status postoperative kidney transplant, maintained on low-dose immunosuppression, became the first recipient of an encapsulated islet transplant. The patient initially received 10,000 IEQ/kg of cadaver islets encapsulated in an alginate microcapsule followed by a repeat infusion of 5,000 IEQ/kg 6 months later. The patients’ insulin requirements were reduced to 1–2 insulin units per day, and eventually he was able to discontinue all exogenous insulin after 9 months (Soon-Shiong et al. 1994). In 2006 Calafiore et al. reported on their encapsulated islet transplants. Human cadavers (400,000–600,000 IEQ) were isolated for encapsulation into sodium alginate beads for intraperitoneal injection. The patients experienced improved daily glucose levels and a decline in daily exogenous insulin intake; however, neither patient became insulin independent (Calafiore et al. 2006).
Living Cell Technologies, a company out of Australia, has achieved the best outcomes for encapsulated islet transplants. In one arm of their studies, xenotransplants from fetal pigs were isolated from a pathogen-free farm in New Zealand. The islets were encapsulated in alginate microcapsules for intraperitoneal injections into human recipients. Several early-phase clinical trials have been performed from this group with promising results. The most significant achievement has been in the reduction of hypoglycemic episodes to around 40 %. Several patients achieved improvements in daily glucose levels and a reduction in exogenous insulin dosing; two patients became insulin independent after 4 months (Elliott et al. 2007, 2010, Elliott and Living cell technologies Ltd-LCT 2011). These are promising results and achievements; however, not all researchers have been able to achieve such results, and the lack of reproducibility is threatening to dampen enthusiasm. For example, a human clinical trial by Tuch et al. used alginate microcapsules for human cadaveric islet transplants and noted the presence of plasma C-peptide levels for up to 2.5 years; however, there was no change in insulin requirements for the recipients (Tuch et al. 2009). Likewise, in a follow-up publication by Elliot et al., from Living Cell Technologies, one recipient experienced early success with a 30 % reduction in insulin dosing; however, at follow-up at 49 weeks, the patient was back on his original insulin dosing regimen (Elliott et al. 2007). Although the purpose of these early-phase clinical trials is to assure safety and determine dosing, it is notable that most encapsulated islet recipients do not achieve sustainable insulin independence. Likewise, there is yet to be a standardized protocol for the type of biomaterial used and the dose of islets to be infused. It is clear, however, based on novel in vivo studies that the type of biomaterial does impact graft survival. King et al. tested several encapsulation methods using alginate with poly-l-lysine (PLL) or without as well as with high glucuronic acid (G) or high mannuronic acid (M) in mouse recipients and revealed that significant results were achieved with PLL-free high M microcapsules, with sustained normoglycemia for 8 weeks (King et al. 2003). Likewise, Lanza et al. revealed that improved capsule integrity and graft function could be achieved by altering the concentration of alginate in their xenotransplants into diabetic Lewis rats (Lanza et al. 1999). Although no consensus is achieved regarding the best encapsulation vehicles for islets transplants, no review would be complete without a discussion regarding biomaterials.
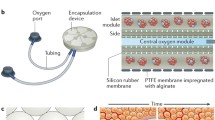
Biomaterials in Transplantation
Chang et al. were one of the first to describe the use of a semipermeable membrane for encapsulation. They postulated that liver enzymes or cells can be delivered in a polymer membrane for treatment of a disorder (Chang 1964). Now 50 years later, several types of encapsulation methods have been developed over the years however; currently the most common employed method are alginate microcapsules (King et al. 2003; Zimmermann et al. 2007; Krishnamurthy and Gimi 2011; Khanna et al. 2012). Capsule vehicles have taken the form of vascular shunts and macro-, micro-, and nanoscale devices. The original vascular device was developed over three decades ago as capillary fibers in culture-coated medium (Chick et al. 1975). Maki et al. performed several studies with vascular devices implanted as arteriovenous shunts into diabetic canines. These devices showed promising results with several canines achieving reduced insulin requirements (Maki et al. 1991, 1996). Ultimately, the difficulty with these devices was the ability to provide enough islets to coat the fibers. When attempts were made to elongate the fibers to include more islets, the devices resulted in clotting and fibrosis. This eventually led to its disuse as the higher dose of islets needed to achieve insulin independence in humans would result in the requirement for multiple devices to be employed Lanza et al. (1992). Macroscale devices have been used by a relative few, due to their increased immunogenicity as well as the larger diffusion parameters required for oxygen and nutrients to reach the cell; however, they offer several advantages including implantation ease and retrievability. In a study using an alginate macroscaled sheet, diabetic canines achieved improved glucose levels for over 80 days (Storrs et al. 2001) (Fig. 1). Nanoencapsulation offers the advantage of improved diffusion parameters, improving the response of insulin to rising blood glucose levels. PEG has been used for nanoencapsulation devices, and when exposed to UV or visible light, it can be cross-linked with minimal damage to the inner cell. However, PEG is not as biocompatible as other hydrogels and does not provide complete protection from cytokines (Jang et al. 2004). Despite these concerns, some success has been attained with these gels (Dong et al. 2012). By far the most common encapsulation device is a microscale vehicle. These beads have mechanical stability, have an improved surface area to volume ratio, and have enhanced immunologic profiles (Krishnamurthy and Gimi 2011; Borg and Bonifacio 2011), but more importantly several companies make so-called encapsulators that can produce uniform capsules using air jet-driven droplet technology (Fiszman et al. 2002; Sun 1988) (Fig. 2). This is highly important when discussing using these capsules for clinical use as standardization, safety, and cost-effectiveness are going to be important aspects for its widespread clinical use. All of the clinical trials discussed in the previous paragraph used microcapsules for encapsulating islets. Along with the different capsule size, various materials have also been tested. Extracellular matrixes have included both synthetic and biologic materials. The most common synthetic agents employed in encapsulation engineering include polyethylene oxide, polyacrylic acid, polyvinyl alcohol, polyphosphazene, polypeptides, and derivatives. Naturally occurring hydrogels include gelatin, fibrin, agarose, hyaluronate, chitosan, and alginate (Nicodemus and Bryant 2008; Lee and Mooney 2000). Polyglycolic and lactic acid polymers continue to be the most commonly used synthetic material used in medical devices. However, several concerns are raised when using synthetic materials as a scaffold, due to the possible potential that the foreign material will elicit a greater response by the body leading to fibrosis and loss of the encased cells. The production of these synthetic constituents would need to occur with nontoxic materials, and these materials would need to be purified in a method that is gentle enough on the cells and the transplant site. These capsules typically are heavily modified so that they can interact with the environment and degrade under physiologic conditions. Consequently, biologic materials are being used with an increasing incidence, with collagen being the most widely used naturally derived polymer in medical devices today. However, these gels exhibit poor strength, are expensive, and display significant variations between collagen batches making standardization of the process a problem (Lee and Mooney 2000). Therefore, alginate has become increasingly utilized for encapsulation due to its excellent biocompatibility, hydrophilic properties, easy gelation process, stable architecture, and relatively low cost. Alginate is a polysaccharide derived from seaweed which has to undergo extensive purification to improve its immunologic profile (O’Sullivan et al. 2010). Impure alginate has been implicated in islet cell necrosis and recruitment of inflammatory mediators (De Vos et al. 1997). Alginate is made up of chains of mannuronic (M) and guluronic (G) acid; the sequence of these units determines many of the encapsulation properties such as mechanical strength, durability, and permeability. For example, high G alginates form gels which are smaller and stronger than high M (O’Sullivan et al. 2010). Wang et al. tested over 200 capsule designs for their islet transplants finally deciding on a polymethyl coguanidine-cellulose sulfate/poly-l-lysine-sodium alginate (PMCG)-CS/PLL (Wang et al. 1997) for their canine syngeneic transplants resulting in normoglycemia achieved for approximately 160 days, with one canine achieving euglycemia for 214 days (Wang et al. 2008). The use of polycations and anions has been shown to provide improved permeability and mechanical strength; however, they also tend to result in an increase biologic response. Some counteract this response by adding another thin layer of alginate to provide a barrier (O’Sullivan et al. 2010). In broad terms, the process of gelation involves cross-linking by covalent, ionic, or physical bonds. For example, alginate converts into a gel form by ionic cross-linking with bivalent cations such as calcium, magnesium, and more commonly barium (King et al. 2001). The diffusion gradient that provides the bidirectional flow of materials is established by the degree of cross-linking. Mesh or pore size is typically much smaller than the encased cells however; hydrogels do not result in uniform pore size, and permselectability has never been clearly defined (O’Sullivan et al. 2010). What is apparent is that an increase in the degree of cross-linking results in gels that have superior mechanical strength but inversely reduces the size of the pores available for diffusion.
Macroencapsulated islets. Islets were isolated from young piglets (average 20 days), matured for 8 days, and injected into a prefabricated islet sheet made of ultrapure high M alginate. Islets were placed in the center of the sheet with a scrim added for greater stability. Encapsulation design by Islet Science, LLC (Picture provided by Dr. Jonathan RT Lakey, UCI)
Microencapsulated islets. Islets were isolated from young pigs (range 18–26 days), matured for 8 days, and then encapsulated in ultrapure high M (UPLVM) alginate microcapsule, using an electrostatic encapsulator. Encapsulated islets were then stained with dithizone. Sample image is an isolated islet, approximately 150 μm in diameter, encapsulated in a 500 μm microcapsule. Scale 100 μm (Picture provided by Dr. Jonathan RT Lakey, UCI)
Several components need to be considered in engineering scaffolds that are safe for the encapsulated cells and the surrounding environment. The process of gelation needs to be mild; the capsule structure and chemistry should be nontoxic and reproducible. The degradation process must follow physiologic tissue growth, and its products must not adversely affect the coated cells or the body. Above all, the process of hydrogel engineering needs to be easily scaled up for industry, acceptable to surgeons, patients, and regulatory committees.
Clearly, capsule engineering is of vital importance for promoting graft survival and function, and many steps need to be considered in capsule construction to promote islet transplantations. Many advances have been achieved, but some studies have illustrated the difficulty with this technology. In animal studies performed by Suzuki et al., Tze, and Duvivier-Kali as well as the human clinical trial by Tuch, capsule fibrosis was a significant problem encountered (Suzuki et al. 1998; Tze et al. 1998; Duvivier-Kali et al. 2004; Tuch et al. 2009). Theoretically immune isolation is achieved by encapsulating cells, however, in these studies; clearly, some component of rejection was experienced. Likewise, oxygen and nutrients are able to freely diffuse across a matrix; however, studies illustrated by De Vos and Xin noted the absence of fibrosis, however, retrieved capsules containing necrotic islets, indicating the lack of appropriate oxygen to promote graft survival (De Vos et al. 1999; Xin et al. 2005). Due to these issues, some researchers have gone on to improve capsule engineering by means of co-encapsulation. These advances will be discussed in future applications as most of these undertakings are currently being performed in vitro.
Future Applications
Improved Capsule Engineering
Co-encapsulation is the process of adding additional molecules to the capsule to enhance the performance of the enveloped cell. In a novel study performed by Bunge, islets encapsulated with dexamethasone witnessed improved islet survival in mice recipients compared to those without the steroid (Bunger et al. 2005). In another study, Baruch encapsulated mouse monocyte macrophage cells and hamster kidney cells with ibuprofen and witnessed improved cell survival in vitro and in vivo (Baruch et al. 2009). Although cells are protected from large molecules such as antibodies, proinflammatory cytokines have a smaller molecular weight and can diffuse across most hydrogel gradients; thus, protection from these cytokines may promote capsule survival. In an in vitro study performed by Leung, capsules with anti-TNF α were able to remove active TNF α from a culture media (Leung et al. 2008). In order to improve oxygen supply to the cell, the access to a rich vascular bed is essential. As such, Khanna showed that the angiogenic factor, fibroblast growth factor 1, was able to be encapsulated and revealed continuous release for a 1 month period in vitro (Khanna et al. 2010). In another study, Pedraza et al. was able to encapsulate solid peroxide within polydimethylsiloxane resulting in sustained oxygen generation for approximately 6 weeks (Pedraza et al. 2012). Clearly, co-encapsulation is possible; however, most of these studies are still in the early phases of investigation, so we are yet to see whether this will improve graft function in vivo. The latest forefront for encapsulation cell technology for the treatment of diabetes involves the use of stem cells as a source of islets. Although some researchers have begun doing in vivo studies, the results have been varied.
Stem Cells
Stem cells are an attractive addition to cell encapsulation as the availability of human cadaveric donors for islet transplants continues to be a major problem. A company out of San Diego, Viacyte LLC, has performed the bulk of encapsulated stem cell-related transplants. Human embryonic stem cells directed down a pancreatic lineage were encapsulated in a device, called TheraCyte, which is made of a double membrane of polytetrafluoroethylene. In a study by Kroon et al., diabetic mice achieved normalization of glucose levels after 2 months (Kroon et al. 2008). Likewise, Lee et al. noted initially low glucose responses and plasma C-peptide levels after 12 weeks; however, after 5 months, improvements in glucose and C-peptide levels were evident, indicating differentiation continued to occur while encapsulated (Lee et al. 2009). In contrast, Matveyenko et al. did not achieve such outcomes, and in fact, their devices were noted to be encased in fibrotic tissue and upon retrieval did not stain positive for endocrine cells (Matveyenko et al. 2010). In a study using mesenchymal stem cells from human amniotic membranes, Kadam et al. were able to produce functional islet-like clusters which were encapsulated in polyurethane-polyvinylpyrrolidone microcapsules for transplantation into diabetic mice which resulted in euglycemia after day 15 until approximately day 30 (Kadam et al. 2010). Although, no study has been able to accomplish sustainable insulin independence using stem cells, improvements in stem cell differentiation are being accomplished and will hopefully improve upon this method (Blyszczuk et al. 2003).
Conclusion
Cell encapsulation of islets for the treatment of T1DM is a promising field that aims to revolutionize the treatment paradigm for diabetics. Although significant achievements have occurred, there are several obstacles that need to be addressed before achieving widespread use of this technology. Improvements in graft viability, biomaterial engineering, and islet isolation techniques will continue to promote success in this field.
References
American Diabetes Association (2004) Diagnosis and classification of diabetes mellitus. Diabetes Care 1:s5–s10
Bachoud-Lévi A, Déglon N, Nguyen JP, Bloch J, Bourdet C, Winkel L, Rémy P, Goddard M, Lefaucheur J, Brugières P, Baudic S, Cesaro P, Peschanski M, Aebischer P (2000) Neuroprotective gene therapy for Huntington’s disease using a polymer encapsulated BHK cell line engineered to secrete human CNTF. Hum Gene Ther 11:1723–1729
Barton FB, Rickels MR, Alejandro R, Hering B, Wease S, Naziruddin B et al (2012) Improvement in outcomes of clinical islet transplantation. Diabetes Care 35:1436–1445
Baruch L, Benny O, Gilbert A, Ukobnik O (2009) Alginate-PLL cell encapsulation system co-entrapping PLGA-microspheres for the continuous release of anti-inflammatory drugs. Biomed Microdevices 11:1103–1113
Bisceglie V (1933) Uber die antineoplastische Immunität; heterologe Einpflanzung von Tumoren in Hühner-embryonen. Z Krebsforsch 40:122–140
Blyszczuk P, Czyz J, Kania G, Wagner M, Roll U, St-Onge L, Wobus AM (2003) Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc Natl Acad Sci U S A 100:998–1003
Borg DJ, Bonifacio E (2011) The use of biomaterials in islet transplantation. Curr Diab Rep 11:434–444
Bunger CM, Tiefenbach B, Jahnkea A, Gerlacha C, Freier T, Schmitz KP, Hopt UT, Schareck W, Klar E, De Vos P (2005) Deletion of the tissue response against alginate-pll capsules by temporary release of co-encapsulated steroids. Biomaterials 26:2353–2360
Calafiore R, Basta G, Luca G, Lemmi A, Montanucci P, Calabrese G, Racanicchi L, Mancuso F, Brunetti P (2006) Microencapsulated pancreatic islet allografts into non immunosuppressed patients with type diabetes: first two cases. Diabetes Care 29:1137–1139
Centers for Disease Control and Prevention (2012) Centers for Disease Control and Prevention: Diabetes Public Health Resources. http://www.cdc.gov/diabetes. Accessed 1 Aug 2012
Chang T (1964) Semipermeable microcapsules. Science 156:524–525
Chick WL, Like AA, Lauris V (1975) 8-cell culture on synthetic capillaries: an artificial endocrine pancreas. Science 187:847–849
De Vos P, De Haan BJ, Wolters GH, Strubbe JH, Van Schilfgaarde R (1997) Improved biocompatibility but limited graft survival after purification of alginate for microencapsulation of pancreatic islets. Diabetologia 40:262–270
De Vos P, Van Straaten JF, Nieuwenhuizen AG, De Groot M, Ploeg RJ, De Haan BJ, Van Schilfgaarde R (1999) Why do microencapsulated islet grafts fail in the absence of fibrotic overgrowth? Diabetes 48:1381–1388
Dong H, Fahmy TM, Metcalfe SM, Morton SL, Dong X, Inverardi L, Adams DB, Gao W, Wang H (2012) Immuno-isolation of pancreatic islet allografts using pegylated nanotherapy leads to long-term normoglycemia in full MHC mismatch recipient mice. PLoS One 7:e50265
Dufrane D, Goebbels RM, Gianello P (2010) Alginate macroencapsulation of pig islets allows correction of streptozotocin-induced diabetes in primates up to 6 months without immunosuppression. Transplantation 90:1054–1062
Duvivier-Kali VF, Omer A, Lopez-Avalos MD, O’Neil JJ, Weir GC (2004) Survival of microencapsulated adult pig islets in mice in spite of an antibody response. Am J Transplant 12:1991–2000
Elliott RB, Living cell technologies Ltd-LCT (2011) In: International pancreas and islet transplant association meeting, Prague
Elliott RB, Escobar L, Tan PL, Garkavenko O, Calafiore R, Basta P, Vasconcellos AV, Emerich DF, Thanos C, Bambra C (2005) Intraperitoneal alginate-encapsulated neonatal porcine islets in a placebo-controlled study with 16 diabetic cynomolgus primates. Transplant Proc 37:3505–3508
Elliott RB, Escobar L, Tan PL et al (2007) Live encapsulated porcine islets from a type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation 14:157–161
Elliott RB, Garkavenko O, Tan P, Skaletsky NN, Guliev A, Draznin B (2010) Transplantation of microencapsulated neonatal porcine islets in patients with type 1 diabetes: safety and efficacy.70th scientific sessions. American Diabetes Association, Orlando
Eriksdotter-Jönhagen M, Linderoth B, Lind G, Aladellie L, Almkvist O, Andreasen N, Blennow K, Bogdanovic N, Jelic V, Kadir A, Nordberg A, Sundström E, Wahlund LO, Wall A, Wiberg M, Winblad B, Seiger A, Almqvist P, Wahlberg L (2012) Encapsulated cell biodelivery of nerve growth factor to the basal forebrain in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord 33:18–28
Fernández M, Barcia E, Fernández-Carballido A, Garcia L, Slowing K, Negro S (2012) Controlled release of rasagiline mesylate promotes neuroprotection in a rotenone-induced advanced model of Parkinson’s disease. Int J Pharm 438:266–278
Fiszman GL, Karara AL, Finocchiaro LM, Glikin GC (2002) A laboratory scale device for microencapsulation of genetically engineered cells into alginate beads. Electron J Biotechnol 5:23–24
Hafiz MM, Faradji RN, Froud T, Pileggi A, Baidal DA, Cure P, Ponte G, Poggioli R, Cornejo A, Messinger S, Ricordi C, Alejandro R (2005) Immunosuppression and procedure-related complications in 26 patients with type 1 diabetes mellitus receiving allogeneic islet cell transplantation. Transplantation 80:1718–1728
Hearing B, Ricordi C (1999) Islet transplantation for patients for patients with type 1 diabetes; results, research priorities, and reasons for optimism. Graft 2:12–27
Huber A, Padrun V, Deglon N, Aebischer P, Hanns M, Boison D (2012) Grafts of adenosine-releasing cells suppress seizures in kindling epilepsy. Proc Natl Acad Sci U S A 98:7611–7616
Jang JY, Lee DY, Park SJ, Byun Y (2004) Immune reactions of lymphocytes and macrophages against PEG-grafted pancreatic islets. Biomaterials 25:3663–3669
Jeon Y (2011) Cell based therapy for the management of chronic pain. Korean J Anesthesiol 60:3–7
Kadam S, Muthyala S, Nair P, Bhonde R (2010) Human placenta-derived mesenchymal stem cells and islet-like cell clusters generated from these cells as a novel source for stem cell therapy in diabetes. Rev Diabet Stud 7:168–182
Khanna O, Moya EC, Opara EC, Brey EM (2010) Synthesis of multilayered alginate microcapsules for the sustained release of fibroblast growth factor-1. J Biomed Mater Res A 95:632–640
Khanna O, Larson JC, Moya ML, Opara EC, Brey EM (2012) Generation of alginate microspheres for biomedical applications. J Vis Exp 66:e3388
King S, Dorian R, Storrs R (2001) Requirements for encapsulation technology and the challenges for transplantation of islets of Langerhans. Graft 4:491–499
King A, Lau J, Nordin A, Sandler S, Andersson A (2003) The effect of capsule composition in the reversal of hyperglycemia in diabetic mice transplanted with microencapsulated allogeneic islets. Diabetes Technol Ther 5:653–663
Kobayashi T, Aomatsu Y, Iwata H, Kin T, Kanehiro H, Hisanaga M, Ko S, Nagao M, Nakajima Y (2003) Indefinite islet protection from autoimmune destruction in nonobese diabetic mice by agarose microencapsulation without immunosuppression. Transplantation 75:619–625
Kobayashi T, Aomatsu Y, Iwata H, Kin T, Kanehiro H, Hisanga M, Ko S, Nagao M, Harb G, Nakajima Y (2006) Survival of microencapsulated islets at 400 days posttransplantation in the omental pouch of NOD mice. Cell Transplant 15:359–365
Krishnamurthy NV, Gimi B (2011) Encapsulated cell grafts to treat cellular deficiencies and dysfunction. Crit Rev Biomed Eng 39:473–491
Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D’Amour KA, Carpenter MK, Baetge EE (2008) Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 26:443–452
Lanza RP, Borland KM, Lodge P, Carretta M, Sullivan SJ, Muller TE, Solomon BA, Maki T, Monaco AP, Chick WL (1992) Treatment of severely diabetic, pancreatectomized dogs using a diffusion-based hybrid pancreas. Diabetes 41:886–889
Lanza RP, Jackson R, Sullivan A, Ringeling J, McGrath C, Kühtreiber W, Chick WL (1999) Xenotransplantation of cells using biodegradable microcapsules. Transplantation 67:1105–1111
Lee K, Mooney D (2000) Hydrogels for tissue engineering. Chem Rev 101:1869–1879
Lee SH, Hao E, Savinov AY, Geron I, Strongin AY, Itkin-Ansari P (2009) Human β-cell precursors mature into functional insulin-producing cells in an immunoisolation device: implications for diabetes cell therapies. Transplantation 87:983–991
Leung A, Lawrie G, Nielson LK, Trau M (2008) Synthesis and characterization of alginate/poly-l-ornithine/alginate microcapsules for local immunosuppression. J Microencapsul 25:387–398
Lim F, Sun AM (1980) Microencapsulated islets as bioartificial pancreas. Science 210:908
Maki T, Ubhi CS, Sanchez-Farpon H, Sullivan SJ, Borland K, Muller TE, Solomon BA, Chick WL, Monaco AP (1991) Successful treatment of diabetes with the biohybrid artificial pancreas in dogs. Transplantation 51:43–51
Maki T, Otsu I, O’Neil JJ, Dunleavy K, Mullon CJ, Solomon BA, Monaco AP (1996) Treatment of diabetes by xenogeneic islets without immunosuppression. Use of a vascularized bioartificial pancreas. Diabetes 45:342–347
Matveyenko AV, Georgia S, Bhushan A, Butler PC (2010) Inconsistent formation and nonfunction of insulin-positive cells from pancreatic endoderm derived from human embryonic stem cells in athymic nude rats. Am J Physiol Endocrinol Metab 299:13–20
Niclauss N, Bosco D, Morel P, Giovannoni L, Berney T, Parnaud G (2011) Rapamycin impairs proliferation of transplants islet β cells. Transplantation 91:714–722
Nicodemus G, Bryant S (2008) Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B Rev 14:149–165
O’Sullivan ES, Johnson AS, Omer A, Hollister-Lock J, Bonner-Weir S, Colton CK, Weir GC (2010) Rat islet cell aggregates are superior to islets for transplantation in microcapsules. Diabetologia 53:937–945
Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL (2012) Preventing hypoxia-induced cell death in β cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc Natl Acad Sci U S A 109:4245–4250
Shapiro AM, Nanji SA, Lakey JR (2003) Clinical islet transplant: current and future directions towards tolerance. Immunol Rev 196:219–236
Soon-Shiong P, Feldman E, Nelson R, Heintz R, Yao Q, Yao Z, Zheng T, Merideth N, Skjak-Braek G, Espevik T et al (1993) Long-term reversal of diabetes by the injection of immunoprotected islets. Proc Natl Acad Sci U S A 90:5843–5847
Soon-Shiong P, Heintz RE, Merideth N, Yao QX, Yao Z, Zheng T, Murphy M, Moloney MK, Schmehl M, Harris M et al (1994) Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet 343:950–951
Storrs R, Dorian R, King SR, Lakey J, Rilo H (2001) Preclinical development of the islet sheet. Ann N Y Acad Sci 944:252–266
Sun AM (1988) Microencapsulation of pancreatic islet cells: a bioartificial endocrine pancreas. Methods Enzymol 137:575–580
Suzuki K, Bonner-Weir S, Trivedi N, Yoon KH, Hollister-Lock J, Colton CK, Weir GC (1998) Function and survival of macroencapsulated syngeneic islets transplanted into streptozocin-diabetic mice. Transplantation 66:21–28
Tuch BE, Keogh GW, Williams LJ et al (2009) Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care 32:1887–1889
Tze WJ, Cheung SC, Tai J, Ye H (1998) Assessment of the in vivo function of pig islets encapsulated in uncoated alginate microspheres. Transplant Proc 30:477–478
Wang T, Lacík I, Brissová M, Anilkumar AV, Prokop A, Hunkeler D, Green R, Shahrokhi K, Powers AC (1997) An encapsulation system for the immunoisolation of pancreatic islets. Nat Biotechnol 15:358–362
Wang T, Adcock J, Kühtreiber W, Qiang D, Salleng KJ, Trenary I, Williams P (2008) Successful allotransplantation of encapsulated islets in pancreatectomized canines for diabetic management without the use of immunosuppression. Transplantation 85:331–337
Xin ZL, Ge SL, Wu XK, Jia YJ, Hu HT (2005) Intracerebral xenotransplantation of semipermeable membrane-encapsuled pancreatic islets. World J Gastroenterol 11:5714–5717
Zimmermann H, Shirley SG, Zimmermann U (2007) Review Alginate-based encapsulation of cells: past, present, and future. Curr Diab Rep 7:314–320
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this entry
Cite this entry
Lakey, J.R.T. et al. (2015). Islet Encapsulation. In: Islam, M. (eds) Islets of Langerhans. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6686-0_29
Download citation
DOI: https://doi.org/10.1007/978-94-007-6686-0_29
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6685-3
Online ISBN: 978-94-007-6686-0
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences