Abstract
CMP-sialic acid is a donor substrate of sialyltransferases. CMP-sialic acid transporter (CST) couples with the antiport of CMP and transports CMP-sialic acid from the cytosol into the lumen of the medial and trans cisternae of the Golgi apparatus, where sialylation is mediated by a variety of sialyltransferases. Therefore, expression of CST regulates the sialylation of glycans on the cell surface. CST is categorized as the first member of subgroup A of Solute carrier family 35 (SLC35A1). CST activity was initially identified in microsomal membrane vesicles prepared from mouse liver. To date, CST genes from the mouse, human and two plant species have been expressed in a yeast expression system and their CST activities proved by an in vitro CMP-sialic acid transport assay using yeast Golgi enriched vesicles. In humans, inactivation of both CST alleles has been found to cause congenital glycosylation type IIf disorder in which the affected patient showed macrothrombocytopenia and neutropenia.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
CMP-sialic acid is a donor substrate of sialyltransferases. CMP-sialic acid transporter (CST) couples with the antiport of CMP and transports CMP-sialic acid from the cytosol into the lumen of the medial and trans cisternae of the Golgi apparatus (Capasso and Hirschberg 1984; Tiralongo et al. 2006; Zhao et al. 2006), where sialylation is mediated by a variety of sialyltransferases (Fig. 121.1). Therefore, expression of CST regulates the sialylation of glycans on the cell surface.
Schematic representation of the CMP-sialic acid transporter (CST/SLC35A1) in the glycosylation pathway. CMP-sialic acid transporter transports CMP-sialic acid from the cytosol into the lumen of the Golgi compartment by coupling with the antiport of CMP, which is produced as the result of a sialyltransferase reaction
CST is categorized as the first member of subgroup A of solute carrier family 35 (SLC35A1) (Ishida and Kawakita 2004). A phylogenetic tree of SLC35A nucleotide sugar transporters is shown in Fig. 121.2. To date, three types of mammalian CST have been identified based on their transporter activity, namely, Cricetulus griseus, Homo sapiens, and Mus musculus, and two types of plant CST have been identified, namely, Arabidopsis thaliana and Oryza sativa (Fig. 121.2 and see section “Databanks”). The plant CSTs cluster on the phylogenetic tree with human SLC35A4 rather than human SLC35A1. Thus, the substrate specificity of a nucleotide sugar transporter cannot easily be predicted from its shared phylogenetic homologies.
Phylogenetic tree of nucleotide sugar transporters of solute carrier family 35 member A (SLC35A) including CMP-sialic acid transporters (CST/SLC35A1). Parentheses indicate transport substrates that have been identified by in vitro activity analysis or complementation analysis. At Arabidopsis thaliana, Bt Bos taurus, Ce Caenorhabditis elegans, Cg Cricetulus griseus, Cl Canis lupus familiaris, Dm Drosophila melanogaster, Hs Homo sapiens, Lm Leishmania major, Mm Mus musculus, Os Oryza sativa Japonica Group, Sp Saccharomyces pombe, Ss Sus scrofa
CST activity was initially identified in microsomal membrane vesicles prepared from mouse liver (Carey et al. 1980). To date, CST genes from the mouse (Eckhardt et al. 1996), human (Eckhardt and Gerardy-Schahn 1997; Ishida et al. 1998) and two plant species (Bakker et al. 2008; Takashima et al. 2009) have been expressed in a yeast expression system and their CST activities proved by an in vitro CMP-sialic acid transport assay using yeast Golgi-enriched vesicles (Aoki et al. 2001, 2003; Bakker et al. 2008; Berninsone et al. 1997; Takashima et al. 2009).
In humans, inactivation of both CST alleles has been found to cause congenital glycosylation type IIf disorder in which the affected patient showed macrothrombocytopenia and neutropenia that probably arose from the lack of sialyl Lewis X structures (Martinez-Duncker et al. 2005).
Databanks
In the case of CMP-sialic acid transporter (CST), solute carrier family 35 member A1 (SLC35A1)
No EC number has been allocated.
CMP-Sialic Acid Transporter (CST) (SLC35A1)
Species | Gene symbol | GenBank accession number | Uniprot ID | PDB accession number |
|---|---|---|---|---|
Arabidopsis thaliana | AT5G41760 | NM_001036915 | Q8LGE9 | N/A |
NM_123541 | ||||
Cricetulus griseus (Chinese hamster) | Slc35a1 | NM_001246755 | O08520 | N/A |
Homo sapiens (Human) | SLC35A1 | NM_001168398 | P78382 | N/A |
NM_006416 | ||||
Mus musculus (Mouse) | Slc35a1 | NM_011895 | Q61420 | N/A |
Oryza sativa Japonica group | Os06g0523400 | NM_001064288 | Q654D9 | N/A |
Name and History
Almost all vertebrate CMP-sialic acids are synthesized in the nucleus by CMP-sialic acid synthetase (Kean et al. 2004) and then moved to the cytosol. However, sialylation reactions occur in the lumen of the Golgi apparatus where they are mediated by a variety of sialyltransferases. SLC35A1/CST is responsible for the transport of CMP-sialic acid from the cytosol into the lumen of the Golgi apparatus (Ishida and Kawakita 2004) (Fig. 121.2). This transporter activity was initially demonstrated in microsomal membrane vesicles prepared from mouse liver (Carey et al. 1980), and subsequent biochemical characterization of Golgi apparatus vesicles and reconstituted proteoliposomes confirmed its exclusive localization to the Golgi apparatus (Capasso and Hirschberg 1984; Milla and Hirschberg 1989).
Lec2 mutant cells, derived from a Chinese hamster ovary (CHO) cell line, lack sialylated oligosaccharides, and their Golgi membrane vesicles are deficient in CST activity (Deutscher et al. 1984). The mouse CST gene was first cloned by complementation of a defect in Lec2 cells (Eckhardt et al. 1996), and, subsequently, the hamster CST gene was identified by the same approach (Eckhardt and Gerardy-Schahn 1997). The human CST gene was cloned using its high homology to the murine CST gene, and its CMP-sialic acid transport activity was analyzed using microsomal vesicles prepared from Lec2 cells that overexpressed the human CST gene (Ishida et al. 1998). These CSTs are localized in the Golgi apparatus. Recently two CST genes, Arabidopsis thaliana and Oryza sativa Japonica group, have also been identified in plants and their activities have been characterized (Bakker et al. 2008; Takashima et al. 2009).
Structure
Mouse and Chinese hamster CSTs consist of 336 amino acids; analyses of the predicted structures suggest that they are multi-pass membrane proteins that have ten membrane-spanning domains. The amino and carboxyl termini are oriented toward the cytosol (Eckhardt et al. 1999). Human CST has two isoforms that consist of 337 amino acids and 278 amino acids, respectively. The longer isoform has the ten membrane-spanning domains and its C-terminus is exposed to the cytosol (Ishida et al. 1998).
Transporter Activity Assay and Substrate Specificity
In the initial assays of CST activity, microsomal membrane vesicles from mouse liver (Capasso and Hirschberg 1984) or reconstituted proteoliposomes from Golgi protein extracts (Milla and Hirschberg 1989) were incubated with CMP-[14C] sialic acid or CMP-[3H] sialic acid and then separated from the incubation mixture by centrifugation or by gel filtration using a Sephadex G-50 column. The amount of incorporated radiolabeled sialic acids in the vesicles or proteoliposomes was measured with a liquid scintillation counter to determine CMP-sialic acid transport activity.
In vitro assays of CST activity are most commonly carried out using a yeast expression system. As yeast (Saccharomyces cerevisiae) microsomal vesicles do not show any strong intrinsic nucleotide sugar transport activity except for GDP-mannose, then incorporation of radiolabeled sialic acids into the vesicles can be used to measure in vitro activity (Abeijon et al. 1989). This in vitro assay system has been used for functional identification and characterization of mouse CST (Berninsone et al. 1997) and plant CSTs (Bakker et al. 2008; Takashima et al. 2009) and for determination of the substrate recognition site in human CST (Aoki et al. 2001, 2003). The first step in the assay is to subclone a cDNA encoding a CST, or a CST tagged with an HA-epitope at the C-terminus, into a yeast expression vector, and the CST is then expressed. Golgi-enriched vesicles are prepared from the yeast incubated with CMP-[3H] sialic acid or CMP-[14C] sialic acid for the appropriate time and separated from the incubation mixture by centrifugation or filtration using a nitrocellulose filter (0.45 μm) (Abeijon et al. 1989). CST activity is measured using a liquid scintillation counter to determine the amount of radiolabeled CMP-sialic acids incorporated into the vesicles. Golgi-enriched vesicles prepared from S. cerevisiae transformed with an empty vector are used as a control. The apparent Km of mouse CST was determined as 2.9 μM by this system (Berninsone et al. 1997).
The E. coli expression system has also been used as an in vitro CST activity assay under zero background conditions (Tiralongo et al. 2006). A CST cDNA tagged with HA-epitopes is first subcloned into the E. coli expression vector, which has an isopropyl-β-D-thiogalactopyranoside- (IPTG) inducible trp/lac promoter, and expression of the CST is induced by adding IPTG to the culture medium. The recombinant HA-tagged CST is expressed in the inclusion body pellet. Washed inclusion body pellets are solubilized in buffer containing 8 M urea, and the CST is renatured by dialysis against a renaturation buffer. The renatured CST is reconstituted into proteoliposomes using the freeze-thawing method (Kasahara and Hinkle 1976) and used for the CST activity assay. The proteoliposomes are incubated with CMP-[3H] sialic acid for the appropriate time and then separated from the incubation mixture using a spin column. A liquid scintillation counter is used to determine CST activity in the collected void volume. Through use of this system, mouse CST has been shown to specifically transport CMP-sialic acid by coupling with the antiport of CMP; there is evidence that mouse CST could not transport any UDP-sugars (Tiralongo et al. 2006).
Preparation
Golgi-enriched vesicles containing CSTs have been prepared from mouse liver (Capasso and Hirschberg 1984), and proteoliposomes containing CSTs have been reconstructed from Golgi protein extracts and egg yolk phosphatidylcholine (Milla and Hirschberg 1989). The CST or HA-tagged CST of mouse (Berninsone et al. 1997), human (Aoki et al. 2001, 2003), and the two plant species (Bakker et al. 2008; Takashima et al. 2009) has been expressed in S. cerevisiae, and Golgi-enriched vesicles including CST have been prepared from the S. cerevisiae. These vesicles still include other nucleotide sugar transporters, although overexpressed CST might be a main component of the vesicles. Functional proteoliposomes have been successfully reconstituted from phosphatidylcholine and renatured mouse CST expressed in E. coli (Tiralongo et al. 2006). The CST was renatured from washed inclusion body pellets that contain unfolded proteins induced or overexpressed in E. coli. Almost all proteins in the inclusion body pellet are induced proteins; thus the purity of the renatured CST is regarded as being high. Therefore, reconstituted proteoliposomes effectively include no other nucleotide sugar transporters.
Biological Aspects
Human CST shows high homology (43 %) to human SLC35A2/UGT that transports UDP-galactose and UDP-N-acetyl galactosamine (Fig. 121.2) (Aoki et al. 2001; Segawa et al. 2002). Functional analyses using chimeric proteins showed that different regions of each transporter provide a critical contribution to recognition of each substrate and that some chimeric proteins could transport both substrates (Aoki et al. 2001, 2003). These findings suggest two possible developmental mechanisms for CSTs: (1) CST and UGT evolved from a common ancestral gene, or (2) CST is derived from UGT. Analyses of chimeric proteins and the substitution of amino acids showed that the Tyr214 and Ser216 residues of the seventh transmembrane region and the Lys272 residue of the fourth cytosolic loop are essential for CST activity (Aoki et al. 2003; Chan et al. 2010; Takeshima-Futagami et al. 2012).
CST is expressed in various tissues where it localizes to the medial and trans cisternae of the Golgi apparatus (Zhao et al. 2006) and supplies a donor substrate, CMP-sialic acid, to sialyltransferases. To date, twenty sialyltransferases have been identified in humans. Defects in CSTs result in abnormal sialylation patterns. Thus, for example, Lec2 CHO mutant cells have no sialylated N-glycans (including either type of sialic acid, N-acetylneuraminic acid, or N-glycolylneuraminic acid) and no sialylated O-glycans (North et al. 2010), because the Gly189Glu mutation in the CTS of Lec2 cells abolishes CTS activity (Eckhardt et al. 1998). Another CHO mutant cell line, MAR-11, shows a decrease in the levels of cell surface sialic acid because of a C to T mutation in the CTS gene that results in a premature stop codon (Lim et al. 2008). In humans, a patient with a congenital disorder of glycosylation type IIf and who had inactivation of both CST alleles (Martinez-Duncker et al. 2005) was found to exhibit marked macrothrombocytopenia and neutropenia caused by the lack of sialyl Lewis X structures (Willig et al. 2001). In the plant Arabidopsis thaliana, T-DNA insertions that disrupt the CST gene cause a lethal phenotype, indicating that CST is essential for plant development (Takashima et al. 2009).
Knockout and Transgenic Mice
To date, no CST knockout or transgenic mice have been reported.
Human Diseases
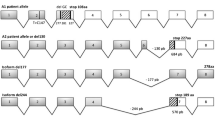
A patient with congenital disorder of glycosylation type IIf, whose polymorphonuclear cells completely lacked sialyl Lewis X antigen (CD15s, NeuAcα2,3Galβ1,4(Fucα1,3)GlcNAc-R), showed macrothrombocytopenia and neutropenia, and died at age 37 months (Martinez-Duncker et al. 2005). The paternally inherited allele of the CST gene contained 2 microdeletions (G277 and C281) that produced a frame shift and premature stop codon at position 327, in addition to a T147C substitution. The maternally inherited allele had a 130 bp deletion in exon 6 that generated a frame shift and premature stop codon at position 684. A common 4 bp insertion in intron 6 found in the mother was responsible for the splice mutation. Neither allele of the patient was able to complement Lec2 mutant cells, demonstrating that they were inactive.
Future Perspectives
Sialic acids are nine carbon sugars that contain an α-keto acid group (2-keto-3-deoxynononic acid) and consist of a large family with more than 50 derivatives. The principal members of this family are N-acetylneuraminic acid, N-glycolylneuraminic acid, and deaminoneuraminic acid. Sialic acids show diversity in their structures, unlike other nucleotide sugars. Currently, the type of sialic acid in many species is unclear. Indeed, it is yet certain whether all species have a sialic acid. To date, sialylated structures have been identified in vertebrates, echinoderms, plants, fungi, Drosophila, and squid; however, CST genes have not yet been identified in fungi, Drosophila, or squid. These genes will undoubtedly be determined in the near future. Such information will provide further insight into the evolution of sialylation.
The structural basis of substrate specificity for CSTs has not yet been clarified, although essential amino acids, Tyr214, Ser 216, and Lys272, have been determined using chimeric proteins and amino acid substitution analyses (Aoki et al. 2003; Chan et al. 2010; Takeshima-Futagami et al. 2012). Three-dimensional structural analyses using X-ray diffraction should provide more precise information on the mechanism for recognition of a specific nucleotide sugar substrate and for its transport.
Further Reading
-
Gerardy-Schahn et al. (2001): Nucleotide sugar transporters: biological and functional aspects.
-
Handford et al. (2006): Nucleotide-sugar transporters: structure, function and roles in vivo.
-
Ishida and Kawakita (2004): Molecular physiology and pathology of the nucleotide sugar transporter family (SLC35).
-
Liu et al. (2010): The role of nucleotide sugar transporters in development of eukaryotes.
References
Abeijon C, Orlean P, Robbins PW, Hirschberg CB (1989) Topography of glycosylation in yeast: characterization of GDP mannose transport and lumenal guanosine diphosphatase activities in Golgi-like vesicles. Proc Natl Acad Sci USA 86:6935–6939
Aoki K, Ishida N, Kawakita M (2001) Substrate recognition by UDP-galactose and CMP-sialic acid transporters. Different sets of transmembrane helices are utilized for the specific recognition of UDP-galactose and CMP-sialic acid. J Biol Chem 276:21555–21561
Aoki K, Ishida N, Kawakita M (2003) Substrate recognition by nucleotide sugar transporters: further characterization of substrate recognition regions by analyses of UDP-galactose/CMP-sialic acid transporter chimeras and biochemical analysis of the substrate specificity of parental and chimeric transporters. J Biol Chem 278:22887–22893
Bakker H, Routier F, Ashikov A, Neumann D, Bosch D, Gerardy-Schahn R (2008) A CMP-sialic acid transporter cloned from Arabidopsis thaliana. Carbohydr Res 343:2148–2152
Berninsone P, Eckhardt M, Gerardy-Schahn R, Hirschberg CB (1997) Functional expression of the murine Golgi CMP-sialic acid transporter in Saccharomyces cerevisiae. J Biol Chem 272:12616–12619
Capasso JM, Hirschberg CB (1984) Mechanisms of glycosylation and sulfation in the Golgi apparatus: evidence for nucleotide sugar/nucleoside monophosphate and nucleotide sulfate/nucleoside monophosphate antiports in the Golgi apparatus membrane. Proc Natl Acad Sci USA 81:7051–7055
Carey DJ, Sommers LW, Hirschberg CB (1980) CMP-N-acetylneuraminic acid: isolation from and penetration into mouse liver microsomes. Cell 19:597–605
Chan KF, Zhang P, Song Z (2010) Identification of essential amino acid residues in the hydrophilic loop regions of the CMP-sialic acid transporter and UDP-galactose transporter. Glycobiology 20:689–701
Deutscher SL, Nuwayhid N, Stanley P, Briles EI, Hirschberg CB (1984) Translocation across Golgi vesicle membranes: a CHO glycosylation mutant deficient in CMP-sialic acid transport. Cell 39:295–299
Eckhardt M, Gerardy-Schahn R (1997) Molecular cloning of the hamster CMP-sialic acid transporter. Eur J Biochem 248:187–192
Eckhardt M, Muhlenhoff M, Bethe A, Gerardy-Schahn R (1996) Expression cloning of the Golgi CMP-sialic acid transporter. Proc Natl Acad Sci USA 93:7572–7576
Eckhardt M, Gotza B, Gerardy-Schahn R (1998) Mutants of the CMP-sialic acid transporter causing the Lec2 phenotype. J Biol Chem 273:20189–20195
Eckhardt M, Gotza B, Gerardy-Schahn R (1999) Membrane topology of the mammalian CMP-sialic acid transporter. J Biol Chem 274:8779–8787
Gerardy-Schahn R, Oelmann S, Bakker H (2001) Nucleotide sugar transporters: biological and functional aspects. Biochimie 83:775–782
Handford M, Rodriguez-Furlan C, Orellana A (2006) Nucleotide-sugar transporters: structure, function and roles in vivo. Braz J Med Biol Res 39:1149–1158
Ishida N, Kawakita M (2004) Molecular physiology and pathology of the nucleotide sugar transporter family (SLC35). Pflugers Arch 447:768–775
Ishida N, Ito M, Yoshioka S, Sun-Wada GH, Kawakita M (1998) Functional expression of human Golgi CMP-sialic acid transporter in the Golgi complex of a transporter-deficient Chinese hamster ovary cell mutant. J Biochem 124:171–178
Kasahara M, Hinkle PC (1976) Reconstitution of D-glucose transport catalyzed by a protein fraction from human erythrocytes in sonicated liposomes. Proc Natl Acad Sci USA 73:396–400
Kean EL, Munster-Kuhnel AK, Gerardy-Schahn R (2004) CMP-sialic acid synthetase of the nucleus. Biochim Biophys Acta 1673:56–65
Lim SF, Lee MM, Zhang P, Song Z (2008) The Golgi CMP-sialic acid transporter: a new CHO mutant provides functional insights. Glycobiology 18:851–860
Liu L, Xu YX, Hirschberg CB (2010) The role of nucleotide sugar transporters in development of eukaryotes. Semin Cell Dev Biol 21:600–608
Martinez-Duncker I, Dupre T, Piller V, Piller F, Candelier JJ, Trichet C, Tchernia G, Oriol R, Mollicone R (2005) Genetic complementation reveals a novel human congenital disorder of glycosylation of type II, due to inactivation of the Golgi CMP-sialic acid transporter. Blood 105:2671–2676
Milla ME, Hirschberg CB (1989) Reconstitution of Golgi vesicle CMP-sialic acid and adenosine 3′-phosphate 5′-phosphosulfate transport into proteoliposomes. Proc Natl Acad Sci USA 86:1786–1790
North SJ, Huang HH, Sundaram S, Jang-Lee J, Etienne AT, Trollope A, Chalabi S, Dell A, Stanley P, Haslam SM (2010) Glycomics profiling of Chinese hamster ovary cell glycosylation mutants reveals N-glycans of a novel size and complexity. J Biol Chem 285:5759–5775
Segawa H, Kawakita M, Ishida N (2002) Human and Drosophila UDP-galactose transporters transport UDP-N-acetylgalactosamine in addition to UDP-galactose. Eur J Biochem 269:128–138
Takashima S, Seino J, Nakano T, Fujiyama K, Tsujimoto M, Ishida N, Hashimoto Y (2009) Analysis of CMP-sialic acid transporter-like proteins in plants. Phytochemistry 70:1973–1981
Takeshima-Futagami T, Sakaguchi M, Uehara E, Aoki K, Ishida N, Sanai Y, Sugahara Y, Kawakita M (2012) Amino acid residues important for CMP-sialic acid recognition by the CMP-sialic acid transporter: analysis of the substrate specificity of UDP-galactose/CMP-sialic acid transporter chimeras. Glycobiology 22:1731–1740
Tiralongo J, Ashikov A, Routier F, Eckhardt M, Bakker H, Gerardy-Schahn R, von Itzstein M (2006) Functional expression of the CMP-sialic acid transporter in Escherichia coli and its identification as a simple mobile carrier. Glycobiology 16:73–81
Willig TB, Breton-Gorius J, Elbim C, Mignotte V, Kaplan C, Mollicone R, Pasquier C, Filipe A, Mielot F, Cartron JP, Gougerot-Pocidalo MA, Debili N, Guichard J, Dommergues JP, Mohandas N, Tchernia G (2001) Macrothrombocytopenia with abnormal demarcation membranes in megakaryocytes and neutropenia with a complete lack of sialyl-Lewis-X antigen in leukocytes–a new syndrome. Blood 97:826–828
Zhao W, Chen TL, Vertel BM, Colley KJ (2006) The CMP-sialic acid transporter is localized in the medial-trans Golgi and possesses two specific endoplasmic reticulum export motifs in its carboxyl-terminal cytoplasmic tail. J Biol Chem 281:31106–31118
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Japan
About this entry
Cite this entry
Nishihara, S. (2014). Solute Carrier Family 35 (CMP-Sialic Acid Transporter), Member A1 (SLC35A1). In: Taniguchi, N., Honke, K., Fukuda, M., Narimatsu, H., Yamaguchi, Y., Angata, T. (eds) Handbook of Glycosyltransferases and Related Genes. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54240-7_98
Download citation
DOI: https://doi.org/10.1007/978-4-431-54240-7_98
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54239-1
Online ISBN: 978-4-431-54240-7
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences