Abstract
Osteochondral lesions of the talus (OLT) are frequently challenging problems for orthopaedic surgeons. Although the talar cartilage has remarkable stiffness to compression and elasticity, it is susceptible to injury and has limited regenerative capability. The treatment strategy mainly is based on classification, diameter, stage, and depth of the lesion as well as patients’ age and level of activity, presence of kissing lesions, and lower limb alignment. Debridement, curettage, antegrade/retrograde drilling, microfracture, and mosaicplasty are the most frequently used treatments for osteochondral talar lesions. Recently a novel treatment method – resurfacing arthroplasty – has become popular. The goal of this chapter is to describe the surgical technique of talar resurfacing arthroplasty, review the current literature, and discuss rehabilitation protocols after talar resurfacing.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Introduction
Osteochondral lesions of the talus (OLT) can be defined as defects of the cartilaginous surface and underlying subchondral bone of the talus. They are commonly located on the medial dome and are often deep and larger than lateral lesions (Elias et al. 2007). A history of trauma has been reported in 7–41 % of patients with OLT (van Dijk et al. 1996). Beside trauma, OLT has been reported rarely with concomitant osteonecrosis, endocrine disorders, and genetic abnormalities (Mandracchia et al. 1999). However, the etiology still remains uncertain (Alexander and Lichtman 1980; Schuman et al. 2002).
Diagnosis of OLTs requires a high index of suspicion because these lesions are rare and the symptoms such as pain, swelling, tenderness, and locking can be present with several ankle disorders. Although the talar cartilage has remarkable stiffness to compression and elasticity, it is susceptible to injury and has limited regenerative capability (Buckwalter and Mankin 1998b; Brittberg 1999). Arthroscopic debridement, antegrade/retrograde drilling, and microfracture are considered the primary treatment options for OLT and yield 85 % success (Zengerink et al. 2010). However, they have been shown to have limited value because of replacing the hyaline cartilage with fibrous cartilage with comparatively poorer biomechanics (Furukawa et al. 1980; Brittberg et al. 1994; Buckwalter and Mankin 1998a). Therefore, for primary treatment failures or large lesions, new techniques have developed to provide hyaline or hyaline-like repair, including autologous osteochondral transplantation (mosaicplasty) and biomaterials and autologous chondrocyte implantation (ACI) (Giannini et al. 2002; Hangody 2003; Kilic et al. 2009; Zengerink et al. 2010). Each of these techniques has disadvantages such as donor site morbidity, technical difficulties, and two-stage surgery (Paul et al. 2009; Reddy et al. 2007). Considering these problems, a new metallic focal resurfacing implant was developed in 2007 as a salvage option (HemiCAP®, Arthrosurface Inc., MA). Its clinical goals are to reduce pain and prevent progression of the lesion.
In this chapter a sample case is presented to describe the surgical technique of talar resurfacing arthroplasty, review the current literature, and discuss the surgical dilemmas posed by this relatively common problem.
Case Presentation
A 37-year-old male was referred to the orthopaedic department with a 2-year history of persistent ankle pain following an initial inversion injury. One year earlier, arthroscopic debridement and retrograde drilling had been done to treat an osteochondral lesion of right talus. At the presentation, there were no abnormal physical examination findings except pain during forced motions. Radiographs and magnetic resonance imaging (MRI) revealed a large cystic defect, measuring 3.5 × 3.3 × 2.9 mm on the medial side of talar dome (Fig. 1).
Surgical Technique
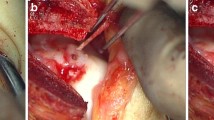
Before the open procedure a diagnostic arthroscopy was performed and a full-thickness chondral defect was observed at the medial part of talar dome (Fig. 2). During the arthroscopy, a subchondral cyst was easily identified with gentle probing. Following the identification of the lesion, an open approach was made just behind the medial malleolus. An anterior arthrotomy was performed, and a retractor was placed into the joint to prevent inadvertent chondral injury during osteotomy. To expose the lesion located in the medial part of the talar dome, an oblique medial malleolar osteotomy was made at the transition between the tibial plafond and medial malleolar joint surface (Gautier et al. 2002; Hangody et al. 2004), which provided excellent exposure of the defect on talus (Fig. 3). At the second stage of the operation, the injured cartilage was debrided with a curette until normal healthy cartilage bordered the debrided defect. Then a pilot hole was created. After drilling and tapping of the pilot hole, the fixation screw was inserted into the subchondral bone up to a precise depth. The surface of the prosthetic device was placed slightly recessed relative to the surrounding surface of the talar cartilage to avoid excessive contact pressure of the implant (van Bergen et al. 2011b). The prosthetic device was implanted 0.5 mm below the adjacent cartilage (Anderson et al. 2010) (Fig. 4). Following joint irrigation, the medial malleolar osteotomy was fixed with two screws (Fig. 5).
Postoperative Rehabilitation Protocol
The postoperative management was started with immediate continuous passive motion the day after surgery and was gradually increased as tolerated. No weight-bearing mobilization was advised for the first two weeks, and then partial weight bearing was allowed with a brace (Walker). During this period non-weight-bearing sagittal range-of-motion exercises were allowed 15 min twice daily, and active plantar flexion/dorsiflexion were encouraged (Reilingh et al. 2012). Radiographs of the operated ankle were obtained to confirm consolidation of the malleolar osteotomy 6 weeks after surgery, and then full weight-bearing mobilization was allowed. Subsequently, to achieve early return to sports in high-demand athletic patients, physical therapy should be used to assist functional recovery with strengthening of ankle muscles and improving proprioceptive ability.
Return to Sports
Sports-specific activities were allowed after bone healing. Before return to activity or return to sport can be considered, it is important to quantify the levels of activity. Van Eekeren et al. described return to activity criteria in patients treated for talar OCDs as four levels of increasing intensity: walking, running, return to non-contact sports, and return to contact sports (van Eekeren et al. 2012). Each of these levels demands specific training and exercises, and each has to be mastered before the next level can be attempted. Considering the patient’s clinical evaluation, running on even ground is permitted after 12 weeks when the patient has no pain and full range of motion in the strengthened ankle. Full return to normal and sporting activities is usually possible four to six months after surgery.
Discussion
Osteochondral lesion of the talus is a clinical challenge to orthopaedic surgeons. These lesions commonly occur in young and active individuals, and the ability to return to their presymptomatic level of function depends on various factors: patient age and activity level, lesion size and stage, and appearance of the lesion. Although multiple treatment modalities are currently available, the best option for restoring joint function, reducing pain, and avoiding early degeneration is not clear (Giannini et al. 2005; Sexton and Labib 2007, pp. 166–171).
Alternative forms of surgical treatment for osteochondral defects of the talus have been described, such as antegrade drilling, retrograde drilling with preservation of the overlying cartilage, osteochondral transplantation, autologous chondrocyte implantation, and metallic focal resurfacing (Angermann and Jensen 1989; Ferkel and Scranton 1993; Hangody et al. 1997; Taranow et al. 1999; Baums et al. 2006; van Bergen et al. 2011a).
The results of bone stimulation techniques that involve penetration of the subchondral plate to obtain stem cells from the marrow cavity for reimplantation into the defect has been well documented, with clinical success rates ranging between 51 % and 88 % (Bryant and Siegel 1993; Taranow et al. 1999). However, replacement of the hyaline cartilage by fibrocartilage, which has comparatively poorer biomechanical properties, is the main disadvantage of these traditional resurfacing techniques (Furukawa et al. 1980; Hangody et al. 1997). Verhagen et al. reported 22 % unsatisfactory results of chondroplasty and 14 % unsatisfactory results of microfracture in patients with OLT (Verhagen et al. 2003). In a more recent study, Gobbi et al. demonstrated 20 % poor results after microfracture (Gobbi et al. 2006). On the other hand, Doral et al. (2012) demonstrated improvement in the postoperative functional and pain scores of patients with OLT treated by microfracture combined with hyaluronan injection. They reported significantly higher (P < 0.001) postoperative AOFAS scores in patients in the injection group compared to those who did not receive an injection.
Currently, new techniques that provide hyaline-like repair tissue have gained great popularity in the treatment of OLT. In 1997 Hangody et al. compared four arthroscopic resurfacing techniques (drilling, abrasion arthroplasty, microfracture, and mosaicplasty) in a multicentric, prospective study involving 413 patients. As the result of this study, they concluded that mosaicplasty, which provides hyaline cartilage-type resurfacing, gives significantly better clinical outcomes than the other techniques, especially after 3, 4, and 5 years (Hangody et al. 1998). An additional three prospective studies have shown favorable clinical results of mosaicplasty for OLT. Hangody et al. reported 94 % good or excellent result (Hangody et al. 2001). Subsequently Al-Shaikh et al. noted a remarkable improvement in ankle function after mosaicplasty, with an average AOFAS score of 88 (Al-Shaikh et al. 2002). In 2006 Kreuz et al. reported 35 patients with osteochondral talar lesions for which previous traditional resurfacing techniques had failed and were then treated with mosaicplasty with an osteochondral graft harvested from the ipsilateral talar articular facet. After a mean follow-up of 48.9 months, they noticed statistically significant improvement between preoperative and postoperative AOFAS scores (Kreuz et al. 2006). Lee et al. reported 88.8 % excellent and 11.8 % good results at 36 months’ follow-up (Lee et al. 2003). Thirteen of their patients (72 %) returned to sports, with six patients (33 %) reaching their previous level of sports activity. Scranton et al. reported 90 % good to excellent score of 80.3 on the Karlsson-Peterson Ankle Score at a mean follow-up of 36 months after knee-to-ankle mosaicplasty (Scranton et al. 2006). In a retrospective study, good results of mosaicplasty in nine patients were documented with a mean AOFAS score of 80.2 at a follow-up of 72 months (Assenmacher et al. 2001). Gautier et al. reported 100 % had good and excellent results in 11 patients 24 months after surgery. The mean AOFAS ankle score was 92, and 9 of 11 patients returned to their preinjury level of activity (82 %) (Gautier et al. 2002). In contrast, Valderrabano et al. (2009) found their results to be less encouraging because of significant restrictions in dorsiflexion of the ankle that were observed after mosaicplasty for OLT surgery (Valderrabano et al. 2009).
Although several studies report good results with arthroscopic debridement and bone marrow stimulation and osteochondral autograft transfer, these techniques are sometimes associated with donor site morbidity (Reddy et al. 2007; Paul et al. 2009). Furthermore, ACI was introduced as a promising technique that brings less surgical morbidity and has the ability to fill bigger lesions with hyaline cartilage (Dozin et al. 2005; Baums et al. 2006; Sexton and Labib 2007, pp. 166–171); however, the cost of preparing a culture of hyaline cartilage cells and the necessity for two operative procedures are the main disadvantages of ACI (Baums et al. 2006).
Recently metallic focal resurfacing implantation has been shown to be a promising option to treat medial OLT after failed primary techniques, especially in middle-aged and older patients (Anderson et al. 2010; van Bergen et al. 2010, 2011a). Although it is not a biologic solution, it restores the joint contour, provides stability, and reproduces normal joint mechanics. The ideal patient for focal metallic resurfacing is still unclear. The indications /contraindications are listed below (van Bergen et al. 2011a):
-
(i)
Indications:
-
Middle-aged/older
-
Medial OLT
-
Chronic ankle pain after primary surgical treatment
-
-
(ii)
Contraindications:
-
Young age (<18 year)
-
OCD size >20 mm
-
Grade II–III ankle osteoarthritis
-
Concomitant ankle pathology
-
Advanced osteoporosis
-
Infection
-
Diabetes mellitus
-
Allergy to implant material
-
Because of the unique geometry of the talus and the relative thinness of its cartilage (1.1 ± 0.18 mm) (Millington et al. 2007), the use of focal resurfacing implants for treatment of talar osteochondral defects is challenging. Decisions about implant design, selection, and surgical approach are more complicated than with other joints (e.g., femoral head, humeral head, femoral condyles). Therefore, precise surgical planning and technique are required in terms of implantation depth, position, and orientation because of the biomechanical properties of the ankle joint (Fig. 6). A proud implant may damage apposing cartilage by causing excessive contact pressures during weight bearing (Custers et al. 2009, 2010). Custers et al. emphasized the importance of optimization of defect-size implants to avoid apposing cartilage degeneration (Custers et al. 2010). Becher et al. determined the peak contact pressure in the tibiofemoral joint with a partial femoral resurfacing device. They reported a statistically significant increase in peak contact pressures with a 1-mm proud implant (Becher et al. 2008). On the other hand, a deep implant might result in resorption of the walls of the defect, the formation of a large cavitary lesion, and the collapse of the surrounding articular cartilage and subchondral bone (Jackson et al. 2001). Hence, the “best available” implantation height aimed to restore the original contact stress distribution in the ankle. Van Bergen et al. demonstrated a significant reduction of total ankle contact pressure on the defect after implantation of the resurfacing metal 0.5 mm below the articular surface (van Bergen et al. 2010). Furthermore, a significant increase in cartilage contact stress was demonstrated after proud implantation of focal resurfacing in a cadaver model. The authors emphasized that even a small degree of proud implantation should be carefully avoided to minimize the risk of excessive cartilage contact stresses acutely after focal resurfacing (Anderson et al. 2010).
There is little information in the literature about postoperative rehabilitation and return to sports issues after surgical treatment of talar osteochondral lesions. The duration of immobilization and weight-bearing strategies depends on the surgical approach and the size and location of the defect. Partial weight bearing is allowed for the first 4 weeks after debridement and bone marrow stimulation (Chuckpaiwong et al. 2008). On the other hand, 8 weeks of non-weight bearing was the postoperative regimen for OCDs treated with osteochondral autograft transfer (Gobbi et al. 2006). After autologous chondrocyte implantation (ACI), patients are kept non-weight bearing and placed in a well-padded short-leg cast; partial weight bearing and gentle ankle range of motion are allowed after the first 2 weeks. Saxena et al. published a case series of 26 microfracture procedures and 20 bone graft procedures of the talus. Patients were kept non-weight bearing for up to 6 weeks, although patients with small lesions (<3 mm in diameter) were allowed to partially bear weight at 3 weeks. It was concluded that both techniques allow patients to return to sports with similar postoperative AOFAS scores. Full weight bearing and return to sports after microfracturing of OCDs of the talus usually are allowed earlier than after other treatment options (Saxena and Eakin 2007).
Summary
In conclusion, resurfacing of talar osteochondral defects is a promising option, especially for revision cases. It restores joint congruency, improves immediate stability of ankle, allows early rehabilitation, and reproduces normal joint mechanics. Even though long-term follow-up results are still unclear, arthrosurfacing has been suggested to be a safe option, with appropriate offset sizes for various talar domes and without excessive pressure on the opposite cartilage, in the treatment of talar osteochondral lesions.
References
Al-Shaikh RA et al (2002) Autologous osteochondral grafting for talar cartilage defects. Foot Ankle Int 23(5):381–389
Alexander AH, Lichtman DM (1980) Surgical treatment of transchondral talar-dome fractures (osteochondritis dissecans). Long-term follow-up. J Bone Joint Surg Am 62(4):646–652
Anderson DD et al (2010) Effect of implantation accuracy on ankle contact mechanics with a metallic focal resurfacing implant. J Bone Joint Surg Am 92(6)):1490–1500
Angermann P, Jensen P (1989) Osteochondritis dissecans of the talus: long-term results of surgical treatment. Foot Ankle 10(3):161–163
Assenmacher JA et al (2001) Arthroscopically assisted autologous osteochondral transplantation for osteochondral lesions of the talar dome: an MRI and clinical follow-up study. Foot Ankle Int 22(7):544–551
Baums MH et al (2006) Autologous chondrocyte transplantation for treating cartilage defects of the talus. J Bone Joint Surg Am 88(2):303–308
Becher C et al (2008) Effects of a contoured articular prosthetic device on tibiofemoral peak contact pressure: a biomechanical study. Knee Surg Sports Traumatol Arthrosc 16(1):56–63
Brittberg M (1999) Autologous chondrocyte transplantation. Clin Orthop Relat Res (367 Suppl):S147–S155
Brittberg M et al (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331(14):889–895
Bryant DD, Siegel MG (1993) Osteochondritis dissecans of the talus: a new technique for arthroscopic drilling. Arthroscopy 9(2):238–241
Buckwalter JA, Mankin HJ (1998a) Articular cartilage repair and transplantation. Arthritis Rheum 41(8):1331–1342
Buckwalter JA, Mankin HJ (1998b) Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr Course Lect 47:477–486
Chuckpaiwong B, Berkson EM, Theodore GH (2008) Microfracture for osteochondral lesions of the ankle: outcome analysis and outcome predictors of 105 cases. Arthroscopy 24(1):106–112
Custers RJ et al (2009) Cartilage damage caused by metal implants applied for the treatment of established localized cartilage defects in a rabbit model. J Orthop Res 27(1):84–90
Custers RJ et al (2010) Cartilage degeneration in the goat knee caused by treating localized cartilage defects with metal implants. Osteoarthritis Cartilage 18(3):377–388
Doral MN et al (2012) Treatment of osteochondral lesions of the talus with microfracture technique and postoperative hyaluronan injection. Knee Surg Sports Traumatol Arthrosc 20(7):1398–1403
Dozin B et al (2005) Comparative evaluation of autologous chondrocyte implantation and mosaicplasty: a multicentered randomized clinical trial. Clin J Sport Med 15(4):220–226
Elias I et al (2007) Osteochondral lesions of the talus: localization and morphologic data from 424 patients using a novel anatomical grid scheme. Foot Ankle Int 28(2):154–161
Ferkel RD, Scranton PE Jr (1993) Arthroscopy of the ankle and foot. J Bone Joint Surg Am 75(8):1233–1242
Furukawa T et al (1980) Biochemical studies on repair cartilage resurfacing experimental defects in the rabbit knee. J Bone Joint Surg Am 62(1):79–89
Gautier E, Kolker D, Jakob RP (2002) Treatment of cartilage defects of the talus by autologous osteochondral grafts. J Bone Joint Surg (Br) 84(2):237–244
Giannini S et al (2005) Surgical treatment of osteochondral lesions of the talus in young active patients. J Bone Joint Surg Am 87(Suppl 2):28–41
Giannini S, Vannini F, Buda R (2002) Osteoarticular grafts in the treatment of OCD of the talus: mosaicplasty versus autologous chondrocyte transplantation. Foot Ankle Clin 7(3):621–633
Gobbi A et al (2006) Osteochondral lesions of the talus: randomized controlled trial comparing chondroplasty, microfracture, and osteochondral autograft transplantation. Arthroscopy 22(10):1085–1092
Hangody L (2003) The mosaicplasty technique for osteochondral lesions of the talus. Foot Ankle Clin 8(2):259–273
Hangody L et al (1997) Treatment of osteochondritis dissecans of the talus: use of the mosaicplasty technique – a preliminary report. Foot Ankle Int 18(10):628–634
Hangody L et al (1998) Mosaicplasty for the treatment of articular cartilage defects: application in clinical practice. Orthopedics 21(7):751–756
Hangody L et al (2001) Mosaicplasty for the treatment of osteochondritis dissecans of the talus: two to seven year results in 36 patients. Foot Ankle Int 22(7):552–558
Hangody L et al (2004) Autologous osteochondral mosaicplasty. Surgical technique. J Bone Joint Surg Am 86-A(Suppl 1):65–72
Jackson DW et al (2001) Spontaneous repair of full-thickness defects of articular cartilage in a goat model. A preliminary study. J Bone Joint Surg Am 83-A(1):53–64
Kilic A et al (2009) Early results of open mosaicplasty in osteochondral lesions of the talus. Acta Orthop Traumatol Turc 43(3):235–242
Kreuz PC et al (2006) Mosaicplasty with autogenous talar autograft for osteochondral lesions of the talus after failed primary arthroscopic management: a prospective study with a 4-year follow-up. Am J Sports Med 34(1):55–63
Lee CH et al (2003) Osteochondral autografts for osteochondritis dissecans of the talus. Foot Ankle Int 24(11):815–822
Mandracchia VJ, Buddecke DE Jr, Giesking JL (1999) Osteochondral lesions of the talar dome. A comprehensive review with retrospective study. Clin Podiatr Med Surg 16(4):725–742
Millington SA et al (2007) Quantification of ankle articular cartilage topography and thickness using a high resolution stereophotography system. Osteoarthritis Cartilage 15(2):205–211
Paul J et al (2009) Donor-site morbidity after osteochondral autologous transplantation for lesions of the talus. J Bone Joint Surg Am 91(7):1683–1688
Reddy S et al (2007) The morbidity associated with osteochondral harvest from asymptomatic knees for the treatment of osteochondral lesions of the talus. Am J Sports Med 35(1):80–85
Reilingh ML, van Bergen CJA, van Dijk CN (2012) Novel metal implantation technique for osteochondral defects of the medial talar dome. Tech Foot Ankle 11:45–49
Saxena A, Eakin C (2007) Articular talar injuries in athletes: results of microfracture and autogenous bone graft. Am J Sports Med 35(10):1680–1687
Schuman L, Struijs PA, van Dijk CN (2002) Arthroscopic treatment for osteochondral defects of the talus. Results at follow-up at 2 to 11 years. J Bone Joint Surg (Br) 84(3):364–368
Scranton PE Jr, Frey CC, Feder KS (2006) Outcome of osteochondral autograft transplantation for type-V cystic osteochondral lesions of the talus. J Bone Joint Surg (Br) 88(5):614–619
Sexton A, Labib SA (2007) Osteochondral lesions of the talus: current opinions on diagnosis and management. Curr Opin Orthop 18(2):166–171
Taranow WS et al (1999) Retrograde drilling of osteochondral lesions of the medial talar dome. Foot Ankle Int 20(8):474–480
Valderrabano V et al (2009) Knee-to-ankle mosaicplasty for the treatment of osteochondral lesions of the ankle joint. Am J Sports Med 37(Suppl 1):105S–111S
van Bergen CJ, Reilingh ML, van Dijk CN (2011a) Tertiary osteochondral defect of the talus treated by a novel contoured metal implant. Knee Surg Sports Traumatol Arthrosc 19(6):999–1003
van Bergen CJ et al (2011b) Direction of the oblique medial malleolar osteotomy for exposure of the talus. Arch Orthop Trauma Surg 131(7):893–901
van Bergen CJ et al (2010) Novel metallic implantation technique for osteochondral defects of the medial talar dome. A cadaver study. Acta Orthop 81(4):495–502
van Dijk CN, Bossuyt PM, Marti RK (1996) Medial ankle pain after lateral ligament rupture. J Bone Joint Surg (Br) 78(4):562–567
van Eekeren IC, Reilingh ML, van Dijk CN (2012) Rehabilitation and return-to-sports activity after debridement and bone marrow stimulation of osteochondral talar defects. Sports Med 42(10):857–870
Verhagen RA et al (2003) Systematic review of treatment strategies for osteochondral defects of the talar dome. Foot Ankle Clin 8(2):233–242
Zengerink M et al (2010) Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc 18(2):238–246
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Doral, M.N., Huri, G., Turhan, E., Dönmez, G., Kaya, D. (2015). Arthrosurfacing in Talar Osteochondral Lesions. In: Doral, M.N., Karlsson, J. (eds) Sports Injuries. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-36569-0_145
Download citation
DOI: https://doi.org/10.1007/978-3-642-36569-0_145
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-36568-3
Online ISBN: 978-3-642-36569-0
eBook Packages: MedicineReference Module Medicine