Abstract
Free radicals possess at least one unpaired electron in the outer electron orbit and usually, but not always, are highly chemically reactive. Molecular dioxygen (O2) is stable; however, the oxygen-centered free radicals, superoxide (O2 •−) and hydroxyl (•OH), are not stable. In biological systems, reactive oxygen species (ROS), such as superoxide (O2 •−) and hydrogen peroxide (H2O2), play important signaling roles but may also contribute to cellular damage and disease development. Nitric oxide (also known as nitrogen oxide, or nitrogen monoxide, or simply NO) is also a free radical, and the existence of an unpaired electron may be reflected by the use of the abbreviation NO• rather than NO. Unless discussing the three-redox forms of nitrogen monoxide (the nitrosonium ion, NO+, the uncharged free radical, NO•, and the nitroxyl anion, NO− or HNO), the abbreviation NO will be used throughout this chapter. Reactive nitrogen species (RNS) are produced in biological systems starting with the reaction of NO with O2 •− to form the highly reactive RNS peroxynitrite (ONOO−) that, unlike NO or O2 •−, is a very strong oxidant and nitrating agent. Thus, despite both NO and O2 •− being free radicals, neither are as reactive as ONOO−, and the toxicity of these two free radicals relates primarily to ONOO−. Understanding how ONOO− modulates different intracellular biochemical pathways and how this may affect normal physiological processes and/or give rise to pathological conditions is an emerging area of great scientific interest. ONOO− exerts its adverse effects by direct interaction with CO2, proteins that contain transition metal centers or thiols, or indirectly by aiding the generation of the highly potent hydroxyl radical. In this chapter, we outline the biochemistry and pathophysiology of ONOO− with a particular reference to cardiovascular disease and diabetes. We also address how scavenging strategies can attenuate the toxic effects of ONOO− and therefore may repress the pathophysiological effects of ONOO− and offer the potential for new therapeutic interventions.
Gnanapragasam Arunachalam and Samson Mathews Samuel contributed equally to the manuscript.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Free radicals
- Hydroxyl radical, •OH
- Nitric oxide, NO
- Peroxynitrite, ONOO−
- Reactive nitrogen species, RNS
- Reactive oxygen species, ROS
- Superoxide, O2 •−
Introduction
A reducing intracellular environment is essential for normal cellular function. Short-lived highly reactive molecules, such as reactive oxygen species (ROS) and reactive nitrogen species (RNS), that are capable of rapidly and drastically changing the normal reduced intracellular environment may therefore be detrimental to the normal function and survival of a cell. However, ROS and other small reactive molecules have been identified as important players in a wide array of physiologic and pathological conditions (Droge 2002). Thus, understanding that these molecules are endogenously produced under tightly controlled conditions suggests that ROS and RNS play significant roles in signal transduction events associated with various physiological processes (Droge 2002).
Oxygen is essential for respiration and energy production in aerobic organisms. However, ROS can turn out to be a serious threat to the cell, and oxidative stress is associated with disease, and thus the reduction of oxidative stress is a target for therapeutic intervention. In aerobic organisms, oxygen undergoes conversion to water in the mitochondria; however, an incomplete one-electron reduction results in the formation of a number of highly reactive (owing to their unpaired valence shell electrons) molecules commonly called ROS (Maulik and Das 2008). The ROS produced in cells include hydrogen peroxide (H2O2), hypochlorous acid (HClO), and free radicals such as the hydroxyl radical (•OH) and the superoxide anion (O2 •−). ROS, such as O2 •− and H2O2, when produced in controlled amounts, have been found to be ideal signaling molecules since they are rapidly generated, easily degradable, and ubiquitously produced in all cell types (Droge 2002; Maulik and Das 2008).
Another gas, the short-lived nitric oxide (NO), which is also a free radical and may be designated as such by the use of the abbreviation NO•, is known to be an omnipresent intercellular messenger, modulating blood flow, neuronal activity, thrombosis, and a multitude of cellular events (Pacher et al. 2007). NO was initially identified in the 1980s as an endothelium-derived relaxation factor, EDRF, capable of causing vascular vasodilatation (Furchgott and Zawadzki 1980; Palmer et al. 1987; Furchgott and Vanhoutte 1989; Ignarro 1990; Thomas et al. 2008). Additionally, macrophages, participating in antitumor and anti-pathogen responses, were also found to produce NO (Stuehr and Nathan 1989; Granger and Hibbs 1996). It was not long before NO was identified to actively participate in pathophysiological responses as well. A number of studies have implied that endogenously generated NO can be toxic, while others have shown that it is protective. NO-associated toxicity was found to be associated with the generation of RNS (see review by Thomas et al. 2008).
The harmful effects of ROS and RNS on the cell are (1) damage to DNA (Wiseman and Halliwell 1996; Cooke et al. 2003; Pacher et al. 2007), (2) oxidation of lipids (Thomas et al. 1985; Radi et al. 1991a; Hall et al. 2004; Pacher et al. 2007), and (3) alteration of amino acids and proteins (Hall et al. 2004; Pacher et al. 2007; Grimsrud et al. 2008). However, during normal cellular activity, neither O2 •− nor NO can be considered particularly toxic since there are normally efficient means to prevent their accumulation and limit their biological half-life (Beckman 1996; Pacher et al. 2007). Different forms of superoxide dismutase (SODs) located in the cytoplasm, SOD1, mitochondria, SOD2, or extracellular matrix, SOD3, rapidly remove O2 •− and convert to H2O, while NO rapidly diffuses through tissues into red blood cells where it is rapidly converted to nitrate (Beckman and Koppenol 1996; Butler et al. 1998; Joshi et al. 2002; Pacher et al. 2007). SOD, the major route for the elimination of O2 •−, removes O2 •− at 2 × 109M−1.s−1 (Beckman and Koppenol 1996). NO can both inhibit and stimulate O2 •−-induced oxidation. Thus, when the sources of O2 •− (NADPH oxidases in the plasma membrane, cytosolic xanthine oxidase, or mitochondrial respiratory complexes) and NO (nitric oxide synthases) are spatially associated and local NO concentrations reach micromolar levels, the superoxide and NO will yield the most potent and highly reactive RNS, ONOO−, in a nonenzymatic reaction that has a rate constant of 6.7 × 109M−1.s−1 that is approximately four times faster than that for removal of O2 •− by SOD (Beckman and Koppenol 1996; Jourd’heuil et al. 2001; see also Kissner et al. 1997). CO2 is present in comparatively high concentrations in cells, and the reaction between ONOO− and the nucleophile CO2 yields nitrogen dioxide (•NO2) and carbonate radical (CO3 •−), which in turn initiates many of the damaging reactions in the biological systems (Ferrer-Sueta and Radi 2009; Pacher et al. 2007). In addition, ONOO− is known to be a strong oxidant and nitrating agent interacting with electron-rich sulfhydryl groups, iron-sulfur centers, zinc thiolates, as well as sulfhydryl sites in tyrosine phosphatase enzymes (Castro et al. 1994; Crow et al. 1995; Radi et al. 1991b; Takakura et al. 1999). Thus, ONOO− can inhibit both phosphorylation and dephosphorylation processes in cell signaling pathways.
ONOO− directly, or indirectly, contributes to pathological conditions such as cardiac diseases, vascular diseases, stroke, cancer, neurodegenerative disorders, and diabetes (see review by Pacher et al. 2007). An accumulation of evidence suggests that ONOO− and its derivatives play critical roles in the progression of diabetes and diabetic-associated vascular complications. In the current chapter, we provide a brief overview of the pathophysiological actions of ONOO− with a particular focus on diabetes and how scavenging ONOO− might aid in alleviating the complications associated with diabetes.
The Biology of ONOO−
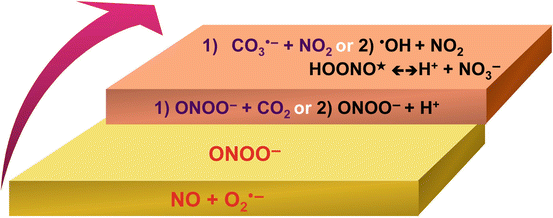
A brief history of the chemistry and biochemistry of ONOO− has been comprehensively reviewed by Koppenol (2001). Co-localization of both NO and O2 •− leads to the formation of ONOO−. The oxidative chemistry is pH dependent and at pH 13 is stable for several days when stored at 4 °C (Bohle et al. 1994). Stability is also increased by hydrogen bonding in the presence of, for instance, mannitol and ethanol (Alvarez et al. 1998). In the cis-conformation, ONOO− is essentially stable; however, the protonated form, peroxynitrous acid (HONOO), has a pKa of 6.8 and decays to nitrate with a rate of 1.3 s−1 at 25 °C (Koppenol et al. 1992; Tsai et al. 1994). HONOO decomposes rapidly at physiological pH, and in the presence of bicarbonate, ONOO− reacts with CO2 to produce NO2 − and carbonate ion radicals (CO3 −) [Reaction 1]. It has been assumed that ONOO− may be reduced to form hydroxide anion (•OH) and NO2 − and that these are the oxidant species (Beckman et al. 1990; Goldstein and Merényi 2008) [Reaction 2]; however, •OH is highly reactive, has a very short half-life of 10−9 s, and, except in organic solvents, would be subjected to extremely rapid scavenging (Sies 1963; Pryor and Squadrito 1995). Thus, most likely, it is an energetic and highly reactive transform of HONOO, termed HONOO*, that is the intermediate for the formation of nitrate, NO3 (Koppenol et al. 1992; Goldstein et al. 1996) [Reaction 2]. For both •OH and NO2 − to be formed, it is argued that this must occur in a “cage” where the radicals are “protected” and held together by solvent molecules (Leffler and Grunwald 1963; Pryor and Squadrito 1995). This caged mechanism explains why it is not possible to completely scavenge the reactive molecule(s), assumed to be HONOO*, that is involved in ONOO−-mediated reactions (Pryor 1966; Pryor and Squadrito 1995) (Fig. 9.1).
Reactions leading to the formation of peroxynitrite and peroxynitrite-derived radicals. Co-localization of both nitric oxide (NO) and superoxide anion (O2 •−) leads to the formation of peroxynitrite (ONOO−). Due to the availability of nucleophilic CO2, ONOO− rapidly reacts with CO2 to yield carbonate radical (CO3 •−) and nitrogen dioxide (NO2)- [Reaction 1]. ONOO− may also be reduced to from hydroxide anion (•OH) and NO2 −; however, in biological systems, it is an energetic form of peroxynitrous acid (HONOO), termed HONOO*, that is likely to be the intermediate for the formation of nitrate (NO3 −) [Reaction 2]
The Biochemistry of ONOO−
Formation
Numerous stimuli such as toxins, stress, ultraviolet light, ischemia/reperfusion injury, and inflammation can result in the production of ONOO− (Ahmad et al. 2009). ONOO− is produced mainly in macrophages, endothelial cells, platelets, leukocytes, neurons, etc., as a result of a rapid nonenzymatic combination reaction between NO and O2 •− (Ahmad et al. 2009). Major cellular sources for the generation of O2 •− are, for instance, mitochondria, NADPH oxidase, xanthine oxidase, and also from oxidation of catechols. Under normal physiological conditions, SODs rapidly remove the O2 •− anion; however, under conditions when the generation of NO (at ∼10 μM) outcompetes the capacity of SODs, NO reacts faster with O2 •− than decomposition by SOD (Su and Groves 2010). It is assumed that the site of ONOO− formation is spatially associated with the sources of O2 •− since O2 •− has restricted diffusion due to its charged nature while NO is uncharged and easily diffusible (Ferrer-Sueta and Radi 2009; Su and Groves 2010).
Interactions of ONOO− with Biological Systems
In addition to local production, ONOO− can also act as a paracrine-like mediator and gain access to cells via anion channels (Denicola et al. 1998; Macfadyen et al. 1999). ONOO−, in common with other oxidants, interacts with DNA, proteins/amino acids, and lipids, leading to distinct cytotoxic effects (Ascenzi et al. 2010). The specifics of the ONOO− modification/biomolecular interactions pertaining to various pathological conditions, especially diabetes, will be detailed later in this chapter. This section briefly highlights the general aspects of ONOO− interactions:
-
(a)
With CO2: CO2 being a nucleophile is a potent target for the strongly anionic ONOO− radical, especially since the concentration of CO2 is particularly high in the biological system (Ferrer-Sueta and Radi 2009). The rapid reaction between CO2 and ONOO− yields CO3 •− and nitrogen dioxide (•NO2), both of which are strong and short-lived oxidant radicals, which in turn target protein thiolates and aromatic residues participating in DNA base modifications (Augusto et al. 2002).
-
(b)
With proteins: ONOO− interacts with various proteins at different levels, and these interactions are primarily responsible for the deleterious effects of ONOO− on biological systems. ONOO− promotes the nitration of tyrosine residues and the oxidation of methionine and cysteine residues (Beckman et al. 1992; Mohr et al. 1994; Pryor et al. 1994). The biological effects of ONOO−-induced modifications of proteins include antioxidant enzyme inhibition/depletion, cytosolic enzyme inhibition, protein aggregation, activation of specific enzymes, membrane channel inhibition, modified/altered cellular signaling events, as well as the accelerated degradation of ONOO−-modified proteins via the proteasome (Milstien and Katusic 1999; Szabo 2003; Szabo et al. 2007). ONOO− can also interact with and impair enzyme cofactors thereby indirectly affecting protein/enzyme function (Milstien and Katusic 1999; Szabo et al. 2007).
-
(i)
Reaction of ONOO− with Specific Amino Acid Residues in Proteins: ONOO− alters protein structure and function by reacting with various amino acids in the polypeptide backbone of the various proteins. Cysteine oxidation (thiol oxidation) by ONOO− is one of the most prominent amino acid modifications in the biological system. ONOO−-linked oxidation of cysteine residues is known to inactivate many enzymes such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Mohr et al. 1994), creatine kinase (Konorev et al. 1998; Mihm et al. 2001), NADH dehydrogenase (complex I) (Radi et al. 2002a, b), succinate dehydrogenase (complex II) (Radi et al. 2002a, b), cytochrome c reductase (complex III) (Radi et al. 2002a, b), and ATP synthase (complex V) (Radi et al. 2002a, b). Phosphotyrosine-dependent signaling pathways may be enhanced by ONOO−-dependent inactivation of tyrosine phosphatase enzymes (Takakura et al. 1999; Lopez et al. 2005). Conversely, ONOO− -associated cysteine oxidation has been shown to activate some enzymes such as matrix metalloproteinases (MMPs) and sarcoendoplasmic reticulum Ca2+-ATPase (SERCA) (Okamoto et al. 2001; Adachi et al. 2004; Migita et al. 2005). ONOO− is also known to oxidize the endogenous antioxidant glutathione, making glutathione an efficient scavenger for ONOO− (Marshall et al. 1999; Bajt et al. 2003).
Tyrosine nitration is another prominent amino acid modification that can result from high levels of ONOO−. Tyrosine nitration has been shown to affect protein structure and function with resultant generation of antigenic epitopes, changes in catalytic efficiency, and impaired signal transduction (Kuhn et al. 1999; Knapp et al. 2001; Pacher et al. 2007). Enzymes such as glyceraldehyde-3-phosphate dehydrogenase, creatine kinase, and the mitochondrial respiratory chain complexes may, in addition to their susceptibility for cysteine oxidation, also be inactivated by tyrosine nitration making them seemingly vulnerable targets for ONOO− (Pacher et al. 2007). The tissue level of nitrotyrosine, resulting from ONOO−-mediated tyrosine nitration, is often used as a marker for nitrative stress. ONOO−-induced tyrosine nitration has been shown to be a key modification associated with altered protein structure and function responsible for clinical manifestations in diabetes-associated heart diseases (Xu et al. 2006), Alzheimer’s disease, and Parkinson’s disease (Aoyama et al. 2000). ONOO−, to a lesser extent, may also oxidize methionine (Whiteman et al. 1996; Alvarez and Radi 2003; Stadtman and Levine 2003; Stadtman et al. 2003), tryptophan (Alvarez and Radi 2003), and histidine residues in certain proteins leading to inactivation of these affected proteins (Alvarez et al. 2004; Yamakura et al. 2005; Yamakura and Ikeda 2006).
-
(ii)
Reaction of ONOO− with Proteins Containing Transition Metal Centers: Proteins with transition metal centers such as hemoglobin (Boccini and Herold 2004), myoglobin (Herold et al. 2003), and cytochrome c (Thomson et al. 1995; Herold et al. 2003) are vulnerable to ONOO−-induced rapid oxidation of their ferrous heme into ferric forms (Ferrer-Sueta and Radi 2009; Su and Groves 2010). In addition, critical metabolic enzymes containing iron-sulfur centers (Djaman et al. 2004) such as mitochondrial aconitase (Castro et al. 1994; Hausladen and Fridovich 1994) and phosphogluconate dehydratase (Keyer and Imlay 1997) and zinc-sulfur motifs such as alcohol dehydrogenase (Crow et al. 1995) have been shown to be inactivated by ONOO− interaction. As previously noted, ONOO−-modified proteins are also candidates for degradation via the proteasome (Szabo 2003).
-
(i)
-
(c)
With lipids: ONOO− triggers lipid peroxidation in membranes, liposomes, and lipoproteins (Radi et al. 1991a; Rubbo et al. 1994; Rubbo and Freeman 1996; Rubbo 1998). The derivatives of ONOO−-lipid interactions such as hydroperoxy radicals, conjugated dienes, and aldehydes in turn attack other lipid moieties thereby propagating the effects of free radical reactions followed by degeneration of membrane lipids and subsequently change membrane fluidity and permeability (Hogg and Kalyanaraman 1999). It has also been shown that ONOO−-modified low-density lipoprotein (LDL) binds with high affinity to the scavenger receptors – a known risk for atherosclerotic plaque formation (Botti et al. 2005; Rubbo and O’Donnell 2005).
-
(d)
With DNA: ONOO−-induced oxidative modifications of nitrogen bases and the sugar phosphates of the nucleotide bases can result in mutagenesis (Ahmad et al. 2009). ONOO− potentiates nitration, nitrosation, and deamination of the purine bases (mainly guanine) in DNA (Douki and Cadet 1996; Douki et al. 1996). 8-nitro-guanine and 8-oxyguanine are prominent ONOO−-induced modifications that occur, leading to single-strand breaks in the DNA (Niles et al. 2006). Additionally interactions of ONOO− with hydrogen atoms on sugar phosphates result in single-strand breaks which can trigger the activation of poly(ADP-ribose) polymerase (PARP), ultimately leading to cell death (Lorch et al. 2002; Ohshima et al. 2002; Szabo 1996; Szabo et al. 1996; Zingarelli et al. 1996).
Physiological Roles of RNS and ONOO−
Substantial progress has been made to understand the routes of formation, mechanisms, cellular actions, and detoxification of RNS such as NO⋅, nitroxyl ion (NO−, HNO), nitrosonium cation (NO+), higher oxides of nitrogen, S-nitrosothiols (RSNOs), and ONOO−. RNS molecules are well recognized for playing dual roles, both beneficial and deleterious, to living organisms (Patel et al. 1999; Ronson et al. 1999; Wolin 2000; Pacher et al. 2007; Triggle and Ding 2010). The primary cellular source of RNS is NO, an important intracellular messenger that exhibits a broad range of physiological properties that are dependent upon both concentration and duration of exposure. NO has both direct and indirect actions, with the indirect actions via RNS formation, which subsequently react with and dysregulate biological targets (Thomas et al. 2008). The physiological level of NO generated following endothelial nitric oxide synthase (eNOS) activation plays a vital role in the regulation of vascular tone, platelet function, angiogenesis, tissue oxygenation, vascular remodeling, and also anti-atherosclerotic actions (Naseem 2005; Pacher et al. 2007; Chen et al. 2008; Luiking et al. 2010). Disruption of NO production and/or bioavailability results in dysregulation of key physiological and cellular processes such as vasodilatation, platelet function, angiogenesis, apoptosis, and smooth muscle cell proliferation (see reviews by Pober et al. 2009; Triggle et al. 2012). The factors such as availability of the substrate L-arginine; elevated levels of circulating nitric oxide synthase (NOS) inhibitors such as asymmetrical dimethylarginine (ADMA); perturbed signal transduction reducing agonist-induced eNOS activation; reduced availability of tetrahydrobiopterin (BH4), an essential cofactor; or the reaction of NO with other free radical species such as O2 •− could potentially affect either the production of NO or the ability of NO to diffuse to its cellular targets (Scott-Burden 1995; Vallance and Leiper 2004; Naseem 2005; Leiper and Nandi 2011). The foremost mechanism thought to be responsible for the detrimental effects of NO is reaction with O2 •− and formation of ONOO− in a diffusion-limited manner. Although most of the pathological effects of NO are mediated by ONOO−, existing data also indicates that ONOO− may be a double-edged sword, as it exerts both cytotoxic as well as cytoprotective effects (Nossaman and Kadowitz 2008). The biological effects (beneficial/deleterious) of ONOO− exposure are greatly dependent on tissue concentration, biological environment in which it is present, and availability of detoxifying agents for conversion of ONOO− to another form. ONOO− exerts beneficial effect under in vivo conditions when thiol-containing agents such as glutathione, albumin, cysteine (Radi et al. 1991b; Wu et al. 1994; Wolin 2000), and uric acid (Skinner et al. 1998) are available to facilitate ONOO− to donate NO (Ronson et al. 1999; Vinten-Johansen 2000; Ferdinandy and Schulz 2001). Putative important physiological roles for ONOO− include vasodilatation, inhibition of platelet aggregation, and inflammatory cell adhesion as well as protection against ischemia/reperfusion injury (Ronson et al. 1999; Vinten-Johansen 2000; Ferdinandy and Schulz 2001; Ferdinandy 2006; Uppu et al. 2007; Nossaman and Kadowitz 2008).
Vasodilatation Properties of ONOO−
ONOO− produces a rapid concentration-dependent and endothelium-independent vasodilatation of aortic ring preparations (Liu et al. 1994; Villa et al. 1994; Wei et al. 1996; Ronson et al. 1999; Nossaman and Kadowitz 2008). Vasodilatation induced by ONOO− can be attributed to an increase in cGMP in vascular smooth muscle cells possibly involving the formation of S-nitrosothiols via the interaction between ONOO− and glutathione (Ronson et al. 1999; Nossaman and Kadowitz 2008). ONOO− can also hyperpolarize the vascular smooth muscle cell membrane via activation of ATP-sensitive potassium channels, myosin light chain phosphatase, and/or interference with calcium entry and release (Wei et al. 1996; Ademoglu et al. 2002; Li et al. 2004b; Graves et al. 2005). Furthermore, ONOO−-mediated vasodilatation may subsequently result in the formation of a stable secondary intermediate(s) following reaction with glucose and/or other functional alcohol groups (Beckman et al. 1990; Moro et al. 1995; Dowell and Martin 1997).
Antithrombotic and Antiplatelet Aggregation Properties of ONOO−
The actions of ONOO− on platelet function are concentration-dependent. ONOO− stimulates platelet aggregation at higher concentrations, whereas at lower concentrations inhibits platelet aggregation (Moro et al. 1994). Within the concentration range of 50–200 μM and in the presence of different platelet aggregation-inducing agents such as collagen, thrombin, and U46619 (thromboxane A2 mimetic), ONOO− inhibited platelet aggregation in a dose-dependent manner. Furthermore, addition of ONOO− could also reverse platelet aggregation previously induced by collagen, ADP, and thrombin. The cellular mechanism of the antiplatelet aggregation property of ONOO− appears to be related in part to a production of NO and/or a nitrosothiol compound. Thus, ONOO− nitrosylates thiols to form nitrosothiols, which then release NO and inhibit platelet aggregation; however, such a protective effect of ONOO− is offset when there is a persistent production of oxidants with the consequent depletion of thiols and a cytotoxic action (Moro et al. 1994; van der Vliet et al. 1995; Yin et al. 1995; Nowak and Wachowicz 2001).
Protective Effect of ONOO− on Ischemic Reperfusion Injury
Various studies have shown the cardio- and vasculoprotective effects of ONOO− in myocardial/reperfusion injury models (Lefer et al. 1997; Nossuli et al. 1997). In animals subjected to myocardial ischemia followed by reperfusion, administration of ONOO− (0.2, 2, or 20 μM) directly into the left ventricle beginning 10 min before and continued throughout the reperfusion period significantly protected the myocardium from developing substantial levels of necrosis. Furthermore, infusions of ONOO−, in the low micromolar range, produced a significant reduction of ventricular fibrillation and ischemia/reperfusion-induced dysrhythmias (Hagar et al. 1991; Vegh et al. 1992; Nossuli et al. 1998; Altug et al. 1999). An attenuated ability of endothelial cells to inhibit adherence of neutrophils is a critical event in ischemic myocardial injury, and ONOO−-mediated cardioprotection has been linked to attenuation and/or modulation of neutrophil-endothelial cell interaction, anti-inflammatory effects via its ability to act as a NO carrier or as an NO donor (Ronson et al. 1999). ONOO− modulates P-selectin expression, a cell adhesion molecule that plays an essential role in neutrophil adherence on the surface of activated endothelial cells (Burns et al. 1999). Pretreatment with ONOO− significantly reduces surface expression of P-selectin and inhibits leukocyte-endothelial cell interactions. Moreover, histological analysis demonstrates that ONOO− significantly attenuates polymorphonuclear leukocytes infiltration into heart tissue and protects the ischemic/reperfused rat heart by inhibiting polymorphonuclear leukocytes accumulation. These data show that ONOO−, in nanomolar concentrations, can inhibit leukocyte-endothelial cell interactions and exert cytoprotective effects, and inhibition of P-selectin is a key mechanism in modulation of ONOO−-mediated inhibition of leukocyte-endothelial cell interactions. It should, however, be stressed that, in these experiments, ONOO− demonstrated marked cardioprotection only when administrated via intraventricular route, whereas with an intravenous method of ONOO− infusion exerted cytotoxic effects (Lefer et al. 1997; Nossuli et al. 1998; Liu et al. 2000). Thus, the contribution of ONOO− to the cardioprotection reported in these studies may have been mediated by nitrosothiols as a result of the ONOO− being premixed with plasma prior to injecting into the animal, and therefore the ONOO− would have reacted with thiols and resulted in the formation of NO donor species (van der Vliet et al. 1998). In contrast to the conclusions reached by Lefer and coworkers (1997), Yasmin et al. (1997) reported that reperfusion of the ischemic rat heart results in the acute production of ONOO−, and the inhibition of its biosynthesis with L-NMMA, or antagonizing its oxidant actions with the NO donor SNAP, may provide a strategy for the protection of the heart from ischemia/reperfusion injury.
Chemical modification of kinases and phosphatases by ONOO− can modify the cell signal transduction pathways and result in the up- or downregulation of signaling cascades in a concentration- and cell-dependent manner. For instance, ONOO−-mediated S-glutathiolation of cysteine residues may result in activation of enzymes, including metalloproteinases, MMPs, which typically occurs at low concentrations of ONOO− [<10 μM], and ONOO−-mediated activation of MMP2 has been shown to be inhibited via the antioxidant effects of acetaminophen (see Klatt and Lamas 2000; Okamoto et al. 2001; Rork et al. 2006; Viappiani et al. 2009). SERCA can also be activated and calcium uptake enhanced by low concentrations of ONOO−, and it has been shown that the key cysteine residue is Cys674 (Adachi et al. 2004). Interestingly S-glutathiolation of SERCA was reduced in atherosclerotic conditions when ROS were elevated, and this was argued to be a result of the irreversible oxidation of key thiols, such as Cys674, effects that were mimicked by mutation of Cys 674 (Adachi et al. 2004). Thus, S-glutathiolation via ONOO− plays an important role in the regulation of enzyme function suggesting that appropriate levels of O2 •− are required to maintain at least some components of NO-mediated cell signaling (Adachi et al. 2004).
ONOO−-induced phosphatidylinositol 3-kinase (PI3K) activation and Akt (protein kinase B) phosphorylation result in the activation of the nuclear factor (erythroid-derived 2)-like 2/antioxidant response element (Nrf2/ARE), transcription factor with subsequent upregulation of the antioxidant and cytoprotective glutathione-S-transferase, heme-oxygenase-1 in neural cells (Kang et al. 2002; Li et al. 2006). Studies with isolated fibroblasts, neutrophils, endothelial and vascular smooth muscle cells, neural cells, cardiomyocytes, and whole lung tissue show that ONOO− activates extracellular signal-regulated kinase (ERK), a critical regulator of myocardial hypertrophy as well as an important cardioprotective signaling molecule (Bapat et al. 2001; Liaudet et al. 2009).
In the vasculature, ONOO−-dependent Janus kinase (JNK) activation, which plays an important prevent anti-atherosclerotic role, has been shown to occur in endothelial cells exposed to laminar shear stress, suggesting that ONOO− may represent a key molecular link between endothelial mechanical stress and endothelial function (Go et al. 1999). The cytoprotective or cytotoxic roles of protein kinase C (PKC) isoforms, PKC epsilon, and delta, respectively, in cardiovascular diseases have been extensively investigated (Churchill and and Mochly-Rosen 2007). NO-mediated PKC epsilon activation is thought to play a protective role during ischemic preconditioning (Ping et al. 1999), and ONOO− rather than NO may be responsible for this effect. Furthermore, ONOO− directly enhances the binding of recombinant PKC epsilon to anchoring protein, receptor for activated C kinase (RACK2), in an in vivo model of NO-mediated cardioprotection, thereby delineating a novel signaling mechanism by which NO activates PKC epsilon in the heart via the generation of ONOO− (Balafanova et al. 2002).
To summarize, ONOO−, at low micromolar concentrations, upregulates multiple signaling cascades by acting as an NO carrier, or NO donor, thereby exhibiting distinctive physiological roles. However, there are several limitations to this generalization including cell specificity, ONOO− concentration within the tissue, the short half-life of ONOO−, the biological environment, and the availability of thiol-containing agents. Concentration-dependent effects of ONOO− are discussed in more detail in the next section.
Pathological Effects of ONOO−
ONOO− toxicity is associated with many diseases (Fig. 9.2). The pathological effects of ONOO− are associated with the source of O2 •− generation, length of formation time, and higher concentrations. Thus, moderate ONOO− generation over a long period can be detrimental to cell constituents, leading to reversible cell injury, irreversible cell injury, and even the induction of cell death through apoptosis/necrosis. Although it is difficult to assign a specific concentration range for the “physiological or enzyme activation” versus “pathophysiological/inactivation” effects of ONOO−, in vitro studies, with MMP2, indicate that 0.3–1.0 μM of ONOO− activated the enzyme, whereas 30–100 μM attenuated enzyme activity (Viappiani et al. 2009). However, activation of MMP2 required the presence of glutathione, and thus the concentration-dependent effects of ONOO− will be very much dependent on the cellular milieu.
Protective and pathological roles of peroxynitrite. At low concentration, ONOO− may play a protective role by acting as an NO carrier or NO donor as well as an activator, via S-glutathiolation, of enzymes such as SERCA (a). At higher concentrations, the effects of ONOO− shift from protective to pathological, and, by acting as pro-oxidant and causing irreversible oxidation/nitration of proteins, ONOO− has been implicated in many disease conditions (b)
An important hallmark of many diseases, including cardiovascular, neurological disorders, cancer, and also aging, is that proteins, lipids, and nucleic acids are oxidized/nitrated by ONOO− (Pacher et al. 2007). Prolonged nitration of several important cardiac proteins, including creatine kinase, SERCA, and inactivation of voltage-gated ion channels, results in the acceleration of cardiac dysfunction. On the other hand, as already noted, the S-glutathiolation of cysteine residues may result in activation of enzymes such as MMPs and SERCA; however, this is concentration-dependent and inactivation occurs at higher concentrations most likely as a result of the irreversible oxidation of key protein thiols (Adachi et al. 2004; Viappiani et al. 2009). Both nitration of cytoskeletal/structural proteins such as desmin, myosin, actinin, and tubulin by ONOO− as well as an increased accumulation of inflammatory cells in the vasculature have also been associated with endothelial and platelet dysfunction (Mihm et al. 2001; Mihm and Bauer 2002; Lee et al. 2003; Li et al. 2004a; Borbely et al. 2005; Lokuta et al. 2005). Moreover, as reviewed by Pacher et al. (2007), ONOO−-mediated modification of amino acids, such as oxidation of tryptophan, methionine, and histidine, may also play an important role in the pathogenesis of neurodegenerative disorders.
The ability of ONOO− to trigger peroxidation in membrane lipids, liposomes, and lipoproteins is also an important aspect of ONOO−-mediated cytotoxic effect of ONOO−. Furthermore, ONOO−-mediated oxidization of LDL and foam cell formation are suggested as critical events in the progression of vascular diseases including atherogenesis. ONOO−-mediated damage to DNA, including oxidation of nucleotides of deoxyribose moieties, strand breaks, is critically involved in induction of cell death and inflammatory pathways and implicated in cancer, diabetes, and cardiovascular disease (see reviews by Pacher et al. 2007; Szabo et al. 2007).
ONOO− and Cardiovascular Disease
The cytotoxic effect of ONOO−, as evidenced by data from both in vitro and in vivo studies, has been implicated in a variety of cardiovascular diseases including myocardial ischemic reperfusion injury, myocarditis, coronary artery disease, and congestive heart failure. ONOO− can activate matrix metalloproteins (MMPs) as well as PARP (Klatt and Lamas 2000; Okamoto et al. 2001; Virag and Szabo 2002). Inhibition of MMPs, notably MMP2, and PARP is a potential target for the treatment of heart failure (Gao et al. 2003; Cena et al. 2010; Pacher et al. 2005; Viappiani et al. 2009). The ability of ONOO− to damage vascular endothelium by increased lipid peroxidation, upregulation of adhesion molecule expression, altered endothelium-dependent vasodilatation, vascular smooth muscle cell proliferation, platelet aggregation, and thrombus formation have all been considered as causative factors of ONOO−-induced vascular dysfunction (see review by Triggle et al. 2012). Furthermore, ONOO−-mediated oxidation of BH4 and uncoupling of eNOS accelerate the onset of endothelial dysfunction, vascular disease pathogenesis, and this aspect of the pathophysiological actions of ONOO− is discussed in more depth in the next section (see reviews by Pacher et al. 2007; Szabo et al. 2007; Triggle et al. 2012).
ONOO−, Diabetes, and Vascular Disease
Growing evidence implicates that ONOO− formation is a major contributor to the initiation, progression, and pathogenesis of diabetes. The greatest contributor to the morbidity and mortality of diabetes is cardiovascular disease, and endothelial dysfunction is the earliest indicator of the onset of vascular dysfunction regardless of whether it is secondary to diabetes or other pathophysiologies such as hypertension (Félétou and Vanhoutte 2006; Pober et al. 2009; Triggle and Ding 2010; Ghiadoni et al. 2011; Triggle et al. 2012).
Endothelial dysfunction can be defined as “Endothelial dysfunction reflects a reduced endothelium-dependent vasodilatation response to either an endothelium-dependent vasodilator, such as acetylcholine (or bradykinin), or to flow-mediated vasodilatation that is accompanied by elevated expression of adhesion molecules, enhanced vascular smooth muscle proliferation and the development of a hypercoagulatory state.” Perhaps more simply endothelial dysfunction can be defined as “The failure of endothelial cells to perform their normal physiological functions” (see Pober et al. 2009; Triggle et al. 2012 for further discussion).
Hyperglycemia leads to an imbalance between the increased production of O2 •− versus NO and results in the formation of ONOO− within the vascular wall. The vasoconstriction, proinflammatory, pro-thrombotic, and atherosclerotic effects of ONOO− have been suggested as critical contributors to diabetes-induced vascular disease (Zou et al. 2004). ONOO− upregulates the expression of proinflammatory cytokines interleukin-1 beta, IL-1β, and tumor necrosis factor-alpha, TNF-α, in pancreatic islet cells, thus promoting β cell destruction (Lakey et al. 2001).
Elevated plasma nitrite/nitrate, nitrotyrosine, and inducible nitric oxide synthase (iNOS) levels are considered as indices of excessive ONOO− formation in type 2 diabetes (Tannous et al. 1999; Ceriello 2002). It has been reported that ONOO−-mediated elevated protein nitration, DNA damage, and oxidative stress eventually lead to β cell dysfunction and apoptosis (Fehsel et al. 1993; Szabo and Ohshima 1997; Hou et al. 2010; Kim et al. 2010; Liang et al. 2010; Stadler 2011). Accelerated ONOO− formation during hyperglycemic episodes results in increased nitrotyrosine formation; increased expression of cellular adhesion molecules, cytokines, and chemokines; PKC-dependent NADP(H) oxidase activation; altered vascular reactivity and diminished vasodilatation with altered activities of Na+/K+-ATPase and Ca2+-ATPase in vascular endothelial cells; and ultimately diabetes-induced endothelial dysfunction (Cosentino et al. 2003a, b; Kossenjans et al. 2000; Soriano et al. 2001; Rabini et al. 2002; Szabo et al. 2002; Zou et al. 2002).
Data from both clinical and experimental studies indicates that enhanced ONOO− formation associated with diabetes results in nitrotyrosine formation in endothelial cells, cardiomyocytes, and fibroblasts and favors accelerated apoptosis and cell death (Frustaci et al. 2000; Kajstura et al. 2001; Ceriello et al. 2002). Exposure of human umbilical vein endothelial cells to ONOO− significantly inhibited both basal and insulin-stimulated Akt phosphorylation at Ser473 and Akt activity in parallel with increased apoptosis (Song et al. 2007). ONOO− can also trigger an impairment of endothelium-dependent vasodilatation, secondarily to a decrease in SERCA2A function (Kobayashi 2008). ONOO−-mediated DNA strand breaks and consequent activation of nuclear enzyme poly(ADP-ribose) polymerase-1, PARP-1 have also been suggested as critical contributors to ONOO−-mediated development of diabetic vascular complications (Garcia Soriano et al. 2001; Pacher et al. 2002; Pacher and Szabo 2005). Data from cell culture and animal studies suggest that diabetes is associated with increased tyrosine nitration of mitochondrial and vascular proteins, lipid peroxidation, activation of angiotensin II, and PARP1 expression in nerves, kidneys, and the retina that subsequently lead to apoptosis and necrosis in diabetes-associated vascular complications such as cardiomyopathy, neuropathy, and retinopathy (Thuraisingham et al. 2000; Ishii et al. 2001; Onozato et al. 2002; El-Remessy et al. 2003; Turko et al. 2003).
There is an accumulation of evidence indicating that both ROS and RNS contribute to endothelial and vascular dysfunction (Kvietys and Granger 2011). Thus, targeting ROS and RNS with the goal of improving endothelial function is a priority for the prevention and treatment of vascular-related diseases such as diabetes (Andrews et al. 2005; Ding and Triggle 2010; Triggle et al. 2012; Sharma et al. 2012). The infusion of a cofactor essential for eNOS function, BH4, improves flow-mediated vasodilatation in forearm blood flow measurements in patients with type 2 diabetes (Heitzer et al. 2000). Furthermore, the direct addition of BH4 to the organ bath in in vitro studies of human vascular tissue improves endothelium-dependent vasodilatation in blood vessels from patients undergoing coronary artery bypass grafting (Heitzer et al. 2000; Verma et al. 2000). Collectively these observations support the hypothesis that endothelial dysfunction is linked to a reduction in the bioavailability of BH4. There is an extensive data set from studies with humans and rodents that supports this hypothesis (Pannirselvam et al. 2002, 2003; Cai et al. 2005; Kietadisorn et al. 2011; Triggle et al. 2012). BH4 is recognized as a key regulator of eNOS and the dysregulation of BH4 synthesis and/or bioavailability, particularly with respect to suboptimal levels of BH4 versus oxidized products of BH4, promotes production of O2 •− from eNOS (and also neuronal NOS, nNOS) and leads to endothelial dysfunction (Vasquez-Vivar et al. 2002; Channon 2004; Aljofan and Ding 2010; Triggle and Ding 2010; Kietadisorn et al. 2011) (Fig. 9.3). In a diabetic milieu, the endothelial cells are exposed to hyperglycemia, which is known to activate the NADPH oxidase leading to the formation of excessive amounts of O2 •−. Since NO is locally produced in endothelial cells by eNOS, the NO and O2 •− and rapidly combine to yield ONOO−. ONOO− oxidizes BH4, an essential cofactor for eNOS, to BH2 (Milstien and Katusic 1999; Alp and Channon 2004; Sasaki et al. 2008). The reduction in the availability of BH4 leads to eNOS uncoupling, shifting the function of eNOS from NO to O2 •− production. Thus, low levels of BH4, which is itself a weak antioxidant, or a reduced ratio of BH4 relative to oxidized biopterins such as BH2, is associated with eNOS functioning as an NADPH oxidase and as such molecular oxygen rather than L-arginine becomes the electron recipient with the resultant production of superoxide, O2 •− (Pannirselvam et al. 2002; Vasquez-Vivar et al. 2002; Cai et al. 2005).
Possible role of peroxynitrite in endothelial dysfunction in diabetes, eNOS uncoupling, and HNO production. In a diabetic milieu, the endothelial cells are constantly exposed to hyperglycemia, which is known to activate the NADPH oxidase leading to the formation of excessive amounts of superoxide anion (O2 •−). Since NO is locally produced in endothelial cells by eNOS, the NO and O2 •− rapidly combine to yield ONOO−. The ONOO− is known to cause the oxidation of tetrahydrobiopterin (BH4, an essential cofactor for eNOS) to dihydrobiopterin (BH2). The lack of availability of BH4 leads to eNOS uncoupling, shifting the function of eNOS from NO to O2 •− production. O2 •− reacts with NO to yield ONOO−. Additionally, uncoupled eNOS is known to catalyze the formation of nitroxyl ion (HNO) that may play a vasculoprotective role and enhance blood flow
As a result of a decrease in the BH4/BH2 ratio, BH2 competes with BH4 for binding to the oxygenase domain of eNOS, and this results in the “uncoupling” of eNOS from the dimeric and free radical NO•-generating enzyme to the O2 •−-producing monomeric enzyme (Kar and Kavdia 2011). As depicted in Fig. 9.3, it is the ratio of BH4/BH2 that is therefore of particular importance for determining whether it is NO or O2 •− that is generated. The uncoupling of eNOS is most likely the key trigger for the acute reduction in flow-mediated vasodilatation that is seen postprandial, or following the ingestion of glucose, and also an important contributor to the reduction in flow-mediated vasodilatation that is seen in patients with diabetes (Kawano et al. 1999; Ihlemann et al. 2003; Crabtree et al. 2008; Aljofan and Ding 2010; Kar and Kavdia 2011).
Uncoupled eNOS catalyzes the formation of NO− rather than NO•. Data in support of the critical importance of the BH4/BH2 ratio rather than the absolute level of BH4 is provided by a study comparing the BH4/BH2 ratio in small mesenteric arteries from the type two diabetic db/db mouse versus nondiabetic control (Pannirselvam et al. 2002). Pannirselvam et al. (2002) reported that the BH4/BH2 ratio, but not the absolute level of BH4, was significantly reduced and the small mesenteric arteries also exhibited profound endothelial dysfunction that, most likely, was due to a reduction in the bioavailability of NO (Pannirselvam et al. 2002, 2003). An uncoupled eNOS, wherein eNOS is in the monomeric rather than the dimeric form, is promoted by S-glutathionylation as well as thiyl radical formation and the oxidation of key cysteine residue(s) in the enzyme (Chen et al. 2010, 2011). BH4 is highly sensitive to oxidation by ONOO− (Kohnen et al. 2001). Hyperglycemia and, paradoxically, hypoglycemia are associated with endothelial dysfunction, and, notably, hyperglycemia-induced endothelial dysfunction can be linked to oxidative stress, a downregulation of antioxidant enzymes, and an uncoupling of eNOS (Ding et al. 2007; Aljofan and Ding 2010; Giacco and Brownlee 2010; Wang et al. 2011; Triggle et al. 2012). It has been shown that increased levels of RNS, in particular ONOO−, can suppress eNOS activity in streptozotocin-induced diabetic rats via the small GTPase, RhoA, in a RhoA/Rho-associated protein kinase (RhoA/ROCK)-dependent manner (El-Remessy et al. 2010; Sharma et al. 2012). Furthermore, treatment with the ONOO− decomposition catalyst FeTTPs improves vasodilatation in response to the endothelium-dependent vasodilator acetylcholine, lowers oxidative-stress and RhoA activity, upregulates eNOS expression, and enhances endothelial levels of NO (El-Remessy et al. 2010; Sharma et al. 2012).
In pathophysiological states, such as hypertension or type 1 and 2 diabetes, where blood glucose levels are raised, the production of ROS is also increased, and the expression and/or activity of enzymes such as NADPH oxidase are also elevated. Thus, such pathophysiological states promote the formation of ONOO− and enhance both oxidative and nitrosative stress (Forstermann and Munzel 2006; Ding et al. 2007; Aljofan and Ding 2010).
ONOO− plays an important role in the development of endothelial dysfunction and vascular disease; however, when BH4 levels are reduced, NOS enzymes may also generate, in addition to O2 •−, the nitroxyl form of NO, HNO/NO−. In Escherichia coli, in the absence of BH4, nNOS is overexpressed and generates NO− from L-arginine, and these data suggest that the most important role for BH4 (Pacher et al. 2007) is to enable nNOS to generate NO• instead of NO− (Rusche et al. 1998; Adak et al. 2000). NO−, which preferably should be called nitrosyl hydride or hydrogen oxonitrate, has a unique and distinctive chemistry and biological profile when compared to NO• (Fukuto et al. 2005; Flores-Santana et al. 2011; Kemp-Harper 2011).
A number of studies have demonstrated that NO− contributes to the responses mediated by NO in the vasculature thus implying that both NO• and NO− must be produced physiologically. Furthermore, the cellular signaling pathways activated by these two chemical species of nitric oxide, notably the nature of the potassium channel subtype activated by NO• versus NO−, are distinct, perhaps suggesting differing, but overlapping, physiological functions and/or cellular compartmentalization (Ellis et al. 2000; Wanstall et al. 2001; Irvine et al. 2003; Andrews et al. 2005; Favaloro and Kemp-Harper 2009; Paolocci and Wink 2009; Kemp-Harper 2011; Yuill et al. 2011) – see Fig. 9.3.
Support for a different profile of physiological actions for NO− versus NO• is provided by studies that show that NO− favors reaction with ferric heme while NO• favors ferrous heme (Miranda et al. 2003a; Fukuto et al. 2005). For instance, the heme-containing soluble guanylyl cyclase would preferentially be activated by NO•, whereas peroxidases require the ferric state and would be preferentially regulated by NO−. The unique chemistry of NO− and its greater preference for thiols and metalloproteins indicate differing cellular roles for these two forms of NO (Liochev and Fridovich 2003; Miranda et al. 2003a, b; Donzelli et al. 2006). Predictably NO− would have greater biological activity in cellular locations, such as membranes, or pathophysiological states when glutathione levels are low (Miranda et al. 2003b). Furthermore, it has been suggested that NO− possesses vascular protective functions and NO− is less susceptible to scavenging by superoxide and less likely to lead to tolerance and endothelial dysfunction (Bullen et al. 2011). In contrast to NO•, NO− also has a positive inotropic action and protects against ischemia/reperfusion injury when applied before ischemia as well as exhibiting vascular antiproliferative actions (Paolocci et al. 2001; Irvine et al. 2003; Bullen et al. 2011; Kemp-Harper 2011; Tocchetti et al. 2011).
The interaction of NO− with thiol proteins in the myocardium may provide the basis for NO−-mediated enhanced Ca2+ cycling and hence the positive inotropic action of NO− (Fukuto and Carrington 2011). The unique chemistry of NO− and differing physiological profile indicate that rather than reflecting a solely pathophysiological role for a BH4-compromised milieu, the generation of NO− is an important facet of the biology of NO that is regulated by ONOO−. ONOO−-mediated regulation of NOS and the generation of NO− versus NO• may therefore have profound effects on cell signaling and, under some circumstances, provide a protective influence on cardiovascular function. In addition, the cytotoxic actions of NO− may also exert antiproliferative effects and reduce tumor progression (Norris et al. 2008).
Collectively, these data indicate that, like many signaling molecules, ONOO−, through its regulation of the production of NO− versus NO•, is also Janus faced.
ONOO− and Inflammation
Several lines of evidence indicate that overproduction of ONOO− critically contributes to pathogenesis of inflammatory disease such as chronic arthritis and inflammatory bowel disease (see review by Pacher et al. 2007). High levels of ONOO− are produced at sites of inflammation and have been implicated in promoting the sustained and/or prolonged development of various disease pathologies. The ability of ONOO− to trigger and enhance NFκB activity, as well as upregulate proinflammatory mediators, plays a critical role in inflammation-associated cell and tissue damage (Matata and Galinanes 2002; Bar-Shai and Reznick 2006). ONOO−-mediated enhanced NFκB activity results in a greater expression of proinflammatory cytokines, chemokines, enzymes such as iNOS and cyclooxygenase 2 (COX2), and cell adhesion molecules (Cooke and Davidge 2002). Additionally, data from animal models of inflammatory disease indicate that ONOO−-mediated PARP activation is a contributory factor to inflammatory diseases such as allergic encephalomyelitis, meningitis, and multiple sclerosis; ocular inflammation uveitis; asthma; and periodontal inflammation gingivitis (Landino et al. 1996; Zouki et al. 2001 and reviewed in Pacher et al. 2007; Szabo et al. 2007).
ONOO− and Cancer
ONOO− may contribute to carcinogenesis and tumor progression by weakening key cellular defense enzymes; increasing the production and infiltration of proinflammatory cytokines, matrix-degrading enzymes, and growth factors; inhibiting DNA repair enzymes; and enhancing cell proliferation, survival, migration, and angiogenesis. The ability of ONOO− to trigger DNA mutation by oxidative modification to, in particular, the nucleobase guanine enhances the potential for single-strand DNA breaks, mutagenesis, and carcinogenesis. Guanine is oxidized by ONOO− to cyanuric acid and oxazolone resulting in guanine fragmentation (Burney et al. 1999; Niles et al. 2006). In addition, studies have also provided evidence of a tumor promoting role of ONOO− resulting from its ability to nitrate and phosphorylate the NFκB inhibitory protein IκB, leading to the activation of NFκB-mediated proinflammatory signaling cascades and, therefore, promoting chronic inflammation and tumor development (Mabley et al. 2004; Gochman et al. 2011).
ONOO− and Neurological Disorders
Neurodegenerative disorders, such as amyotrophic lateral sclerosis, ALS, have also been associated with elevated generation/bioavailability of NO and accelerated formation of ONOO− (Verge et al. 1992; Solodkin et al. 1992; Beckman et al. 1993). Furthermore, ONOO−-induced protein nitration, neuron degeneration, and DNA damage have all been implicated in neuronal cell death and necrosis (Torreilles et al. 1999; Souza et al. 2000; Ischiropoulos and Beckman 2003; Reynolds et al. 2005; Pacher et al. 2007; Singh et al. 2007). ONOO−-mediated protein nitration/oxidation and lipid peroxidation of neuronal synaptic and structural proteins such as α-synuclein, tubulin, and myelin have all been highlighted as critical steps in the pathogenesis of neurodegenerative diseases such as multiple sclerosis and Parkinson’s and Alzheimer’s diseases, and therefore ONOO− is a potential therapeutic target (see review by Tao et al. 2012).
Scavenging ONOO− and its Derivatives
The rate of disappearance of ONOO− follows first-order kinetics with a half-life of ∼1 s at 37 °C and pH 7.4 although, as noted, the cis-conformation of ONOO− is quite stable (Koppenol et al. 1992; Tsai et al. 1994; Pryor and Squadrito 1995; Goldstein et al. 1996). Thus, the fate of the ONOO− radical will either be decomposition if no proximal oxidizable targets are available, or ONOO− will rapidly oxidize available proximal targets in most cases yielding secondary reactive species (Crow 2002). However, in a cellular environment, with an abundant supply of molecules that directly react with ONOO−, then ONOO− will be consumed through direct reactions with these biomolecules.
In light of the multifaceted reaction capabilities of ONOO− that can cause adverse effects in biological systems, there is obvious therapeutic potential for the identification of potential ONOO− scavengers as protective agents. An ideal scavenger of ONOO− should (1) rapidly react with ONOO−, (2) “contain” reactive intermediates, (3) be stable under in vivo conditions, (4) be able to traverse the plasma membrane and evenly distribute in the tissues, and (5) be nontoxic itself as well as when irreversibly modified upon its reaction with ONOO− (Crow 2002).
Natural and dietary products that contain antioxidants such as carotenoids (Panasenko et al. 2000), polyphenolic compounds (Arteel and Sies 1999), and epicatechin (Schroeder et al. 2003) have been shown to be protective against ONOO−-induced toxicity (Arteel et al. 1999). However, the mechanisms by which these antioxidants render their protective effect are still unclear since they tend to react rather slowly with ONOO− and may also react with other reactive species including ONOO−-derived radicals.
Various molecules have been identified that can react directly with ONOO− or with the ONOO−-derived reactive radicals. In the biological system, peroxiredoxins are efficient scavengers of ONOO− (Bryk et al. 2000). The reactive thiol active site of peroxiredoxins is oxidized during the catalytic reduction of ONOO− to nitrite. The oxidized peroxiredoxins is then reduced back to its resting state by thioredoxin (Maulik and Das 2008). Owing to the capability of ONOO− to cause rapid cysteine oxidation, ONOO− also oxidizes the thiol group in glutathione, making glutathione an efficient scavenger of the ONOO− radical (Arteel et al. 1999; Bajt et al. 2003). Thiol-based antioxidants such as N-acetyl cysteine (Cuzzocrea et al. 2000) and dihydrolipoic acid have been shown to reduce ONOO−-mediated toxicity (Szabo 2003).
Heme proteins (hemoglobin and methemoglobin) are also known to be efficient scavengers of ONOO− in biological systems, thereby protecting cells from the detrimental effects of ONOO− (Herold et al. 2002; Herold and Fago 2005; Romero and Radi 2005; Ascenzi et al. 2008, 2010; Ascenzi and Visca 2008). ONOO− isomerizes to form nitrate (NO3 −) upon its reaction with ferrous deoxygenated heme proteins (Ascenzi et al. 2010).
Interest has grown in certain synthetic molecules (such as iron and manganese metalloporphyrins) that react directly with and are potent catalysts of ONOO− decomposition with potential as therapeutic agents (Salvemini et al. 1998; see also review by Szabo et al. 2007). These are commonly called ONOO− decomposition catalysts. Iron and manganese porphyrins are advantageous of being much effective at much lower concentrations and ability to be recycled.
ONOO− rapidly causes tyrosine nitration of proteins, and therefore tyrosine-containing peptides may also act as effective scavengers of ONOO− (Ye et al. 2007). Additionally, various studies have reported uric acid to be an efficient scavenger of ONOO− and its derived radicals such as CO3 •− and •NO2 (Kooy et al. 1994; Hooper et al. 1998; Squadrito et al. 2000). Seleno-compounds (Klotz and Sies 2003) and selenium-containing proteins such as glutathione peroxidase (Sies and Masumoto 1997; Sies et al. 1997; Arteel et al. 1998) have been reported to exhibit ONOO− scavenging properties. SOD mimetics have also exhibited ONOO− scavenging and protective effects (Batinic-Haberle et al. 2009).
Tempol, a well-studied compound used to protect cells and animals from oxidative stress, has also been shown to be an effective protective agent against ONOO−-induced cytotoxicity (Fernandes et al. 2005a). Tempol is oxidized by ONOO−-derived CO3 •− yielding an oxoammonium cation, which then is reduced back to tempol by reacting with ONOO− and yielding oxygen and NO, thereby diverting the ONOO−/CO2 reactivity from protein tyrosine nitration to cysteine nitrosation (Fernandes et al. 2005a).
Finally, several commonly used drugs such as acetaminophen, propofol, simvastatin, pindolol, as well as herbal extracts have been demonstrated to possess ONOO− scavenging actions (Acquaviva et al. 2004; Choi et al. 2002; Fernandes et al. 2005b; Rork et al. 2006; Selley 2005; see also review by Szabo et al. 2007).
In general, the catalytic scavenger would be preferred over “sacrificial” scavengers (such as glutathione), which cannot be regenerated. In the future, as more knowledge is gained on the formation of ONOO− and its intermediates, more specific scavengers may be designed to combat the various pathological conditions where ONOO− and its derivatives play a significant role.
Conclusion and Future Prospects
The current chapter has focused on how ONOO− is formed from NO and O2 •− and briefly summarized both the physiological and the pathological targets and effects of ONOO−. However, as for any other ROS, it is difficult to interpret what level of ONOO− formation is beneficial and where and what threshold levels need to be exceeded to initiate detrimental cellular actions. In order to develop a better therapeutic strategy to combat the adverse effects of excessive ONOO− levels in biological systems, it is necessary to also better understand the cellular processes that regulate the formation and degradation of ONOO−. Clearly, however, studies on the protective effects of scavengers of ONOO− are warranted in disease models (diabetes, cardiovascular disease, cancer, etc.) to investigate questions related to dosage, formulation, and bioavailability.
Abbreviations
- (•NO2):
-
Nitrogen dioxide
- (•OH):
-
Hydroxyl radical
- (CO3 •−):
-
Carbonate radical
- (O2 •−):
-
Superoxide anion
- ARE:
-
Antioxidant response element
- BH2 :
-
Dihydrobiopterin
- BH4 :
-
Tetrahydrobiopterin
- cGMP:
-
Cyclic guanosine monophosphate
- eNOS:
-
Endothelial nitric oxide synthase
- ERKs:
-
Extracellular signal-regulated kinases
- H2O2 :
-
Hydrogen peroxide
- HClO:
-
Hypochlorous acid
- HONOO:
-
Peroxynitrous acid
- IκB:
-
IκB kinase
- JNK:
-
c-Jun N-terminal kinases
- NO:
-
Nitrogen (mon)oxide
- NO+ :
-
Nitrosonium ion
- NO− (HNO):
-
Nitroxyl ion nitrosyl hydride, or hydrogen oxonitrate
- NO• :
-
Nitric oxide free radical
- NRF2:
-
Nuclear factor (erythroid-derived 2)-like 2
- ONOO− :
-
Peroxynitrite
- PARP:
-
Poly(ADP-ribose) polymerase
- PKC:
-
Protein kinase C
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- SERCA:
-
Sarcoendoplasmic reticulum Ca2+-ATPase
- SOD:
-
Superoxide dismutases
- TNF-α:
-
Tumor necrosis factor-alpha
References
Acquaviva R, Campisi A, Murabito P, Raciti G, Avola R, Mangiameli S, Musumeci I, Barcellona ML, Vanella A, Li Volti G (2004) Propofol attenuates peroxynitrite-mediated DNA damage and apoptosis in cultured astrocytes: an alternative protective mechanism. Anesthesiology 101:1363–1371
Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schöneich C, Cohen RA (2004) S-glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med 10:1200–1207
Adak S, Wang Q, Stuehr DJ (2000) Arginine conversion to nitroxide by tetrahydrobiopterin-free neuronal nitric-oxide synthase. Implications for mechanism. J Biol Chem 275:33554–33561
Ademoglu F, Basgut B, Abacioglu N, Cakici I (2002) The mechanisms of peroxynitrite-induced relaxations in isolated rat anococcygeus muscle. Pharmacology 66:1–4
Ahmad R, Rasheed Z, Ahsan H (2009) Biochemical and cellular toxicology of peroxynitrite: implications in cell death and autoimmune phenomenon. Immunopharmacol Immunotoxicol 31:388–396
Aljofan M, Ding H (2010) High glucose increases expression of cyclooxygenase-2, increases oxidative stress and decreases the generation of nitric oxide in mouse microvessel endothelial cells. J Cell Physiol 222:669–675
Alp NJ, Channon KM (2004) Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol 24:413–420
Altug S, Demiryurek AT, Cakici I, Kanzik I (1999) The beneficial effects of peroxynitrite on ischaemia-reperfusion arrhythmias in rat isolated hearts. Eur J Pharmacol 384:157–162
Alvarez B, Radi R (2003) Peroxynitrite reactivity with amino acids and proteins. Amino Acids 25:295–311
Alvarez B, Ferrer-Sueta G, Radi R (1998) Slowing of peroxynitrite decomposition in the presence of mannitol and ethanol. Free Radic Biol Med 24:1331–1337
Alvarez B, Demicheli V, Duran R, Trujillo M, Cervenansky C, Freeman BA, Radi R (2004) Inactivation of human Cu, Zn superoxide dismutase by peroxynitrite and formation of histidinyl radical. Free Radic Biol Med 37:813–822
Andrews KL, Pannirselvam M, Anderson TJ, Jenkins AJ, Triggle CR, Hill MA (2005) The vascular endothelium in diabetes: a practical target for drug treatment? Expert Opin Ther Targets 9:101–117
Aoyama K, Matsubara K, Fujikawa Y, Nagahiro Y, Shimizu K, Umegae N, Hayase N, Shiono H, Kobayashi S (2000) Nitration of manganese superoxide dismutase in cerebrospinal fluids is a marker for peroxynitrite-mediated oxidative stress in neurodegenerative diseases. Ann Neurol 47:524–527
Arteel GE, Sies H (1999) Protection against peroxynitrite by cocoa polyphenol oligomers. FEBS Lett 462:167–170
Arteel GE, Mostert V, Oubrahim H, Briviba K, Abel J, Sies H (1998) Protection by selenoprotein P in human plasma against peroxynitrite-mediated oxidation and nitration. Biol Chem 379:1201–1205
Arteel GE, Briviba K, Sies H (1999) Protection against peroxynitrite. FEBS Lett 445:226–230
Ascenzi P, Visca P (2008) Scavenging of reactive nitrogen species by mycobacterial truncated hemoglobins. Methods Enzymol 436:317–337
Ascenzi P, De Marinis E, Coletta M, Visca P (2008) H2O2 and (.)NO scavenging by Mycobacterium leprae truncated hemoglobin O. Biochem Biophys Res Commun 373:197–201
Ascenzi P, di Masi A, Sciorati C, Clementi E (2010) Peroxynitrite – an ugly biofactor? Biofactors 36:264–273
Augusto O, Bonini MG, Amanso AM, Linares E, Santos CC, De Menezes SL (2002) Nitrogen dioxide and carbonate radical anion: two emerging radicals in biology. Free Radic Biol Med 32:841–859
Bajt ML, Knight TR, Farhood A, Jaeschke H (2003) Scavenging peroxynitrite with glutathione promotes regeneration and enhances survival during acetaminophen-induced liver injury in mice. J Pharmacol Exp Ther 307:67–73
Balafanova Z, Bolli R, Zhang J, Zheng Y, Pass JM, Bhatnagar A, Tang XL, Wang O, Cardwell E, Ping P (2002) Nitric oxide (NO) induces nitration of protein kinase Cepsilon (PKCepsilon), facilitating PKCepsilon translocation via enhanced PKCepsilon -RACK2 interactions: a novel mechanism of no-triggered activation of PKCepsilon. J Biol Chem 277:15021–15027
Bapat S, Verkleij A, Post JA (2001) Peroxynitrite activates mitogen-activated protein kinase (MAPK) via a MEK-independent pathway: a role for protein kinase C. FEBS Lett 499:21–26
Bar-Shai M, Reznick AZ (2006) Peroxynitrite induces an alternative NF-kappaB activation pathway in L8 rat myoblasts. Antioxid Redox Signal 8:639–652
Batinic-Haberle I, Cuzzocrea S, Reboucas JS, Ferrer-Sueta G, Mazzon E, Di Paola R, Radi R, Spasojevic I, Benov L, Salvemini D (2009) Pure MnTBAP selectively scavenges peroxynitrite over superoxide: comparison of pure and commercial MnTBAP samples to MnTE-2-PyP in two models of oxidative stress injury, an SOD-specific Escherichia coli model and carrageenan-induced pleurisy. Free Radic Biol Med 46:192–201
Beckman JS (1996) Oxidative damage and tyrosine nitration from peroxynitrite. Chem Res Toxicol 9:836–844
Beckman JS, Koppenol WH (1996) Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol 271:C1424–C1437
Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA (1990) Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 87:1620–1624
Beckman JS, Ischiropoulos H, Zhu L, van der Woerd M, Smith C, Chen J, Harrison J, Martin JC, Tsai M (1992) Kinetics of superoxide dismutase- and iron-catalyzed nitration of phenolics by peroxynitrite. Arch Biochem Biophys 298:438–445
Beckman JS, Carson M, Smith CD, Koppenol WH (1993) ALS, SOD and peroxynitrite. Nature 364:584
Boccini F, Herold S (2004) Mechanistic studies of the oxidation of oxyhemoglobin by peroxynitrite. Biochemistry 43:16393–16404
Bohle DS, Hansert B, Paulson SC, Smith BD (1994) Biomimetic synthesis of the putative cytotoxin peroxynitrite ONOO-, and its characterization as a tetramethylammonium salt. J Am Chem Soc 116:7423–7424
Borbely A, Toth A, Edes I, Virag L, Papp JG, Varro A, Paulus WJ, van der Velden J, Stienen GJ, Papp Z (2005) Peroxynitrite-induced alpha-actinin nitration and contractile alterations in isolated human myocardial cells. Cardiovasc Res 67:225–233
Botti H, Trostchansky A, Batthyany C, Rubbo H (2005) Reactivity of peroxynitrite and nitric oxide with LDL. IUBMB Life 57:407–412
Bryk R, Griffin P, Nathan C (2000) Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature 407:211–215
Bullen ML, Miller AA, Andrews KL, Irvine JC, Ritchie RH, Sobey CG, Kemp-Harper BK (2011) Nitroxyl (HNO) as a vasoprotective signaling molecule. Antioxid Redox Signal 14:1675–1686
Burney S, Caulfield JL, Niles JC, Wishnok JS, Tannenbaum SR (1999) The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat Res 424:37–49
Burns AR, Bowden RA, Abe Y, Walker DC, Simon SI, Entman ML, Smith CW (1999) P-selectin mediates neutrophil adhesion to endothelial cell borders. J Leukoc Biol 65:299–306
Butler AR, Megson IL, Wright PG (1998) Diffusion of nitric oxide and scavenging by blood in the vasculature. Biochim Biophys Acta 1425:168–176
Cai S, Khoo J, Mussa S, Alp NJ, Channon KM (2005) Endothelial nitric oxide synthase dysfunction in diabetic mice: importance of tetrahydrobiopterin in eNOS dimerisation. Diabetologia 48:1933–1940
Castro L, Rodriguez M, Radi R (1994) Aconitase is readily inactivated by peroxynitrite, but not by its precursor, nitric oxide. J Biol Chem 269:29409–29415
Cena JJ, Lalu MM, Cho WJ, Chow AK, Bagdan ML, Daniel EE, Castro MM, Schulz R (2010) Inhibition of matrix metalloproteinase activity in vivo protects against vascular hyporeactivity in endotoxemia. Am J Physiol Heart Circ Physiol 298:H45–H51
Ceriello A (2002) Nitrotyrosine: new findings as a marker of postprandial oxidative stress. Int J Clin Pract Suppl 129:51–58
Ceriello A, Quagliaro L, D’Amico M, Di Filippo C, Marfella R, Nappo F, Berrino L, Rossi F, Giugliano D (2002) Acute hyperglycemia induces nitrotyrosine formation and apoptosis in perfused heart from rat. Diabetes 51:1076–1082
Channon KM (2004) Tetrahydrobiopterin: regulator of endothelial nitric oxide synthase in vascular disease. Trends Cardiovasc Med 14:323–327
Chen K, Pittman RN, Popel AS (2008) Nitric oxide in the vasculature: where does it come from and where does it go? A quantitative perspective. Antioxid Redox Signal 10:1185–1198
Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, Chen YR, Druhan LJ, Zweier JL (2010) S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 468:1115–1118
Chen CA, Lin CH, Druhan LJ, Wang TY, Chen YR, Zweier JL (2011) Superoxide induces endothelial nitric-oxide synthase protein thiyl radical formation, a novel mechanism regulating eNOS function and coupling. J Biol Chem 286:29098–29107
Choi HR, Choi JS, Han YN, Bae SJ, Chung HY (2002) Peroxynitrite scavenging activity of herb extracts. Phytother Res 16:364–367
Churchill EN, Mochly-Rosen D (2007) The roles of PKCdelta and epsilon isoenzymes in the regulation of myocardial ischaemia/reperfusion injury. Biochem Soc Trans 35:1040–1042
Cooke CL, Davidge ST (2002) Peroxynitrite increases iNOS through NF-kappaB and decreases prostacyclin synthase in endothelial cells. Am J Physiol Cell Physiol 282:C395–C402
Cooke MS, Evans MD, Dizdaroglu M, Lunec J (2003) Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J 17:1195–1214
Cosentino F, Eto M, De Paolis P, van der Loo B, Bachschmid M, Ullrich V, Kouroedov A, Delli Gatti C, Joch H, Volpe M, Luscher TF, Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A (2003a) Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes 52:2795–2804
Cosentino F, Eto M, De Paolis P, van der Loo B, Bachschmid M, Ullrich V, Kouroedov A, Delli Gatti C, Joch H, Volpe M, Lüscher TF (2003b) High glucose causes upregulation of cyclooxygenase-2 and alters prostanoid profile in human endothelial cells: role of protein kinase C and reactive oxygen species. Circulation 107:1017–1023
Crabtree MJ, Smith CL, Lam G, Goligorsky MS, Gross SS (2008) Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production by eNOS. Am J Physiol Heart Circ Physiol 294:H1530–H1540
Crow JP (2002) Peroxynitrite: scavenging for survival. www.sfrbm.org/frs/CrowONOO.pdf
Crow JP, Beckman JS, McCord JM (1995) Sensitivity of the essential zinc-thiolate moiety of yeast alcohol dehydrogenase to hypochlorite and peroxynitrite. Biochemistry 34:3544–3552
Cuzzocrea S, Mazzon E, Costantino G, Serraino I, Dugo L, Calabro G, Cucinotta G, De Sarro A, Caputi AP (2000) Beneficial effects of n-acetylcysteine on ischaemic brain injury. Br J Pharmacol 130:1219–1226
Denicola A, Souza JM, Radi R (1998) Diffusion of peroxynitrite across erythrocyte membranes. Proc Natl Acad Sci USA 95:3566–3571
Ding H, Triggle CR (2010) Endothelial dysfunction in diabetes: multiple targets for treatment. Pflugers Arch 459:977–994
Ding H, Aljofan M, Triggle CR (2007) Oxidative stress and increased eNOS and NADPH oxidase expression in mouse microvessel endothelial cells. J Cell Physiol 212:682–689
Djaman O, Outten FW, Imlay JA (2004) Repair of oxidized iron-sulfur clusters in Escherichia coli. J Biol Chem 279:44590–44599
Donzelli S, Espey MG, Thomas DD, Mancardi D, Tocchetti CG, Ridnour LA, Paolocci N, King SB, Miranda KM, Lazzarino G, Fukuto JM, Wink DA (2006) Discriminating formation of HNO from other reactive nitrogen oxide species. Free Radic Biol Med 40:1056–1066
Douki T, Cadet J (1996) Peroxynitrite mediated oxidation of purine bases of nucleosides and isolated DNA. Free Radic Res 24:369–380
Douki T, Cadet J, Ames BN (1996) An adduct between peroxynitrite and 2′-deoxyguanosine: 4,5-dihydro-5-hydroxy-4-(nitrosooxy)-2′-deoxyguanosine. Chem Res Toxicol 9:3–7
Dowell FJ, Martin W (1997) The effects of peroxynitrite on rat aorta: interaction with glucose and related substances. Eur J Pharmacol 338:43–53
Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Ellis A, Li CG, Rand MJ (2000) Differential actions of L-cysteine on responses to nitric oxide, nitroxyl anions and EDRF in the rat aorta. Br J Pharmacol 129:315–322
El-Remessy AB, Behzadian MA, Abou-Mohamed G, Franklin T, Caldwell RW, Caldwell RB (2003) Experimental diabetes causes breakdown of the blood-retina barrier by a mechanism involving tyrosine nitration and increases in expression of vascular endothelial growth factor and urokinase plasminogen activator receptor. Am J Pathol 162:1995–2004
El-Remessy AB, Tawfik HE, Matragoon S, Pillai B, Caldwell RB, Caldwell RW (2010) Peroxynitrite mediates diabetes-induced endothelial dysfunction: possible role of Rho kinase activation. Exp Diabetes Res 2010:247861
Favaloro JL, Kemp-Harper BK (2009) Redox variants of NO (NO{middle dot} and HNO) elicit vasorelaxation of resistance arteries via distinct mechanisms. Am J Physiol Heart Circ Physiol 296:H1274–H1280
Fehsel K, Jalowy A, Qi S, Burkart V, Hartmann B, Kolb H (1993) Islet cell DNA is a target of inflammatory attack by nitric oxide. Diabetes 42:496–500
Félétou M, Vanhoutte PM (2006) Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture). Am J Physiol Heart Circ Physiol 291:H985–H1002
Ferdinandy P (2006) Peroxynitrite: just an oxidative/nitrosative stressor or a physiological regulator as well? Br J Pharmacol 148:1–3
Ferdinandy P, Schulz R (2001) Peroxynitrite: toxic or protective in the heart? Circ Res 88:E12–E13
Fernandes DC, Medinas DB, Alves MJ, Augusto O (2005a) Tempol diverts peroxynitrite/carbon dioxide reactivity toward albumin and cells from protein-tyrosine nitration to protein-cysteine nitrosation. Free Radic Biol Med 38:189–200
Fernandes E, Gomes A, Costa D, Lima JL (2005b) Pindolol is a potent scavenger of reactive nitrogen species. Life Sci 77:1983–1992
Ferrer-Sueta G, Radi R (2009) Chemical biology of peroxynitrite: kinetics, diffusion, and radicals. ACS Chem Biol 4:161–177
Flores-Santana W, Salmon DJ, Donzelli S, Switzer CH, Basudhar D, Ridnour L, Cheng R, Glynn SA, Paolocci N, Fukuto JM, Miranda KM, Wink DA (2011) The specificity of nitroxyl chemistry is unique among nitrogen oxides in biological systems. Antioxid Redox Signal 14:1659–1674
Forstermann U, Munzel T (2006) Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113:1708–1714
Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P (2000) Myocardial cell death in human diabetes. Circ Res 87:1123–1132
Fukuto JM, Carrington SJ (2011) HNO signaling mechanisms. Antioxid Redox Signal 14:1649–1657
Fukuto JM, Bartberger MD, Dutton AS, Paolocci N, Wink DA, Houk KN (2005) The physiological chemistry and biological activity of nitroxyl (HNO): the neglected, misunderstood, and enigmatic nitrogen oxide. Chem Res Toxicol 18:790–801
Furchgott RF, Vanhoutte PM (1989) Endothelium-derived relaxing and contracting factors. FASEB J 3:2007–2018
Furchgott RF, Zawadzki JV (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288:373–376
Gao CQ, Sawicki G, Suarez-Pinzon WL, Csont T, Wozniak M, Ferdinandy P, Schulz R (2003) Matrix metalloproteinase-2 mediates cytokine-induced myocardial contractile dysfunction. Cardiovasc Res 57:426–433
Garcia Soriano F, Virag L, Jagtap P, Szabo E, Mabley JG, Liaudet L, Marton A, Hoyt DG, Murthy KG, Salzman AL, Southan GJ, Szabo C (2001) Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat Med 7:108–113
Ghiadoni L, Taddei S, Virdis A (2011) Hypertension and endothelial dysfunction: therapeutic approach. Curr Vasc Pharmacol 10(1):42–60
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107:1058–1070
Go YM, Patel RP, Maland MC, Park H, Beckman JS, Darley-Usmar VM, Jo H (1999) Evidence for peroxynitrite as a signaling molecule in flow-dependent activation of c-Jun NH(2)-terminal kinase. Am J Physiol 277:H1647–H1653
Gochman E, Mahajna J, Reznick AZ (2011) NF-kappaB activation by peroxynitrite through IkappaBalpha-dependent phosphorylation versus nitration in colon cancer cells. Anticancer Res 31:1607–1617
Goldstein S, Merényi G (2008) The chemistry of peroxynitrite: implications for biological activity. Methods Enzymol 436:49–61
Goldstein S, Squadrito GL, Pryor WA, Czapski G (1996) Direct and indirect oxidations by peroxynitrite, neither involving the hydroxyl radical. Free Radic Biol Med 21:965–974
Granger DL, Hibbs JB Jr (1996) High-output nitric oxide: weapon against infection? Trends Microbiol 4:46–47
Graves JE, Lewis SJ, Kooy NW (2005) Loss of K+ATP-channel-mediated vasodilation after induction of tachyphylaxis to peroxynitrite. J Cardiovasc Pharmacol 46:646–652
Grimsrud PA, Xie H, Griffin TJ, Bernlohr DA (2008) Oxidative stress and covalent modification of protein with bioactive aldehydes. J Biol Chem 283:21837–21841
Hagar JM, Hale SL, Kloner RA (1991) Effect of preconditioning ischemia on reperfusion arrhythmias after coronary artery occlusion and reperfusion in the rat. Circ Res 68:61–68
Hall ED, Detloff MR, Johnson K, Kupina NC (2004) Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. J Neurotrauma 21:9–20
Hausladen A, Fridovich I (1994) Superoxide and peroxynitrite inactivate aconitases, but nitric oxide does not. J Biol Chem 269:29405–29408
Heitzer T, Krohn K, Albers S, Meinertz T (2000) Tetrahydrobiopterin improves endothelium-dependent vasodilation by increasing nitric oxide activity in patients with Type II diabetes mellitus. Diabetologia 43:1435–1438
Herold S, Fago A (2005) Reactions of peroxynitrite with globin proteins and their possible physiological role. Comp Biochem Physiol A Mol Integr Physiol 142:124–129
Herold S, Shivashankar K, Mehl M (2002) Myoglobin scavenges peroxynitrite without being significantly nitrated. Biochemistry 41:13460–13472
Herold S, Exner M, Boccini F (2003) The mechanism of the peroxynitrite-mediated oxidation of myoglobin in the absence and presence of carbon dioxide. Chem Res Toxicol 16:390–402
Hogg N, Kalyanaraman B (1999) Nitric oxide and lipid peroxidation. Biochim Biophys Acta 1411:378–384
Hooper DC, Spitsin S, Kean RB, Champion JM, Dickson GM, Chaudhry I, Koprowski H (1998) Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci USA 95:675–680
Hou R, Zhang J, Yin T, Cao H, Zhang N, Li X, Wang L, Xing Y, Li D, Ji Q (2010) Upregulation of PTEN by peroxynitrite contributes to cytokine-induced apoptosis in pancreatic beta-cells. Apoptosis 15:877–886
Ignarro LJ (1990) Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol 30:535–560
Ihlemann N, Rask-Madsen C, Perner A, Dominguez H, Hermann T, Kober L, Torp-Pedersen C (2003) Tetrahydrobiopterin restores endothelial dysfunction induced by an oral glucose challenge in healthy subjects. Am J Physiol Heart Circ Physiol 285:H875–H882
Irvine JC, Favaloro JL, Kemp-Harper BK (2003) NO- activates soluble guanylate cyclase and Kv channels to vasodilate resistance arteries. Hypertension 41:1301–1307
Ischiropoulos H, Beckman JS (2003) Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J Clin Invest 111:163–169
Ishii N, Patel KP, Lane PH, Taylor T, Bian K, Murad F, Pollock JS, Carmines PK (2001) Nitric oxide synthesis and oxidative stress in the renal cortex of rats with diabetes mellitus. J Am Soc Nephrol 12:1630–1639
Joshi MS, Ferguson TB Jr, Han TH, Hyduke DR, Liao JC, Rassaf T, Bryan N, Feelisch M, Lancaster JR Jr (2002) Nitric oxide is consumed, rather than conserved, by reaction with oxyhemoglobin under physiological conditions. Proc Natl Acad Sci USA 99:10341–10346
Jourd’heuil D, Jourd’heuil FL, Kutchukian PS, Musah RA, Wink DA, Grisham MB (2001) Reaction of superoxide and nitric oxide with peroxynitrite. Implications for peroxynitrite-mediated oxidation reactions in vivo. J Biol Chem 276:28799–28805
Kajstura J, Fiordaliso F, Andreoli AM, Li B, Chimenti S, Medow MS, Limana F, Nadal-Ginard B, Leri A, Anversa P (2001) IGF-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin II-mediated oxidative stress. Diabetes 50:1414–1424
Kang KW, Choi SH, Kim SG (2002) Peroxynitrite activates NF-E2-related factor 2/antioxidant response element through the pathway of phosphatidylinositol 3-kinase: the role of nitric oxide synthase in rat glutathione S-transferase A2 induction. Nitric Oxide 7:244–253
Kar S, Kavdia M (2011) Modeling of biopterin-dependent pathways of eNOS for nitric oxide and superoxide production. Free Radic Biol Med 51:1411–1427
Kawano H, Motoyama T, Hirashima O, Hirai N, Miyao Y, Sakamoto T, Kugiyama K, Ogawa H, Yasue H (1999) Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J Am Coll Cardiol 34:146–154
Kemp-Harper BK (2011) Nitroxyl (HNO): a novel redox signaling molecule. Antioxid Redox Signal 14:1609–1613
Keyer K, Imlay JA (1997) Inactivation of dehydratase [4Fe-4S] clusters and disruption of iron homeostasis upon cell exposure to peroxynitrite. J Biol Chem 272:27652–27659
Kietadisorn R, Juni RP, Moens AL (2011) Tackling endothelial dysfunction by modulating NOS-uncoupling: new insights in pathogenesis and therapeutic possibilities. Am J Physiol Endocrinol Metab 302:E481–E495
Kim JY, Song EH, Lee HJ, Oh YK, Park YS, Park JW, Kim BJ, Kim DJ, Lee I, Song J, Kim WH (2010) Chronic ethanol consumption-induced pancreatic {beta}-cell dysfunction and apoptosis through glucokinase nitration and its down-regulation. J Biol Chem 285:37251–37262
Kissner R, Nauser T, Bugnon P, Lye PG, Koppenol WH (1997) Formation and properties of peroxynitrite as studied by laser flash photolysis, high-pressure stopped-flow technique, and pulse radiolysis. Chem Res Toxicol 10:1285–1292
Klatt P, Lamas S (2000) Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem 267:4928–4944
Klotz LO, Sies H (2003) Defenses against peroxynitrite: selenocompounds and flavonoids. Toxicol Lett 140–141:125–132
Knapp LT, Kanterewicz BI, Hayes EL, Klann E (2001) Peroxynitrite-induced tyrosine nitration and inhibition of protein kinase C. Biochem Biophys Res Commun 286:764–770
Kobayashi T (2008) Possible involvement of insulin and oxidative stress in vascular dysfunction of diabetic mellitus. Yakugaku Zasshi 128:1013–1021
Kohnen SL, Mouithys-Mickalad AA, Deby-Dupont GP, Deby CM, Lamy ML, Noels AF (2001) Oxidation of tetrahydrobiopterin by peroxynitrite or oxoferryl species occurs by a radical pathway. Free Radic Res 35:709–721
Konorev EA, Hogg N, Kalyanaraman B (1998) Rapid and irreversible inhibition of creatine kinase by peroxynitrite. FEBS Lett 427:171–174
Kooy NW, Royall JA, Ischiropoulos H, Beckman JS (1994) Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic Biol Med 16:149–156
Koppenol WH (2001) 100 years of peroxynitrite chemistry and 11 years of peroxynitrite biochemistry. Redox Rep 6:339–341
Koppenol WH, Moreno JJ, Pryor WA, Ischiropoulos H, Beckman JS (1992) Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem Res Toxicol 5:834–842
Kossenjans W, Eis A, Sahay R, Brockman D, Myatt L (2000) Role of peroxynitrite in altered fetal-placental vascular reactivity in diabetes or preeclampsia. Am J Physiol Heart Circ Physiol 278:H1311–H1319
Kuhn DM, Aretha CW, Geddes TJ (1999) Peroxynitrite inactivation of tyrosine hydroxylase: mediation by sulfhydryl oxidation, not tyrosine nitration. J Neurosci 19:10289–10294
Kvietys PR, Granger DN (2011) Role of reactive oxygen and nitrogen species in the vascular responses to inflammation. Free Radic Biol Med 52(3):556–592
Lakey JR, Suarez-Pinzon WL, Strynadka K, Korbutt GS, Rajotte RV, Mabley JG, Szabo C, Rabinovitch A (2001) Peroxynitrite is a mediator of cytokine-induced destruction of human pancreatic islet beta cells. Lab Invest 81:1683–1692
Landino LM, Crews BC, Timmons MD, Morrow JD, Marnett LJ (1996) Peroxynitrite, the coupling product of nitric oxide and superoxide, activates prostaglandin biosynthesis. Proc Natl Acad Sci USA 93:15069–15074
Lee WH, Gounarides JS, Roos ES, Wolin MS (2003) Influence of peroxynitrite on energy metabolism and cardiac function in a rat ischemia-reperfusion model. Am J Physiol Heart Circ Physiol 285:H1385–H1395
Lefer DJ, Scalia R, Campbell B, Nossuli T, Hayward R, Salamon M, Grayson J, Lefer AM (1997) Peroxynitrite inhibits leukocyte-endothelial cell interactions and protects against ischemia-reperfusion injury in rats. J Clin Invest 99:684–691
Leffler JE, Grunwald E (1963) Rates and equilibria of organic reactions. Wiley, New York
Leiper J, Nandi M (2011) The therapeutic potential of targeting endogenous inhibitors of nitric oxide synthesis. Nat Rev Drug Discov 10:277–291
Li H, Gutterman DD, Rusch NJ, Bubolz A, Liu Y (2004a) Nitration and functional loss of voltage-gated K+ channels in rat coronary microvessels exposed to high glucose. Diabetes 53:2436–2442
Li J, Li W, Altura BT, Altura BM (2004b) Peroxynitrite-induced relaxation in isolated canine cerebral arteries and mechanisms of action. Toxicol Appl Pharmacol 196:176–182
Li MH, Cha YN, Surh YJ (2006) Peroxynitrite induces HO-1 expression via PI3K/Akt-dependent activation of NF-E2-related factor 2 in PC12 cells. Free Radic Biol Med 41:1079–1091
Liang JH, Li YN, Qi JS, Jia XX (2010) Peroxynitrite-induced protein nitration is responsible for renal mitochondrial damage in diabetic rat. J Endocrinol Invest 33:140–146
Liaudet L, Vassalli G, Pacher P (2009) Role of peroxynitrite in the redox regulation of cell signal transduction pathways. Front Biosci 14:4809–4814
Liochev SI, Fridovich I (2003) The mode of decomposition of Angeli’s salt (Na2N2O3) and the effects thereon of oxygen, nitrite, superoxide dismutase, and glutathione. Free Radic Biol Med 34:1399–1404
Liu S, Beckman JS, Ku DD (1994) Peroxynitrite, a product of superoxide and nitric oxide, produces coronary vasorelaxation in dogs. J Pharmacol Exp Ther 268:1114–1121
Liu P, Xu B, Quilley J, Wong PY (2000) Peroxynitrite attenuates hepatic ischemia-reperfusion injury. Am J Physiol Cell Physiol 279:C1970–C1977
Lokuta AJ, Maertz NA, Meethal SV, Potter KT, Kamp TJ, Valdivia HH, Haworth RA (2005) Increased nitration of sarcoplasmic reticulum Ca2+-ATPase in human heart failure. Circulation 111:988–995
Lopez CJ, Qayyum I, Mishra OP, Delivoria-Papadopoulos M (2005) Effect of nitration on protein tyrosine phosphatase and protein phosphatase activity in neuronal cell membranes of newborn piglets. Neurosci Lett 386:78–81
Lorch S, Lightfoot R, Ohshima H, Virag L, Chen Q, Hertkorn C, Weiss M, Souza J, Ischiropoulos H, Yermilov V, Pignatelli B, Masuda M, Szabo C (2002) Detection of peroxynitrite-induced protein and DNA modifications. Methods Mol Biol 196:247–275
Luiking YC, Engelen MP, Deutz NE (2010) Regulation of nitric oxide production in health and disease. Curr Opin Clin Nutr Metab Care 13:97–104
Mabley JG, Pacher P, Bai P, Wallace R, Goonesekera S, Virag L, Southan GJ, Szabo C (2004) Suppression of intestinal polyposis in Apcmin/+ mice by targeting the nitric oxide or poly(ADP-ribose) pathways. Mutat Res 548:107–116
Macfadyen AJ, Reiter C, Zhuang Y, Beckman JS (1999) A novel superoxide dismutase-based trap for peroxynitrite used to detect entry of peroxynitrite into erythrocyte ghosts. Chem Res Toxicol 12:223–229
Marshall KA, Reist M, Jenner P, Halliwell B (1999) The neuronal toxicity of sulfite plus peroxynitrite is enhanced by glutathione depletion: implications for Parkinson’s disease. Free Radic Biol Med 27:515–520
Matata BM, Galinanes M (2002) Peroxynitrite is an essential component of cytokines production mechanism in human monocytes through modulation of nuclear factor-kappa B DNA binding activity. J Biol Chem 277:2330–2335
Maulik N, Das DK (2008) Emerging potential of thioredoxin and thioredoxin interacting proteins in various disease conditions. Biochim Biophys Acta 1780:1368–1382
Migita K, Maeda Y, Abiru S, Komori A, Yokoyama T, Takii Y, Nakamura M, Yatsuhashi H, Eguchi K, Ishibashi H (2005) Peroxynitrite-mediated matrix metalloproteinase-2 activation in human hepatic stellate cells. FEBS Lett 579:3119–3125
Mihm MJ, Bauer JA (2002) Peroxynitrite-induced inhibition and nitration of cardiac myofibrillar creatine kinase. Biochimie 84:1013–1019
Mihm MJ, Coyle CM, Schanbacher BL, Weinstein DM, Bauer JA (2001) Peroxynitrite induced nitration and inactivation of myofibrillar creatine kinase in experimental heart failure. Cardiovasc Res 49:798–807
Milstien S, Katusic Z (1999) Oxidation of tetrahydrobiopterin by peroxynitrite: implications for vascular endothelial function. Biochem Biophys Res Commun 263:681–684
Miranda KM, Nims RW, Thomas DD, Espey MG, Citrin D, Bartberger MD, Paolocci N, Fukuto JM, Feelisch M, Wink DA (2003a) Comparison of the reactivity of nitric oxide and nitroxyl with heme proteins. A chemical discussion of the differential biological effects of these redox related products of NOS. J Inorg Biochem 93:52–60
Miranda KM, Paolocci N, Katori T, Thomas DD, Ford E, Bartberger MD, Espey MG, Kass DA, Feelisch M, Fukuto JM, Wink DA (2003b) A biochemical rationale for the discrete behavior of nitroxyl and nitric oxide in the cardiovascular system. Proc Natl Acad Sci USA 100:9196–9201
Mohr S, Stamler JS, Brune B (1994) Mechanism of covalent modification of glyceraldehyde-3-phosphate dehydrogenase at its active site thiol by nitric oxide, peroxynitrite and related nitrosating agents. FEBS Lett 348:223–227
Moro MA, Darley-Usmar VM, Goodwin DA, Read NG, Zamora-Pino R, Feelisch M, Radomski MW, Moncada S (1994) Paradoxical fate and biological action of peroxynitrite on human platelets. Proc Natl Acad Sci USA 91:6702–6706
Moro MA, Darley-Usmar VM, Lizasoain I, Su Y, Knowles RG, Radomski MW, Moncada S (1995) The formation of nitric oxide donors from peroxynitrite. Br J Pharmacol 116:1999–2004
Naseem KM (2005) The role of nitric oxide in cardiovascular diseases. Mol Aspects Med 26:33–65
Niles JC, Wishnok JS, Tannenbaum SR (2006) Peroxynitrite-induced oxidation and nitration products of guanine and 8-oxoguanine: structures and mechanisms of product formation. Nitric Oxide 14:109–121
Norris AJ, Sartippour MR, Lu M, Park T, Rao JY, Jackson MI, Fukuto JM, Brooks MN (2008) Nitroxyl inhibits breast tumor growth and angiogenesis. Int J Cancer 122:1905–1910
Nossaman BD, Kadowitz PJ (2008) Potential benefits of peroxynitrite. Open Pharmacol J 2:31–53
Nossuli TO, Hayward R, Scalia R, Lefer AM (1997) Peroxynitrite reduces myocardial infarct size and preserves coronary endothelium after ischemia and reperfusion in cats. Circulation 96:2317–2324
Nossuli TO, Hayward R, Jensen D, Scalia R, Lefer AM (1998) Mechanisms of cardioprotection by peroxynitrite in myocardial ischemia and reperfusion injury. Am J Physiol 275:H509–H519
Nowak P, Wachowicz B (2001) Studies on pig blood platelet responses to peroxynitrite action. Platelets 12:376–381
Ohshima H, Virag L, Souza J, Yermilov V, Pignatelli B, Masuda M, Szabo C (2002) Detection of certain peroxynitrite-induced DNA modifications. Methods Mol Biol 186:77–88
Okamoto T, Akaike T, Sawa T, Miyamoto Y, van der Vliet A, Maeda H (2001) Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J Biol Chem 276:29596–29602
Onozato ML, Tojo A, Goto A, Fujita T, Wilcox CS (2002) Oxidative stress and nitric oxide synthase in rat diabetic nephropathy: effects of ACEI and ARB. Kidney Int 61:186–194
Pacher P, Szabo C (2005) Role of poly (ADP-ribose) polymerase-1 activation in the pathogenesis of diabetic complications: endothelial dysfunction, as a common underlying theme. Antioxid Redox Signal 7:1568–1580
Pacher P, Liaudet L, Soriano FG, Mabley JG, Szabo E, Szabo C (2002) The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes 51:514–521
Pacher P, Schulz R, Liaudet L, Szabo C (2005) Nitrosative stress and pharmacological modulation of heart failure. Trends Pharmacol Sci 26:302–310
Pacher P, Beckman JS, Liaudet L (2007) Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87:315–424
Palmer RM, Ferrige AG, Moncada S (1987) Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327:524–526
Panasenko OM, Sharov VS, Briviba K, Sies H (2000) Interaction of peroxynitrite with carotenoids in human low density lipoproteins. Arch Biochem Biophys 373:302–305
Pannirselvam M, Verma S, Anderson TJ, Triggle CR (2002) Cellular basis of endothelial dysfunction in small mesenteric arteries from spontaneously diabetic (db/db −/−) mice: role of decreased tetrahydrobiopterin bioavailability. Br J Pharmacol 136:255–263
Pannirselvam M, Simon V, Verma S, Anderson T, Triggle CR (2003) Chronic oral supplementation with sepiapterin prevents endothelial dysfunction and oxidative stress in small mesenteric arteries from diabetic (db/db) mice. Br J Pharmacol 140:701–706
Paolocci N, Wink DA (2009) The shy Angeli and his elusive creature: the HNO route to vasodilation. Am J Physiol Heart Circ Physiol 296:H1217–H1220
Paolocci N, Saavedra WF, Miranda KM, Martignani C, Isoda T, Hare JM, Espey MG, Fukuto JM, Feelisch M, Wink DA, Kass DA (2001) Nitroxyl anion exerts redox-sensitive positive cardiac inotropy in vivo by calcitonin gene-related peptide signaling. Proc Natl Acad Sci USA 98:10463–10468
Patel RP, McAndrew J, Sellak H, White CR, Jo H, Freeman BA, Darley-Usmar VM (1999) Biological aspects of reactive nitrogen species. Biochim Biophys Acta 1411:385–400
Ping P, Takano H, Zhang J, Tang XL, Qiu Y, Li RC, Banerjee S, Dawn B, Balafonova Z, Bolli R (1999) Isoform-selective activation of protein kinase C by nitric oxide in the heart of conscious rabbits: a signaling mechanism for both nitric oxide-induced and ischemia-induced preconditioning. Circ Res 84:587–604
Pober JS, Min W, Bradley JR (2009) Mechanisms of endothelial dysfunction, injury, and death. Annu Rev Pathol 4:71–95
Pryor WA (1966) Free radicals. McGraw-Hill, New York
Pryor WA, Squadrito GL (1995) The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am J Physiol 268:L699–L722
Pryor WA, Jin X, Squadrito GL (1994) One- and two-electron oxidations of methionine by peroxynitrite. Proc Natl Acad Sci USA 91:11173–11177
Rabini RA, Vignini A, Salvolini E, Staffolani R, Martarelli D, Moretti N, Mazzanti L (2002) Activation of human aortic endothelial cells by LDL from Type 1 diabetic patients: an in vitro study. Atherosclerosis 165:69–77
Radi R, Beckman JS, Bush KM, Freeman BA (1991a) Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys 288:481–487
Radi R, Beckman JS, Bush KM, Freeman BA (1991b) Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem 266:4244–4250
Radi R, Cassina A, Hodara R (2002a) Nitric oxide and peroxynitrite interactions with mitochondria. Biol Chem 383:401–409
Radi R, Cassina A, Hodara R, Quijano C, Castro L (2002b) Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med 33:1451–1464
Reynolds MR, Berry RW, Binder LI (2005) Site-specific nitration and oxidative dityrosine bridging of the tau protein by peroxynitrite: implications for Alzheimer’s disease. Biochemistry 44:1690–1700
Romero N, Radi R (2005) Hemoglobin and red blood cells as tools for studying peroxynitrite biochemistry. Methods Enzymol 396:229–245
Ronson RS, Nakamura M, Vinten-Johansen J (1999) The cardiovascular effects and implications of peroxynitrite. Cardiovasc Res 44:47–59
Rork TH, Hadzimichalis NM, Kappil MA, Merrill GF (2006) Acetaminophen attenuates peroxynitrite-activated matrix metalloproteinase-2-mediated troponin I cleavage in the isolated guinea pig myocardium. J Mol Cell Cardiol 40:553–561
Rubbo H (1998) Nitric oxide and peroxynitrite in lipid peroxidation. Medicina (B Aires) 58:361–366
Rubbo H, Freeman BA (1996) Nitric oxide regulation of lipid oxidation reactions: formation and analysis of nitrogen-containing oxidized lipid derivatives. Methods Enzymol 269:385–394
Rubbo H, O’Donnell V (2005) Nitric oxide, peroxynitrite and lipoxygenase in atherogenesis: mechanistic insights. Toxicology 208:305–317
Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman BA (1994) Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem 269:26066–26075
Rusche KM, Spiering MM, Marletta MA (1998) Reactions catalyzed by tetrahydrobiopterin-free nitric oxide synthase. Biochemistry 37:15503–15512
Salvemini D, Wang ZQ, Stern MK, Currie MG, Misko TP (1998) Proc Natl Acad Sci USA 95:2659–2663
Sasaki N, Yamashita T, Takaya T, Shinohara M, Shiraki R, Takeda M, Emoto N, Fukatsu A, Hayashi T, Ikemoto K, Nomura T, Yokoyama M, Hirata K, Kawashima S (2008) Augmentation of vascular remodeling by uncoupled endothelial nitric oxide synthase in a mouse model of diabetes mellitus. Arterioscler Thromb Vasc Biol 28:1068–1076
Schroeder P, Klotz LO, Sies H (2003) Amphiphilic properties of (−)-epicatechin and their significance for protection of cells against peroxynitrite. Biochem Biophys Res Commun 307:69–73
Scott-Burden T (1995) Regulation of nitric oxide production by tetrahydrobiopterin. Circulation 91:248–250
Selley ML (2005) Simvastatin prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced striatal dopamine depletion and protein tyrosine nitration in mice. Brain Res 1037(1–2):1–6
Sharma A, Bernatchez PN, de Haan JB (2012) Targeting endothelial dysfunction in vascular complications associated with diabetes. Int J Vasc Med 2012:750126
Sies H (1963) Strategies of antioxidant defense. Eur J Biochem 215:213–219
Sies H, Masumoto H (1997) Ebselen as a glutathione peroxidase mimic and as a scavenger of peroxynitrite. Adv Pharmacol 38:229–246
Sies H, Sharov VS, Klotz LO, Briviba K (1997) Glutathione peroxidase protects against peroxynitrite-mediated oxidations. A new function for selenoproteins as peroxynitrite reductase. J Biol Chem 272:27812–27817
Singh IN, Sullivan PG, Hall ED (2007) Peroxynitrite-mediated oxidative damage to brain mitochondria: protective effects of peroxynitrite scavengers. J Neurosci Res 85:2216–2223
Skinner KA, White CR, Patel R, Tan S, Barnes S, Kirk M, Darley-Usmar V, Parks DA (1998) Nitrosation of uric acid by peroxynitrite. Formation of a vasoactive nitric oxide donor. J Biol Chem 273:24491–24497
Solodkin A, Traub RJ, Gebhart GF (1992) Neuroscience 51:495–499
Song P, Wu Y, Xu J, Xie Z, Dong Y, Zhang M, Zou MH (2007) Reactive nitrogen species induced by hyperglycemia suppresses Akt signaling and triggers apoptosis by upregulating phosphatase PTEN (phosphatase and tensin homologue deleted on chromosome 10) in an LKB1-dependent manner. Circulation 116:1585–1595
Soriano FG, Virag L, Szabo C (2001) Diabetic endothelial dysfunction: role of reactive oxygen and nitrogen species production and poly- (ADP-ribose) polymerase activation. J Mol Med 79:437–448
Souza JM, Giasson BI, Chen Q, Lee VM, Ischiropoulos H (2000) Dityrosine cross-linking promotes formation of stable alpha -synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J Biol Chem 275:18344–18349
Squadrito GL, Cueto R, Splenser AE, Valavanidis A, Zhang H, Uppu RM, Pryor WA (2000) Reaction of uric acid with peroxynitrite and implications for the mechanism of neuroprotection by uric acid. Arch Biochem Biophys 376:333–337
Stadler K (2011) Peroxynitrite-driven mechanisms in diabetes and insulin resistance – the latest advances. Curr Med Chem 18:280–290
Stadtman ER, Levine RL (2003) Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 25:207–218
Stadtman ER, Moskovitz J, Levine RL (2003) Oxidation of methionine residues of proteins: biological consequences. Antioxid Redox Signal 5:577–582
Stuehr DJ, Nathan CF (1989) Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med 169:1543–1555
Su J, Groves JT (2010) Mechanisms of peroxynitrite interactions with heme proteins. Inorg Chem 49:6317–6329
Szabo C (1996) DNA strand breakage and activation of poly-ADP ribosyltransferase: a cytotoxic pathway triggered by peroxynitrite. Free Radic Biol Med 21:855–869
Szabo C (2003) Multiple pathways of peroxynitrite cytotoxicity. Toxicol Lett 140–141:105–112
Szabo C, Ohshima H (1997) DNA damage induced by peroxynitrite: subsequent biological effects. Nitric Oxide 1:373–385
Szabo C, Zingarelli B, O’Connor M, Salzman AL (1996) DNA strand breakage, activation of poly (ADP-ribose) synthetase, and cellular energy depletion are involved in the cytotoxicity of macrophages and smooth muscle cells exposed to peroxynitrite. Proc Natl Acad Sci USA 93:1753–1758
Szabo C, Zanchi A, Komjati K, Pacher P, Krolewski AS, Quist WC, LoGerfo FW, Horton ES, Veves A (2002) Poly(ADP-ribose) polymerase is activated in subjects at risk of developing type 2 diabetes and is associated with impaired vascular reactivity. Circulation 106:2680–2686
Szabo C, Ischiropoulos H, Radi R (2007) Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov 6:662–680
Takakura K, Beckman JS, MacMillan-Crow LA, Crow JP (1999) Rapid and irreversible inactivation of protein tyrosine phosphatases PTP1B, CD45, and LAR by peroxynitrite. Arch Biochem Biophys 369:197–207
Tannous M, Rabini RA, Vignini A, Moretti N, Fumelli P, Zielinski B, Mazzanti L, Mutus B (1999) Evidence for iNOS-dependent peroxynitrite production in diabetic platelets. Diabetologia 42:539–544
Tao RR, Ji YL, Lu YM, Fukunaga K, Han F (2012) Targeting nitrosative stress for neurovascular protection: new implications in brain diseases. Curr Drug Targets 13:272–284
Thomas CE, Morehouse LA, Aust SD (1985) Ferritin and superoxide-dependent lipid peroxidation. J Biol Chem 260:3275–3280
Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, Hussain P, Vecoli C, Paolocci N, Ambs S, Colton CA, Harris CC, Roberts DD, Wink DA (2008) The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med 45:18–31
Thomson L, Trujillo M, Telleri R, Radi R (1995) Kinetics of cytochrome c2+ oxidation by peroxynitrite: implications for superoxide measurements in nitric oxide-producing biological systems. Arch Biochem Biophys 319:491–497
Thuraisingham RC, Nott CA, Dodd SM, Yaqoob MM (2000) Increased nitrotyrosine staining in kidneys from patients with diabetic nephropathy. Kidney Int 57:1968–1972
Tocchetti CG, Stanley BA, Murray CI, Sivakumaran V, Donzelli S, Mancardi D, Pagliaro P, Gao WD, van Eyk J, Kass DA, Wink DA, Paolocci N (2011) Playing with cardiac “redox switches”: the “HNO way” to modulate cardiac function. Antioxid Redox Signal 14:1687–1698
Torreilles F, Salman-Tabcheh S, Guerin M, Torreilles J (1999) Neurodegenerative disorders: the role of peroxynitrite. Brain Res Brain Res Rev 30:153–163
Triggle CR, Ding H (2010) A review of endothelial dysfunction in diabetes: a focus on the contribution of a dysfunctional eNOS. J Am Soc Hypertens 4:102–115
Triggle CR, Samuel SM, Ravishankar S, Marei I, Arunachalam G, Ding H (2012) The endothelium: influencing vascular smooth muscle in many ways. Can J Physiol Pharmacol 90:713–738
Tsai J-HM, Hamilton TP, Harrison JG, Jablowski M, van der Woerd M, Martin JC, Beckman JS (1994) Role of peroxynitrite conformation with its stability and toxicity. J Am Chem Soc 116:4115–4116
Turko IV, Li L, Aulak KS, Stuehr DJ, Chang JY, Murad F (2003) Protein tyrosine nitration in the mitochondria from diabetic mouse heart. Implications to dysfunctional mitochondria in diabetes. J Biol Chem 278:33972–33977
Uppu RM, Nossaman BD, Greco AJ, Fokin A, Murthy SN, Fonseca VA, Kadowitz PJ (2007) Cardiovascular effects of peroxynitrite. Clin Exp Pharmacol Physiol 34:933–937
Vallance P, Leiper J (2004) Cardiovascular biology of the asymmetric dimethylarginine:dimethylarginine dimethylaminohydrolase pathway. Arterioscler Thromb Vasc Biol 24:1023–1030
van der Vliet A, Eiserich JP, O’Neill CA, Halliwell B, Cross CE (1995) Tyrosine modification by reactive nitrogen species: a closer look. Arch Biochem Biophys 319:341–349
van der Vliet A, Hoen PA, Wong PS, Bast A, Cross CE (1998) Formation of S-nitrosothiols via direct nucleophilic nitrosation of thiols by peroxynitrite with elimination of hydrogen peroxide. J Biol Chem 273:30255–30262
Vasquez-Vivar J, Martasek P, Whitsett J, Joseph J, Kalyanaraman B (2002) The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogues controls superoxide release from endothelial nitric oxide synthase: an EPR spin trapping study. Biochem J 362:733–739
Vegh A, Szekeres L, Parratt J (1992) Preconditioning of the ischaemic myocardium; involvement of the L-arginine nitric oxide pathway. Br J Pharmacol 107:648–652
Verge VMK, Xu Z, Xu XJ, Wiesenfeld-Hallin Z, Hökfelt T (1992) Proc Natl Acad Sci USA 89:11617–11621
Verma S, Lovren F, Dumont AS, Mather KJ, Maitland A, Kieser TM, Triggle CR, Anderson TJ (2000) Tetrahydrobiopterin improves endothelial function in human saphenous veins. J Thorac Cardiovasc Surg 120:668–671
Viappiani S, Nicolescu AC, Holt A, Sawicki G, Crawford BD, Leon H, van Mulligen T, Schulz R (2009) Activation and modulation of 72 kDa matrix metalloproteinase-2 by peroxynitrite and glutathione. Biochem Pharmacol 77:826–834
Villa LM, Salas E, Darley-Usmar VM, Radomski MW, Moncada S (1994) Peroxynitrite induces both vasodilatation and impaired vascular relaxation in the isolated perfused rat heart. Proc Natl Acad Sci USA 91:12383–12387
Vinten-Johansen J (2000) Physiological effects of peroxynitrite: potential products of the environment. Circ Res 87:170–172
Virag L, Szabo C (2002) The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev 54:375–429
Wang J, Alexanian A, Ying R, Kizhakekuttu TJ, Dharmashankar K, Vasquez-Vivar J, Gutterman DD, Widlansky ME (2011) Acute exposure to low glucose rapidly induces endothelial dysfunction and mitochondrial oxidative stress: role for AMP kinase. Arterioscler Thromb Vasc Biol 32(3):712–720
Wanstall JC, Jeffery TK, Gambino A, Lovren F, Triggle CR (2001) Vascular smooth muscle relaxation mediated by nitric oxide donors: a comparison with acetylcholine, nitric oxide and nitroxyl ion. Br J Pharmacol 134:463–472
Wei EP, Kontos HA, Beckman JS (1996) Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide, and peroxynitrite. Am J Physiol 271:H1262–H1266
Whiteman M, Kaur H, Halliwell B (1996) Protection against peroxynitrite dependent tyrosine nitration and alpha 1-antiproteinase inactivation by some anti-inflammatory drugs and by the antibiotic tetracycline. Ann Rheum Dis 55:383–387
Wiseman H, Halliwell B (1996) Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J 313(Pt 1):17–29
Wolin MS (2000) Interactions of oxidants with vascular signaling systems. Arterioscler Thromb Vasc Biol 20:1430–1442
Wu M, Pritchard KA Jr, Kaminski PM, Fayngersh RP, Hintze TH, Wolin MS (1994) Involvement of nitric oxide and nitrosothiols in relaxation of pulmonary arteries to peroxynitrite. Am J Physiol 266:H2108–H2113
Xu S, Ying J, Jiang B, Guo W, Adachi T, Sharov V, Lazar H, Menzoian J, Knyushko TV, Bigelow D, Schoneich C, Cohen RA (2006) Detection of sequence-specific tyrosine nitration of manganese SOD and SERCA in cardiovascular disease and aging. Am J Physiol Heart Circ Physiol 290:H2220–H2227
Yamakura F, Ikeda K (2006) Modification of tryptophan and tryptophan residues in proteins by reactive nitrogen species. Nitric Oxide 14:152–161
Yamakura F, Matsumoto T, Ikeda K, Taka H, Fujimura T, Murayama K, Watanabe E, Tamaki M, Imai T, Takamori K (2005) Nitrated and oxidized products of a single tryptophan residue in human Cu, Zn-superoxide dismutase treated with either peroxynitrite-carbon dioxide or myeloperoxidase-hydrogen peroxide-nitrite. J Biochem 138:57–69
Yasmin W, Strynadka KD, Schulz R (1997) Generation of peroxynitrite contributes to ischemia-reperfusion injury in isolated rat hearts. Cardiovasc Res 33:422–432
Ye Y, Quijano C, Robinson KM, Ricart KC, Strayer AL, Sahawneh MA, Shacka JJ, Kirk M, Barnes S, Accavitti-Loper MA, Radi R, Beckman JS, Estevez AG (2007) Prevention of peroxynitrite-induced apoptosis of motor neurons and PC12 cells by tyrosine-containing peptides. J Biol Chem 282:6324–6337
Yin K, Lai PS, Rodriguez A, Spur BW, Wong PY (1995) Antithrombotic effects of peroxynitrite: inhibition and reversal of aggregation in human platelets. Prostaglandins 50:169–178
Yuill KH, Yarova P, Kemp-Harper BK, Garland CJ, Dora KA (2011) A novel role for HNO in local and spreading vasodilatation in rat mesenteric resistance arteries. Antioxid Redox Signal 14:1625–1635
Zingarelli B, O’Connor M, Wong H, Salzman AL, Szabo C (1996) Peroxynitrite-mediated DNA strand breakage activates poly-adenosine diphosphate ribosyl synthetase and causes cellular energy depletion in macrophages stimulated with bacterial lipopolysaccharide. J Immunol 156:350–358
Zou MH, Shi C, Cohen RA (2002) High glucose via peroxynitrite causes tyrosine nitration and inactivation of prostacyclin synthase that is associated with thromboxane/prostaglandin H(2) receptor-mediated apoptosis and adhesion molecule expression in cultured human aortic endothelial cells. Diabetes 51:198–203
Zou MH, Cohen R, Ullrich V (2004) Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium 11:89–97
Zouki C, Zhang SL, Chan JS, Filep JG (2001) Peroxynitrite induces integrin-dependent adhesion of human neutrophils to endothelial cells via activation of the Raf-1/MEK/Erk pathway. FASEB J 15:25–27
Acknowledgments
The authors acknowledge the generous support of the Qatar Foundation in part via a project grant through the National Priorities Research Program (NPRP: 08-165-3-054; 4-910-3-244) as well as a BMRP establishment grant.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Arunachalam, G., Samuel, S.M., Ding, H., Triggle, C.R. (2014). Peroxynitrite Biology. In: Laher, I. (eds) Systems Biology of Free Radicals and Antioxidants. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-30018-9_5
Download citation
DOI: https://doi.org/10.1007/978-3-642-30018-9_5
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-30017-2
Online ISBN: 978-3-642-30018-9
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences