Abstract:
Lipids and long chain fatty acids (LCFA) are energy-rich compounds that support the growth of microorganisms that thrive in environments with a low redox potential. This chapter describes the properties of LCFA-degrading sulfate-reducing bacteria and LCFA-degrading methanogenic communities. The methanogenic conversion of LCFA requires syntrophic communities of acetogenic bacteria and methanogenic archaea. In this syntrophic cooperation, interspecies hydrogen transfer plays a key role. The understanding of the microbial interactions involved in LCFA degradation is essential to optimize the methane formation from LCFA-containing waste streams in bioreactors. Sulfate-reducing and methanogenic communities degrade LCFA by β-oxidation, but the differences and similarities between the degradation of saturated and unsaturated LCFA are not yet fully understood. Generally, bacteria that degrade unsaturated fatty acids degrade saturated fatty acids also, but the opposite does not always seem to be the case.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Long Chain Fatty Acid
- Methanogenic Community
- Acetogenic Bacterium
- Midpoint Redox Potential
- Unsaturated Long Chain Fatty Acid
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Lipids, natural oils, and fats are abundantly present in nature. Lipids are constituents of membranes of bacteria, archaea, and eukaryotes, while oils and fats are storage compounds for carbon and energy in all kinds of living organisms. Lipids are mostly esters of glycerol and long-chain fatty acids (LCFA) or, in the case of archaea, ethers of glycerol and long-chain alcohols. A substantial part of the dry weight of biomass is lipids, oils, and fats. These energy-rich compounds can be anaerobically degraded by methanogenic and sulfate-reducing communities. Methanogens and sulfate-reducing bacteria are not known to hydrolyze lipids. This suggests that other anaerobic microorganisms are responsible for the synthesis and excretion of lipases for the initial attack of lipids. A variety of sulfate-reducing bacteria is able to degrade LCFA coupled to the reduction of sulfate. However, methanogens do not degrade LCFA. In methanogenic environments, LCFA are degraded by proton-reducing acetogenic bacteria to acetate and hydrogen, which are substrates for the methanogens. For energetic reasons, the growth of proton-reducing acetogenic bacteria on LCFA is possible only if acetate and, in particular, hydrogen are efficiently removed by methanogens. This results in an obligately syntrophic growth driven by interspecies hydrogen transfer (Schink, 1997; Schink and Stams, 2006). In this chapter, we present information on the physiology of sulfate-reducing bacteria and syntrophic methanogenic communities that are able to grow on LCFA.
2 General Biochemical Pathway of LCFA Degradation
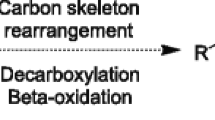
LCFA degradation pathways have not been studied extensively in methanogenic and sulfate-reducing communities at a biochemical or genetic level. Experiments with 14C-labeled LCFA indicated that degradation occurs by β-oxidation (Nuck and Federle, 1996; Weng and Jeris, 1976). Detailed studies on LCFA metabolism have been done in model organisms like Escherichia coli (DiRusso et al., 1999). LCFA biodegradation occurs through sequential steps: (1) LCFA adsorption to the cell surface, (2) LCFA uptake, and (3) LCFA conversion to lower molecular weight components via β-oxidation. A scheme of the β-oxidation cycle is shown in Fig. 1 . The end product in this cycle is acetyl-CoA.
E. coli possesses a metabolic pathway to degrade LCFA anaerobically in the presence of nitrate, fumarate, or trimethylamine-N-oxide that is distinct from the Fad enzymes used for aerobic LCFA metabolism (Campbell et al., 2003; Klein et al., 1971). FadIJ, like FadAB (Pramanik et al., 1979), appear to form a multienzyme complex that contains enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase, and 3-oxoacyl-CoA thiolase activities (Campbell et al., 2003; Snell et al., 2002). In contrast to the gene organization in E. coli, genomic analyses of Syntrophus aciditrophicus (McInerney et al., 2007) and Syntrophomonas wolfei (http://www.jgi.doe.gov) indicate that separate genes encode for enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase, and 3-oxoacyl-CoA thiolase activities. Both the S. aciditrophicus and S. wolfei genomes contain multiple homologs encoding not only for enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase, and 3-oxoacyl-CoA thiolase activities but also for acetyl-CoA synthetase (AMP-forming) and acyl-CoA dehydrogenase activities (Table 1 ). Presumably, the homologs differ in chain-length or substrate (saturated vs. unsaturated fatty acids) specificity.
In sulfate-reducing bacteria that degrade LCFA completely to CO2, acetyl-CoA is further degraded via the acetyl-CoA cleavage pathway or a modified citric acid cycle (Schauder et al., 1986). Acetyl-CoA can also be converted to acetate as is the case for LCFA-degrading bacteria in methanogenic environments and in several sulfate-reducing bacteria. Acetate is then a substrate for acetoclastic methanogens (Methanosarcina and Methanosaeta) or acetate-utilizing sulfate-reducing bacteria (Desulfobacter, Desulfobacterium, Desulforhabdus and Desulfobacca).
3 Hydrogenation of Unsaturated Fatty Acids
Some fermentative microorganisms are capable of hydrogenating unsaturated fatty acids, without being able to grow by these conversions (Mackie et al., 1991). Several anaerobic ruminal and human gut bacteria with biohydrogenation capabilities, belonging to the genera Butyrivibrio, Pseudobutyrivibrio, “Fusocillus,” Borrelia, Roseburia, and Clostridium, have been isolated and characterized (Devillard et al., 2007; Fukuda et al., 2005; Hunter et al., 1976; Kemp et al., 1984; Kopecny et al., 2003; Maia et al., 2007; Moon et al., 2008; Sachan & Davis, 1969; van de Vossenberg and Joblin, 2003; Wallace et al., 2006).
Early studies suggested that the degradation of unsaturated LCFA, such as linoleic (C18:2) and oleic (C18:1) acids, proceeded via β-oxidation only after chain saturation (Novak and Carlson, 1970; Weng and Jeris, 1976). Canovas-Dias et al. (1991) detected palmitoleate (C16:1) as a transient product during oleate (C18:1) degradation, suggesting the occurrence of one β-oxidation step without prior chain saturation. Thus far, experimental data are lacking to define the exact pathway involved in the degradation of unsaturated LCFA. Nevertheless, an analysis of the free energy variation of different possible reactions involved in LCFA degradation can provide some insight into which pathways are most likely as illustrated for oleate in (Table 2 ).
The hydrogenation step is thermodynamically favorable as indicated by the Gibbs free energy change of oleate (C18:1) conversion to stearate (C18:0). The direct β-oxidation of oleate (C18:1) to palmitoleate (C16:1) is thermodynamically unfavorable under standard conditions (ΔG0′> 0). Oleate (C18:1) degradation by a combined hydrogenation and β-oxidation to form palmitate (C16:0) seems most likely, as the endergonic oxidation reaction could be driven by the exergonic hydrogenation reaction. In studies performed with anaerobic sludges, palmitate was indeed a main intermediate product in oleate degradation (Lalman and Bagley, 2001; Pereira et al., 2002).
4 LCFA Degradation by Sulfate-Reducing Communities
Baars (1930) described Vibrio rübentschickii, a sulfate-reducing bacterium that is able to grow on LCFA and short-chain fatty acids. However, it never became really clear whether or not this culture was a pure culture of a sulfate-reducing bacterium (Postgate and Campbell, 1966). Later, the ability of pure cultures of sulfate-reducing bacteria to grow on LCFA and short-chain fatty acids was shown by Widdel (1980). Currently, representatives of several genera of sulfate-reducing microorganisms are known to be able to grow on LCFA (Fig. 2 ). Table 3 lists some properties of sulfate-reducing bacteria that can grow on LCFA. Some of the sulfate-reducing bacteria that have been tested grow also on acetate, indicating that these sulfate-reducing bacteria degrade LCFA completely to CO2. However, many LCFA-degrading sulfate-reducing bacteria produce acetate as a side product.
Phylogenetic tree of bacterial 16S rRNA gene sequences of the fatty-acid degrading sulfate-reducing and acetogenic bacteria described in the Tables 3 and 4 (fatty-acid degrading bacteria are shown in bold). The tree was based on 16S rRNA gene sequences and calculated using the ARB software package (Ludwig et al., 2004). Thermotoga lettingae (AF355615) was used as outgroup.
5 LCFA Degradation by Methanogenic Communities
Syntrophomonas sapovorans was the first-described LCFA-degrading bacterium that grows in syntrophy with methanogens (Roy et al., 1986). The first-defined butyrate-degrading culture, consisting of Syntrophomonas wolfei and Methanospirillum hungatei, was described earlier by McInerney et al. (1981). This bacterium is able to degrade fatty acids with a chain length of up to 8 carbon atoms. Syntrophomonas sapovorans grows on LCFA with more than 12 and up to 18 carbon atoms and is able to utilize unsaturated LCFA, such as oleate (C18:1) and linoleate (C18:2). To date, 14 syntrophic LCFA-degrading microorganisms have been obtained in pure culture or in coculture with hydrogen-consuming microorganisms (Table 4 ; Fig. 2 ). All these acetogenic bacteria are capable of anaerobically degrading fatty acids with more than 4 carbon atoms and up to 18 carbon atoms . They all belong to the families Syntrophomonadacea (McInerney, 1992; Zhao et al., 1993; Wu et al., 2006a) and Syntrophaceae (Jackson et al., 1999). LCFA higher than lauric acid (i.e., with more than 12 carbon atoms) are utilized only by Syntrophomonas sapovorans, S. saponavida, S. curvata, S. zehnderi, S. palmitatica, Thermosyntrophica lipolytica, and Syntrophus aciditrophicus. T. lipolytica also grows syntrophically with methanogens on lipids such as olive oil, utilizing only the liberated fatty acid moieties and releasing the glycerol. Two lipases are excreted by this bacterium (Salameh and Wiegel, 2007).
The LCFA degradation in methanogenic environments requires the syntrophic cooperation of LCFA-degrading bacteria and methanogens. Interspecies hydrogen transfer is a key process in methanogenesis. In 1967, Bryant and coworkers described for the first time an obligate syntrophic association between two microbial species (Bryant et al., 1967). They discovered that Methanobacillus omelianskii, originally believed to be a pure culture, (Stadtman and Barker, 1949; Barker, 1956) was not axenic. It was actually a coculture of a bacterium fermenting ethanol to acetate and H2 (the “S-organism”), and an archaeon (later named Methanobacterium bryantii; Balch et al., 1979) that uses H2 to reduce carbon dioxide to methane. The conversion of ethanol to acetate and H2 by the “S-organism” was thermodynamically possible only in the presence of M. bryantii, which keeps the H2 concentration low. The concept of interspecies hydrogen transfer originating from these observations was fundamental to understand how compounds such as propionate, butyrate, and LCFA are degraded in methanogenic environments. Table 5 summarizes the reactions involved in syntrophic LCFA degradation.
To sustain their metabolism, LCFA-degrading bacteria have to couple the oxidation of NADH and FADH2 to proton reduction, which is energetically difficult. The midpoint redox potential of the couple H+/H2 at pH 7 is −414 mV, whereas the midpoint redox potential of the couples of NAD+/NADH and FAD/FADH2 are −320 mV and −220 mV respectively (Thauer et al., 1977). The ΔG0′ values of the reactions NADH + H+ → NAD+ + H2 and FADH2 → FAD + H2 are about 18 and 38 kJ respectively. Methanogens are able to create a hydrogen partial pressure as low as 1 Pa. Under these conditions, the ΔG′ values of NADH and FADH2 oxidation are approximately −11 and + 9 kJ respectively. Thus, NADH oxidation, but not FADH2 oxidation, is feasible by the creation of a low hydrogen partial pressure by methanogens. It is not clear how LCFA-degrading bacteria solve the energetic problem of FADH2 oxidation coupled to hydrogen formation. Likely, a reversed electron transport mechanism is involved. The sequence analysis of the genes near fadK on the E. coli chromosome provides a clue about the nature of the reverse electron transport system involved in the syntrophic metabolism of LCFA. fadK mutants are impaired in anaerobic growth with fatty acids, and FadK is a putative acyl-CoA sythetase (Campbell et al., 2003). Genes ydiQRST are clustered with fadK on the E. coli chromosome, and ydiQRST have high sequence homology to fixABCX involved in the anaerobic carnitine metabolism in E. coli (Buchet et al., 1998; Walt and Kahn, 2002) and the reverse electron transport in N2-fixing bacteria (Earl et al., 1987; Edgren and Nordlund, 2004, 2006; Lindblad et al., 1996). The S. wolfei genome contains homologs to fixABCX, while S. aciditrophicus apparently uses another system for reverse electron transport (McInerney et al., 2007). Further research is needed to unravel the biochemistry of reverse electron transport.
6 Biogas Formation from LCFA Containing Waste Materials
LCFA are energy-rich compounds that are abundantly present in raw and waste materials (Table 6 ). Thus, biogas formation from LCFA-containing waste represents a sustainable technology. About 1 m3 methane can be produced from 1 kg of LCFA. Lipids and LCFA are present in domestic and industrial wastewaters. In domestic wastewater, generally, lipids represent 20–25% of the total organic matter with concentrations ranging from 40 to 100 mg/L (Quémeneur and Marty, 1994). Lipids/LCFA concentrations in industrial wastewaters are more variable and highly dependent of the industrial process. Concentrations of 11.2–22.4 g lipids/L were found in an industrial wastewater from a wool-scouring process (Becker et al., 1999). Also, a relatively high concentration of lipids, that is, 6.6 g/L, was measured in olive oil mill effluents (Beccari et al., 1998). Lower concentrations (0.4–1.7 g lipids/L) were detected in a sunflower oil mill wastewater with LCFA concentrations in the range 0.2–1.3 g/L (Saatci et al., 2003). Total lipids in dairy wastewaters range from 0.9 to 2.0 g/L (Kim et al., 2004).
Wastewaters and waste streams that contain high concentrations of lipids and LCFA may yield high levels of methane in an anaerobic digestion process. A problem associated with anaerobic treatment of lipids and LCFA is their poor solubility. LCFA were found to be inhibitory for methanogens (Lalman and Bagley, 2001, 2002; Kim et al., 2004; Pereira et al., 2003, 2004). The inhibitory effects are reversible and are often associated with the interactions of lipids and LCFA with the cell wall, preventing the conversion of other compounds due to their physical interaction (Pereira et al., 2005). However, by applying a sequence of loading and digestion phases in anaerobic reactors, high rates of methanogenesis and complete methanogenesis could be obtained (Pereira et al., 2002, 2003, 2004). Recently, it was demonstrated that continuous high rate anaerobic treatment of LCFA was possible by applying a relatively short start-up period with three feeding and three feed less phases. Methane recovery of up to 72% was obtained when a bioreactor was fed with an organic loading rate of 21 kgCOD m−3 day−1 and retention time of 9h (Cavaleiro et al., 2009). These findings offer excellent prospects for a sustainable energy production from lipids and LCFA.
7 Research Needs
Numerous strains of LCFA-degrading sulfate-reducing bacteria and LCFA-degrading acetogenic bacteria that grow in syntrophy with methanogens have been isolated and characterized. Although these bacteria have been relatively well studied from the physiological point of view, biochemical and genetic studies of the pathways involved in LCFA degradation are scarce. The genomes of Desulfatibacillum alkenivorans and the acetogenic bacterium Syntrophomonas zehnderi are presently being sequenced (US Department of Energy, Joint Genome Institute). Comparison of the genomes of these bacteria and the genomes of related bacteria that are not well able to grow on LCFA may provide insight into LCFA-degrading pathways and the regulation of these pathways. In addition, it may allow an elucidation of the differences and similarities between degradation of saturated and unsaturated LCFA. Studies with methanogenic communities have shown that bacteria that were enriched with unsaturated fatty acids are able to degrade a wide range of saturated fatty acids, but the opposite is not the case (Sousa et al., 2007a, b).
As lipids and LCFA are highly energetic compounds, further research is needed to get insight into how such compounds can be efficiently and completely converted to biogas. The challenge is to implement the syntrophic nature of the microorganisms involved in the most appropriate reactor configuration and the most optimal process operation.
References
Baars JK (1930) Over sulfaatreductie door bacteriën. The Netherlands: Delft, WD Meinema NV.
Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS (1979) Methanogens: reevaluation of a unique biological group. Microbiol Rev 43: 260–296.
Balk M, Altınbaş M, Rijpstra WIC, Damsté JS, Stams AJM (2008) Desulfatirhabdium butyrativorans gen. nov., sp. nov., a novel butyrate-oxidizing sulfate-reducing bacterium isolated from an anaerobic bioreactor. Int J Syst Evol Microbiol 58: 110–115.
Barker HA (1956) Bacterial Fermentation. New York: Wiley.
Beccari M, Majone M, Torrisi L (1998) Two-reactor system with partial phase separation for anaerobic treatment of olive oil mill effluents. Water Sci Technol 38: 53–60.
Becker P, Koster D, Popov MN, Markossian S, Antranikian G, Markl H (1999) The biodegradation of olive oil and the treatment of lipid-rich wool scouring wastewater under aerobic thermophilic conditions. Water Res 33: 653–660.
Beeder J, Torsvik T, Lien T (1995) Thermodesulforhabdus norvegicus gen. nov., sp. nov., a novel thermophilic sulfate-reducing bacterium from oil field water. Arch Microbiol 64: 331–336.
Brandt KK, Patel BKC, Ingvorsen K (1999) Desulfocella halophila gen. nov., sp. nov., a halophilic, fatty-acid-oxidizing, sulfate reducing bacterium isolated from sediments of the Great Salt Lake. Int J Syst Bacteriol 49: 193–200.
Bryant MP, Wolin EA, Wolin MJ, Wolfe RS (1967) Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch Microbiol 59: 20–31.
Brysch K, Schneider C, Fuchs G, Widdel F (1987) Lithoautotrophic growth of sulfate-reducing bacteria, and description of Desulfobacterium autotrophicum gen. nov., sp. nov. Arch Microbiol 148: 264–274.
Buchet AK, Eichler K, Mandrand-Berthelot MA (1998) Regulation of the carnitine pathway in Escherichia coli: investigation of the cai-fix divergent promoter region. J Bacteriol 180: 2599–2608.
Campbell JW, Morgan-Kiss RM, and Cronan JE (2003) A new Escherichia coli metabolic competency: growth on fatty acids by a novel anaerobic β-oxidation pathway. Mol Microbiol 47: 793–805.
Canovas-Diaz M, Sanchez-Roig MJ, Iborra JL (1991) Myristic and oleic acid degradation by an acclimated anaerobic consortia: synergistic behavior. In Biomass for Energy, Industry and Environment 6th EC Conference. G Grassi, A Collina, and H Zibetta (eds.). London, UK: Elseviers Applied Science, pp. 580–584.
Cavaleiro AJ, Salvador AF, Alves JI, Alves MM (2009) Continuous high rate anaerobic treatment of oleic acid based wastewater is possible after a step feeding start-up. Environ Sci Technol 43: 2931–2936.
Cord-Ruwisch R, Garcia JL (1985) Isolation and characterization of an anaerobic benzoate-degrading spore-forming sulfate-reducing bacterium, Desulfotomaculum sapomandens sp. nov. FEMS Microb Lett 29: 325–330.
Cravo-Laureau C, Matheron R, Cayol JL, Joulian C, Hirschler-Réa A (2004a) Desulfatibacillum aliphaticivorans gen. nov., sp. nov., an n-alkane- and n-alkene-degrading, sulfate-reducing bacterium. Int J Syst Evol Microbiol 54: 77–83.
Cravo-Laureau C, Matheron R, Joulian C, Cayol J, Hirschler-Réa A (2004b) Desulfatibacillum alkenivorans sp. nov., a novel n-alkene-degrading, sulfate-reducing bacterium, and emended description of the genus Desulfatibacillum. Int J Syst Evol Microbiol 54: 1639–1642.
Cravo-Laureau C, Labat C, Joulian C, Matheron R, Hirschler-Réa A (2007) Desulfatiferula olefinivorans gen. nov., sp. nov., a long-chain n-alkene-degrading, sulfate-reducing bacterium. Int J Syst Evol Microbiol 57: 2699–2702.
Daumas S, Cord-Ruwisch R, Garcia JL (1988) Desulfotomaculum geothermicum sp. nov., a thermophilic, fatty acid-degrading, sulfate-reducing bacterium isolated with H2 from geothermal ground water. Antonie van Leeuwenhoek 54: 165–178.
Devillard E, McIntosh FM, Duncan SH, Wallace RJ (2007) Metabolism of linoleic acid by human gut bacteria: Different routes for biosynthesis of conjugated linoleic acid. J Bacteriol 189: 2566–2570.
DiRusso CC, Black PN, Weimar JD (1999) Molecular inroads into the regulation and metabolism of fatty acids, lessons from bacteria. Prog Lipid Res 38: 129–197.
Earl CD, Ronson CW, Ausubel FM (1987) Genetic and structural analysis of the Rhizobium meliloti fixA, fixB, fixC, and fixX genes. J Bacteriol 169: 1127–1136.
Edgren T, Nordlund S (2004) The fixABCX genes in Rhodospirillum rubrum encode a putative membrane complex participating in electron transfer to nitrogenase. J Bacteriol 186: 2052–2060.
Edgren T, Nordlund S (2006) Two pathways of electron transport to nitrogenase in Rhodospirillum rubrum: the major pathway is dependent on the fix gene products. FEMS Microbiol Lett 260: 30–35.
Finster K, Liesack W, Tindall BJ (1997) Desulfospira joergensenii, gen. nov., sp. nov., a new sulfate-reducing bacterium isolated from marine surface sediment. Syst Appl Microbiol 20: 201–208.
Fukuda S, Furuya H, Suzuki Y, Asanuma N & Hino T (2005) A new strain of Butyrivibrio fibrisolvens that has high ability to isomerize linoleic acid to conjugated linoleic acid. J Gen Appl Microbiol 51: 105–113.
Galushko AS, Rozanova EP (1991) Desulfobacterium cetonicum sp. nov.: a sulfate-reducing bacterium which oxidizes fatty acids and ketones. Mikrobiologiya 60: 742–746.
Hanaki K, Nagase M, Matsuo T (1981) Mechanism of inhibition caused by long-chain fatty acids in anaerobic digestion process. Biotechnol Bioeng 23: 1591–1610.
Hatamoto M, Imachi H, Fukayo S, Ohashi A, Harada H (2007) Syntrophomonas palmitatica sp. nov., an anaerobic, syntrophic, long-chain fatty-acid-oxidizing bacterium isolated from methanogenic sludge. Int J Syst Evol Microbiol 57: 2137–2142.
Hunter WJ, Baker FC, Rosenfeld IS, Keyser JB, Tove SB (1976) Biohydrogenation of unsaturated fatty-acids: hydrogenation by cell-free preparations of Butyrivibrio fibrisolvens. J Biol Chem 251: 2241–2247.
Hwu C-S (1997) Enhancing anaerobic treatment of wastewater containing oleic acid. Ph.D. thesis, Wageningen Agriculture University, The Netherlands.
Jackson BE, Bhupathiraju VK, Tanner RS, Woese CR, McInerney MJ (1999) Syntrophus aciditrophicus sp. nov., a new anaerobic bacterium that degrades fatty acids and benzoate in syntrophic association with hydrogen-using microorganisms. Arch Microbiol 171: 107–114.
Jakobsen TF, Kjeldsen KU, Ingvorsen K (2006) Desulfohalobium utahense sp. nov., a moderately halophilic, sulfate-reducing bacterium isolated from Great Salt Lake. Int J Syst Evol Microbiol 56: 2063–2069.
Kemp P, Lander DJ, Gunstone FD (1984) The hydrogenation of some cis-octadecenoic and trans-octadecenoic acids to stearic-acid by a rumen Fusocillus sp. Br J Nutr 52: 165–170.
Kim SH, Han SK, Shin HS (2004) Kinetics of LCFA Inhibition on acetoclastic methanogenesis, propionate degradation and beta-oxidation. J Env Sci Health, part A 39: 1025–1038.
Klein K, Steinberg R, Fiethen B, Overath P (1971) Fatty acid degradation in Escherichia coli. An inducible system for the uptake of fatty acids and further characterization of old mutants. Eur J Biochem 19: 442–450.
Kopecny J, Zorec M, Mrazek J, Kobayashi Y & Marinsek-Logar R (2003) Butyrivibrio hungatei sp. nov. and Pseudobutyrivibrio xylanivorans sp. nov., butyrate-producing bacteria from the rumen. Int J Syst Evol Microbiol 53: 201–209.
Kuever J, Konneke M, Galushko A, Drzyzga O (2001) Reclassification of Desulfobacterium phenolicum as Desulfobacula phenolica comb. nov. and description of strain Sax(T) as Desulfotignum balticum gen. nov., sp. nov. Int J Syst Evol Microbiol 51: 171–177.
Kuever J, Rainey F, Widdel F (2005) Bergey’s Manual of Systematic Bacteriology, 2nd edn. Part: Class IV, Subpart: Order IV. US: Springer, pp. 1004–1005.
Lalman JA, Bagley DM (2000) Anaerobic degradation and inhibitory effects of linoleic acid. Water Res 34: 4220–4228.
Lalman JA, Bagley DM (2001) Anaerobic degradation and methanogenic inhibitory effects of oleic and stearic acids. Water Res 35: 2975–2983.
Lindblad A, Jansson J, Brostedt E, Johansson M, Hellman U, Nordlund S (1996) Identification and sequence of a nifJ-like gene in Rhodospirillum rubrum: partial haracterization of a mutant unaffected in nitrogen fixation. Mol Microbiol 20: 559–568.
Lorowitz WH, Zhao HX, Bryant MP (1989) Syntrophomonas wolfei subsp saponavida subsp. nov., a long chain fatty-acid degrading, anaerobic, syntrophic bacterium - Syntrophomonas wolfei subsp wolfei subsp. nov. - and emended descriptions of the genus and species. Int J Syst Bacteriol 39: 122–126.
Ludwig W, Strunk O, Westram R et al. (2004) ARB: a software environment for sequence data. Nucleic Acids Res 32: 1363–1371.
Mackie RI, White BA, Bryant MP (1991) Lipid metabolism in anaerobic ecosystems. Crit Rev Microbiol 17: 449–479.
Maia MRG, Chaudhary LC, Figueres L & Wallace RJ (2007) Metabolism of polyunsaturated fatty acids and their toxicity to the microflora of the rumen. Antonie Van Leeuwenhoek 91: 303–314.
Mavrovouniotis ML (1991) Estimation of standard Gibbs energy changes of biotransformations. J Biol Chem 266: 14440–14445.
McInerney MJ (1992) The genus Syntrophomonas, and other syntrophic bacteria. In The Prokaryotes, 2nd edn. A Balows, et al. (eds.). New York: Springer, pp. 2048–2057.
McInerney MJ, Bryant MP, Hespell RB, Costerton JW (1981) Syntrophomonas wolfei gen. nov. sp. nov., an anaerobic, syntrophic, fatty-acid oxidizing bacterium. Appl Environ Microbiol 41: 1029–1039.
McInerney MJ, Rohlin L, Mouttaki H, Kim UM, Krupp RS, Rios-Hernandez L, Sieber J, Struchtemeyer CG, Bhattacharyya A, Campbell JW (2007) The genome of Syntrophus aciditrophicus: Life at the thermodynamic limit of microbial growth. Proc Natl Acad Sci USA 104: 7600–7605.
Moon CD, Pacheco DM, Kelly WJ, Leahy SC, Li D, Kopecny J & Attwood GT (2008) Reclassification of Clostridium proteoclasticum as Butyrivibrio proteoclasticus comb. nov., a butyrate-producing ruminal bacterium. Int J Syst Evol Microbiol 58: 2041–2045.
Novak JT, Carlson DA (1970) Kinetics of anaerobic long chain fatty acid degradation. J Water Pollut Control Fed 42: 1932–1943.
Nuck BA, Federle TW (1996) Batch test for assessing the mineralization of C-14-radiolabeled compounds under realistic anaerobic conditions. Environ Sci Technol 30: 3597–3603.
Pereira MA, Cavaleiro AJ, Mota M, Alves MM (2003) Accumulation of long chain fatty acids onto anaerobic sludge under steady state and shock loading conditions: effect on acetogenic and methanogenic activity. Water Sci Technol 48: 33–40.
Pereira MA, Pires OC, Mota M, Alves MM (2002) Anaerobic degradation of oleic acid by suspended and granular sludge: identification of palmitic acid as a key intermediate. Water Sci Technol 45: 139–144.
Pereira MA, Pires OC, Mota M, Alves MM (2005) Anaerobic biodegradation of oleic and palmitic acids: Evidence of mass transfer limitations caused by long chain fatty acid accumulation onto the anaerobic sludge. Biotechnol Bioeng 92: 15–23.
Pereira MA, Sousa DZ, Mota M, Alves MM (2004) Mineralization of LCFA associated with anaerobic sludge: Kinetics, enhancement of methanogenic activity, and effect of VFA. Biotechnol Bioeng 88: 502–511.
Postgate JR, Campbell LL (1966) Classification of Desulfovibrio species, the nonsporulating sulfate-reducing bacteria. Bacteriol Rev 30: 732–738.
Pramanik A, Pawar S, Antonian E, Schulz H (1979) Five different enzymatic activities are associated with the multienzyme complex of fatty acid oxidation in Escherichia coli. J Bacteriol 137: 369–473.
Quémeneur M, Marty Y (1994) Fatty-acids and sterols in domestic wastewaters. Water Res 28: 1217–1226.
Rees GN, Patel BKC (2001) Desulforegula conservatrix gen. nov., sp. nov., a long-chain fatty acid-oxidizing, sulfate reducing bacterium isolated from sediments of a freshwater lake. Int J Syst Evol Microbiol 51: 1911–1916.
Roy F, Samain E, Dubourguier HC, Albagnac G (1986) Synthrophomonas sapovorans sp. nov., a new obligately proton reducing anaerobe oxidizing saturated and unsaturated long-chain fatty acids. Arch Microbiol 145: 142–147.
Rueter P, Rabus R, Wilkest H, Aeckersberg F, Rainey FA, Jannasch HW, Widdel F (1994) Anaerobic oxidation of hydrocarbons in crude oil by new types of sulphate-reducing bacteria. Nature 372: 455–458.
Saatci Y, Arslan EI, Konar V (2003) Removal of total lipids and fatty acids from sunflower oil factory effluent by UASB reactor. Biores Technol 87: 269–272.
Sachan DS & Davis CL (1969) Hydrogenation of linoleic acid by a rumen spirochete. J Bacteriol 98: 300–301.
Salameh MA, Wiegel J (2007) Purification and characterization of two highly thermophilic alkaline lipases from Thermosyntropha lipolytica. Appl Environ Microbiol 73: 7725–7731.
Schauder R, Eikmanns B, Thauer TK, Widdel F, Fuchs G (1986) Acetate oxidation to CO2 in anaerobic bacteria via a novel pathway not involving reactions of the citric acid cycle. Arch Microbiol 145: 162–172.
Schink B (1997) Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev 61: 262–280.
Schink B, Stams AJM (2006) Syntrophism among prokaryotes. In The Prokaryotes, vol. 2. M Dworkin, K-H Schleifer, and E Stackebrandt (eds.). New York: Springer, pp. 309–335.
Sekiguchi Y, Kamagata Y, Nakamura K, Ohashi A, Harada H (2000) Syntrophothermus lipocalidus gen. nov., sp. nov., a novel thermophilic, syntrophic, fatty-acid-oxidizing anaerobe which utilizes isobutyrate. Int J Syst Evol Microbiol 50: 771–779.
Snell KD, Feng F, Zhong L, Martin D, Madison LL (2002) YfcX enables medium-chain-length poly(3-hydroxyalkanoate) formation from fatty acids in recombinant Escherichia coli fadB strains. J Bacteriol 184: 5696–5705.
Sousa DZ, Pereira MA, Smidt H, Stams AJM, Alves MM (2007a) Molecular assessment of complex microbial communities degrading long chain fatty acids (LCFA) in methanogenic bioreactors. FEMS Microbiol Ecol 60: 252–265.
Sousa DZ, Pereira MA, Stams AJM, Alves MM, Smidt H (2007b) Microbial communities involved in anaerobic degradation of unsaturated or saturated long chain fatty acids (LCFA). Appl Environ Microbiol 73: 1054–1064.
Sousa DZ, Smidt H, Alves MM, Stams AJM (2007c) Syntrophomonas zehnderi sp. nov., an anaerobe that degrades long chain fatty acids in co-culture with Methanobacterium formicicum. Int J Syst Evol Microbiol 57: 609–615.
Stadtman TC, Barker HA (1949) Studies on the methane fermentation. VII. Tracer experiments on the mechanism of methane formation. Arch Biochem 21: 256–264.
Stieb M, Schink B (1985) Anaerobic oxidation of fatty-acids by Clostridium bryantii sp. nov., a sporeforming, obligately syntrophic bacterium. Arch Microbiol 140: 387–390.
Svetlitshnyi V, Rainey F, Wiegel J (1996) Thermosyntropha lipolytica gen. nov., sp. nov., a lipolytic, anaerobic, alkalitolerant, thermophilic bacterium utilizing short- and long-chain fatty acids in syntrophic coculture with a methanogenic archaeum. Int J Syst Bacteriol 46: 1131–1137.
Taylor RJ (1965) The Chemistry of Glycerides. England: Unilever Ltd.
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotropic anaerobic bacteria. Bacteriol Rev 41: 100–180.
van de Vossenberg JLCM, Joblin KN (2003) Biohydrogenation of C18 unsaturated fatty acids to stearic acid by a strain of Butyrivibrio hungatei from the bovine rumen. Lett Appl Microbiol 37: 424–428.
Wallace JR, Chaudhary LC, McKain N, McEwan NR, Richardson AJ, Vercoe PE, Walker ND, Paillard D (2006) Clostridium proteoclasticum: A ruminal bacterium that forms stearic acid from linoleic acid. FEMS Microbiol Lett 265: 195–201.
Walt A, Kahn MJ (2002) The fixA and fixB genes are necessary for anaerobic carnitine reduction in Escherichia coli. J Bacteriol 184: 4044–4047.
Weng C, Jeris JS (1976) Biochemical mechanisms in methane fermentation of glutamic and oleic acids. Water Res 10: 9–18.
Widdel F (1980) Anaerober Abbau von Fettsäuren und Benzoesäure durch neu Isolierte Arten Sulfat-reduzierender Bakterien. Ph.D. thesis, Göttingen University, Göttingen.
Widdel F, Kohring GW, Mayer F (1983) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids III. Characterization of the filamentous gliding Desulfonema limicola gen. nov. sp. nov., and Desulfonema magnum sp. nov. Arch Microbiol 134: 286–294.
Wu C, Liu X, Dong X (2006a) Syntrophomonas cellicola sp. nov., a novel spore-forming syntrophic bacterium isolated from a distilled-spirit-fermenting cellar and assignment of Syntrophospora bryantii to Syntrophomonas bryantii sp. nov., comb. nov. Int J Syst Evol Microbiol 56: 2331–2335.
Wu C, Liu X, Dong X (2006b) Syntrophomonas erecta subsp. sporosyntropha subsp. nov., a spore-forming bacterium that degrades short chain fatty acids in co-culture with methanogens. Syst Appl Microbiol 29: 457–462.
Wu CG, Dong XZ, Liu XL (2007) Syntrophomonas wolfei subsp. methylbutyratica subsp. nov., and assignment of Syntrophomonas wolfei subsp. saponavida to Syntrophomonas saponavida sp. nov comb. nov. Syst Appl Microbiol 30: 376–380.
Zhang CY, Liu XL, Dong XZ (2004) Syntrophomonas curvata sp. nov., an anaerobe that degrades fatty acids in co-culture with methanogens. Int J Syst Evol Microbiol 54: 969–973.
Zhang CY, Liu XL, Dong XZ (2005) Syntrophomonas erecta sp. nov., a novel anaerobe that syntrophically degrades short-chain fatty acids. Int J Syst Evol Microbiol 55: 799–803.
Zhao H, Yang D, Woese CR, Bryant MP (1993) Assignment of fatty acid-β-oxidizing syntrophic bacteria to Syntrophomonadaceae fam. nov. on the basis of 16S rRNA sequence analysis. Int J Syst Bacteriol 43: 278–286.
Acknowledgments
Our research on anaerobic LCFA degradation was made possible by the grants of the Fundação para a Ciência e Tecnologia (FCT) and the Fundo Social Europeu (FSE) (SFRH/BD/8726/2002), the Wageningen Institute for Environmental and Climate Research (WIMEK), the Darwin Center for Biogeology of Netherlands Organization for Scientific Research (NWO), the NWO divisions Earth and Life Sciences (ALW) and Chemical Sciences (CW), and the Technology Foundation STW.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Sousa, D.Z. et al. (2010). Degradation of Long-Chain Fatty Acids by Sulfate-Reducing and Methanogenic Communities. In: Timmis, K.N. (eds) Handbook of Hydrocarbon and Lipid Microbiology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-77587-4_69
Download citation
DOI: https://doi.org/10.1007/978-3-540-77587-4_69
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-77584-3
Online ISBN: 978-3-540-77587-4
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences