Abstract
Over the last few decades, the worldwide growth in industrial activity has resulted in the discharge of significant pollutant quantities in the aquatic environment. These contaminants are generally characterized by their toxicity for living organisms and the environment. In response to these dangers, environmental regulations are imposing limitations on a wide variety of pollutants within industrial wastewaters. The great diversity of raw materials and production operations employed by industries poses a matter of concern for scientific community attempting to define proficient control technologies. Biological treatment technologies were viewed as attractive alternatives to conventional methods. Indeed, biofilm-mediated processes for industrial wastewater treatment are among the most proficient technologies due to their distinct advantages over conventional methods. Biofilm mode proved its capacity to enhance the overall pollutant degradation efficiency. Expanding the knowledge regarding biofilm wastewater treatment would contribute to its full-scale use. This chapter presents an overview of the beneficial use of biofilm mode in depollution technologies and a critical discussion of a relevant number of recent investigations that have dealt with biofilm-based processes. It also provides a review of the various types of bioreactors and biofilm supports currently used.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
Introduction
The rescue of industrial wastewaters has become a significant problem throughout the world (Segura et al. 2015). These wastewaters generally contain high quantities of hazardous pollutants such as persistent organic pollutants, heavy metals, phenols, dyes, pesticides, humic substances, and detergents (Ferronato et al. 2016). These contaminants are mostly characterized by their toxicity for living organisms, their persistence against chemical or biological decomposition, their high environmental mobility, and their extreme bioaccumulation tendency in the food chain (Bahafid et al. 2013; Liu et al. 2013b).
In response to these dangers, environmental regulations are imposing limitations in a wide variety of pollutants within industrial wastewaters (Day 1993). In order to remove or transform undesirable elements from industrial effluents, various physicochemical treatment processes such as electrochemical, coagulation–flocculation, adsorption, and membrane treatment are currently employed depending on the wastewater characteristics (Shammas 2005; Leyva-Ramos et al. 2008).
Biological treatment technologies were viewed as an appealing approach compared to these conventional methods. Among these, biosorption defined as the capacity of biological materials to accumulate and/or transform pollutants from contaminated sites had gained especial attention (Fomina and Gadd 2014). This technology can use different materials such as plant biomass or animal polymers. Nevertheless, biodepollution plants mainly employ microorganisms.
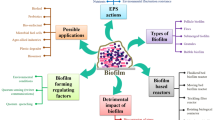
Microorganisms can be employed in different forms within wastewater treatment systems, as living or dead (Bahafid et al. 2013), suspended or biofilm-immobilized biomass (Pan et al. 2014), etc. Biofilm is a growth mode distinct from planktonic form of microorganisms. It can be defined as a complex structure of cells and extracellular products that can be formed spontaneously as dense granules (Lettinga et al. 1980). It can also be fixed on a solid surface or suspended carrier supported on particles (Davey and O’toole 2000). This microbial mode of development is of great interest in biotechnology applications. Bioreactors based on fixed microorganisms in biofilm growth mode are increasingly employed for bioremediation applications (Lewandowski and Boltz 2011). They represent a recent proficient alternative for assessing environmental effects. This is by means of their significant capacity to resist to stressful conditions and their various catabolic pathways allowing the degradation of various hazardous contaminants. The extracellular products of biofilms are of great importance. They form a barrier for microorganisms against the toxicity of wastewaters and thus enhance the pollutant immobilization (Quintelas et al. 2011).
This review summarizes existing knowledge on various aspects of the implication of biofilm in wastewater treatment. It (i) provides essential insight into the benefits of biofilms in wastewater treatment technologies, (ii) presents an overview on the supports currently used in these biofilm-based technologies, and (iii) gives a rundown of the commonly employed biofilm-mediated processes. This chapter provides a source of useful information for future areas of research and efforts, aiming to fully implement these methods for wastewater treatment.
Wastewater Characteristics
Wastewater treatment process should be decided based on a balance between technical and economical aspects and customized according to the wastewater characteristics.
Industrial wastewaters are commonly characterized by the basic parameters which are represented by chemical oxygen demand (COD), biochemical oxygen demand (BOD), suspended solids (SS), ammonium nitrogen, heavy metal concentration, pH, turbidity, color, and biological parameters. These characteristics vary in a wide range depending on the industrial activity. Industrial processes constituting the major sources of polluted effluents include petroleum, textile, tannery, food processing, pharmaceutical, and manufacturing industries. Typical characteristics of industrial wastewaters are summarized in many previous reviews (Lin et al. 2012). It offers a useful guideline for the decision of the treatment process despite the variation of wastewater characteristics.
The wastewater from industries varies so greatly in both flow and pollution strength. Thus, it is impossible to give fixed values to their composition. Conventionally, industrial wastewaters may contain suspended, colloidal, and dissolved (mineral and organic) solids. In addition, it may contain colored matter at varied concentrations. It may also contain inert, organic, or toxic materials and possibly pathogenic microorganisms. Compared with municipal wastewaters, industrial effluents generally present a higher organic strength (>1000 mg COD/L) and an extreme physicochemical nature with extreme acid or alkaline pH values, high salinity (e.g., from petroleum refining, textile processing, leather processing), and a variable temperature. It may also contain high concentrations of toxic substances (natural or synthetic) which may present an obstacle to biological treatment plants of the effluents (Lin et al. 2012). The pH variation and salinity were always the serious challenges of biological treatment. These conditions lead to the inhibition of both microbial cell activity and the flocculation of sludge flocs (Lefebvre and Moletta 2006). In the case of industrial wastewater with high concentration of toxic compounds (heavy metals, phenols, surfactants, pesticides, etc.) mainly characterized by marked persistence against chemical or biological degradation, a pre- or posttreatment should be carried using performing microorganisms able to remove the pollutant of concern or in some cases the biological treatment should be combined to other physic-chemical treatment strategy for an optimal treatment efficiency (Lin et al. 2012).
With an appropriate analysis and control of the effluent, generally wastewaters with a BOD/COD ration of 0.5 or greater may be treated with a biological treatment system (Metcalf and Eddy 2003). Compared to other wastewater treatment strategies, biological treatment strategies present the advantages of being eco-friendly and economic. It can be assessed by either aerobic or anaerobic processes. Aerobic processes refer to the use of dissolved oxygen by microbial cells in the conversion of the organic matter to biomass and CO2, while anaerobic process involves the degradation of organic wastes into methane, CO2, and H2O in the absence of oxygen. Each of the treatments presents various merits. In fact, both systems are able to remove effectively organic pollution. However, generally, aerobic systems are preferably dedicated to the treatment of wastewaters containing biodegradable COD concentrations less than 1000 mg/L. A crossover point ranging from 300 to 700 mg/L influent wastewater ultimate BOD (BODu) was determined by Cakir and Stenstrom (2005) as a crucial interval for an effective treatment of wastewaters using aerobic systems, while anaerobic systems become favorable for the treatment of higher strength wastewater (COD concentration over 4000 mg/L) (Chan et al. 2009).
Biofilm Fundamentals
Since the first paper, where the term “biofilm” was coined and described by Costerton et al. (1978), great efforts have been made to deeply and properly understand this complex structure. Thus, many definitions were attributed to the term biofilm in the literature. It is commonly defined as being a complex coherent structure of cells (aggregates) and cellular products (Nicolella 2000). Most definitions include the attachment of microorganisms to a solid surface or carriers (Quintelas et al. 2008; Yang et al. 2015); though few definitions do not consider the surface as an essential element in the biofilm mode, it can be formed spontaneously as granules (Lettinga et al. 1980). Biofilms are heterogeneous and enormously complex communities of microbial cells suspended in a matrix of extracellular polymeric substances (EPS) (Quintelas et al. 2011). They are highly affected by the surrounding environmental and mechanical conditions. According to these latter, the biofilm may use its ability to regulate many activities such as attachment, mobility, and detachment through the cell-to-cell molecular signaling, called “quorum sensing” (Singh et al. 2006).
Biofilm is a predominant microbial growth mode in the environment distinct from planktonic growth mechanism. It is commonly found in soil and aquatic systems. It has been proved to be an advantageous form offering protection against different dangers including predation, and chemical and biological toxicants. It also offers resistance to stressing conditions such as dehydration and lack of nutrients (Singh et al. 2006). It is hence increasingly employed in wastewater treatment systems (Butler and Boltz 2014). Nevertheless, these microbial aggregates are with relevance to serious problems in diverse fields, essentially in terms of material corrosion and degradation (Hamadi et al. 2005).
Potentiality of Biofilm in Wastewater Treatment
The use of biofilm-based technology in wastewater treatment dates from 1893 with the first utilization of trickling filters in England (Lohmeyer 1957). Although microbial biofilms cause detrimental effects in various environments, they are still considered as useful in biodegradation of complex pollutants. Biofilm-mediated depollution presents an efficient and cost-effective option rather than the use of planktonic microbial cells. This is due to the better survival and adaptation ability to stressing conditions. Indeed, biofilm-forming microorganisms are good competitors with nutrients and oxygen. They are also known to survive and manage the most stressful conditions and harsh hydrodynamics forces. It makes them excellent candidates for bioremediation applications. In fact, depending on the environmental conditions, the biofilm via the EPS matrix can develop different structures to overcome environmental challenges (Kreft and Wimpenny 2001; Miqueleto et al. 2010; Jung et al. 2013). Thus, it forms mushroomlike shapes in the case of static water and appears in filamentous structure in fast-moving water flux (Edwards et al. 2000; Reysenbach and Cady 2001).
In fact, in recent years, biofilm-based processes are being increasingly employed as an appealing strategy representing an environment-friendly and cost-effective option for pollutant removal (Das et al. 2012). The degradation of xenobiotics is more effective within biofilm systems, owing to the close, mutually beneficial physical and physiological interactions among biofilm-forming cells. The use of the biofilm-mediated bioremediation plants became hence common for the removal of pollutants (Das et al. 2012). The beneficial use of biofilm processes for the adsorption, immobilization, and degradation of various pollutants was pointed out (Quintelas et al. 2010). In fact, these technologies have been widely used for the removal of both organic and inorganic compounds from aqueous media (Chen et al. 2008; Quintelas et al. 2013; Hai et al. 2015). Many contaminants including heavy metals, petroleum, dyes, and pesticides have been successfully remediated using microbial biofilms (Mitra and Mukhopadhyay 2016).
In order to resist predatory protozoa, in biofilm mode, microbial cells are immobilized in a self-synthesized matrix which offers protection from stress, contaminants, and predatory protozoa (Quintelas et al. 2011). They also tend to form large inedible microcolonies that offers a better resistance to protozoa under harsh environment (Matz and Kjelleberg 2005; Mitra and Mukhopadhyay 2016).
Furthermore, the gene expression within biofilm mode is distinct from planktonic form of microbial cells. The heterogeneous assemblage of microorganisms in the biofilm in terms of microbial species offers a diversity of metabolic pathways. It enables the biofilm to degrade several types of pollutants either individually or correctively (Gieg et al. 2013; Horemans et al. 2013).
Various microbial species were successfully employed as biofilm form for the wastewater treatment, including bacteria (Abzazou et al. 2016), yeast (Cong et al. 2014), fungi (Badia-Fabregat et al. 2017), and algae (Hoh et al. 2016). The introduction of algae into the biofilm reactors is considered by some researchers as a nuisance due to the clogging issues, while it is viewed by others as a source of great amounts of oxygen beneficial to the growth of the other species constituting the biofilm (Kesaano and Sims 2014). Biofilm processes favor selective development of slow-growing microorganisms such as autotrophs (i.e., nitrogen-oxidizing bacteria) and phosphorus-accumulating microorganisms by the maintenance of high biomass age, which reduces their washout from the system (Lee et al. 2006).
Since the researchers became aware of the importance of biofilm in treatment systems, the growth conditions were thoroughly studied for their great influence on the system efficiency. This included temperature, nutrients, substrata, extracellular polymeric substances (EPS), species interactions, and light for algal based systems (Kesaano and Sims 2014). These studies are of extreme importance for the design and scale-up of effective treatment systems.
To successfully achieve the treatment of wastewater using biofilm-based systems, optimal conditions must be provided to the microbial consortia presenting the ability to remove or transform the pollutant of concern.
Biofilm Application in Wastewater Treatment
Supports in Biofilm-Based Processes
In biofilm reactors, microorganisms may be supported on various materials. The depollution efficiency is strongly related to the properties and the nature of these supports and the ability of the biofilm to be attached to the chosen support (Asri et al. 2017). Indeed, the commonly used supports are of various natures including ceramic, clays including zeolithes, seashell and charcoal, plastic materials, sintered glass, fire bricks, sand, natural stones like limestone and gravel, pumice, and rocky aggregates (Silva et al. 2008; Tarjányi-Szikora et al. 2013). These supports were used directly without any modification or after treatment to modify certain properties (porosity, surface charges, etc.) in order to enhance their effectiveness, while others can be commercially synthesized. For microbial fuel cells (MFCs), the used material should present not only an adhesive property but also an electrical conductivity and a chemical stability. The commonly used supports in this technology are carbon-based materials such as carbon cloth, carbon paper, or carbon felt (Liu et al. 2013a; Zhang et al. 2013; Alatraktchi et al. 2014).
The effective depollution of the biofilm systems was related to support properties mainly the surface area, surface morphology, and capacity of microbial adhesion. Numerous works mainly focused on microbial adhesion studies aiming the determination of the influence of surface characteristics on biofilm formation. Indeed, this step is viewed as a key element promoting the cell attachment and the long-term stability of the biofilm (Zainul Akmar et al. 2007). Many surface properties were proved to influence microbial initial attachment to various substrata. For instance, roughness is among the most reported parameters (El Abed et al. 2012). In fact, rough or porous surfaces were more favorable for cell adhesion than regular surfaces, which was ascribed to the increased surface area and the protection against hydraulic shear forces (Hoh et al. 2016).
Surface energy components were also showed to strongly influence microbial initial attachment and treatment performance of the biofilm-based system (Asri et al. 2017). Among these components, hydrophobicity character was reported to be of extreme importance in wastewater applications. Indeed, a greater adhesion of different microbial species to hydrophobic surfaces such as titanium, Perspex, and stainless steel was showed (Kesaano and Sims 2014). Conversely, Irving and Allen (2011) showed no correlation between surface hydrophobicity of the substratum and cell adhesion of microbial biofilms grown in wastewater. On this basis, there is no recommended standard material as support for biofilm formation in wastewater treatment scale-up operation. However, in order to select adequate materials for biofilm development for successful application of this technology, the support must be ideally chosen. Thus, many factors should be taken into consideration such as its availability, cost, durability, and compatibility to the selected microorganisms from a thermodynamical point of view (Asri et al. 2017).
In the majority of the wastewater treatment plants using microbial biofilm, granular activated carbon (GAC) was used as a support material (Quintelas et al. 2010; Muhamad et al. 2013). However, its high cost and the challenges associated to its regeneration have strongly limited its utilization. At present, the works are mostly oriented toward the utilization of cheap alternatives. Hence, works in this regard have been significantly increased. This recent approach for the choice of supports is attempting to use natural wastes as supports; this adsorbent-support category is called low-cost adsorbents especially lignocellulosic wastes and by-products as an economical and eco-friendly alternative to conventional supports (Abdolali et al. 2014). This class includes a large variety of adsorbents such as fruit peel (Babel and Kurniawan 2004; Memon et al. 2009) and wood husk (Zainul Akmar et al. 2007; Asri et al. 2017).
Various used support materials within different biofilm-based wastewater treatment systems are illustrated in Table 1.
Bioreactors
Aerobic/Anaerobic Treatment
In order to achieve a high degree of treatment efficiency, organic wastewaters are preferably treated in aerobic biological processes. These processes allow the achievement of a higher removal of soluble biodegradable organic material compared to the anaerobic treatment. Furthermore, the produced biomass is easily separated from the aqueous media presenting a better flocculation property which provides an effluent of lower suspended solid concentration and thus higher quality. However, anaerobic treatments overcome the aerobic approach when treating influents with higher COD concentration. Anaerobic treatment is more suitable for the treatment of highly contaminated industrial effluents because of the high COD concentration. It also requires less energy and nutrient recovery with a low sludge production. Whereas anaerobic treatment is a promising approach from the resource recovery and utilization point of view, more efforts are still required for a better pollution control (Seghezzo et al. 1998; Chan et al. 2009). Indeed, this treatment suffers from a low growth rate of microbial cells, a and low quality of effluent due to the low flocculation ability and settling rate of produced biomass. In practical applications, anaerobic treatment suffers also from the difficulty of complete stabilization of organic matter because of the high COD level which provides a final effluent containing solubilized organic matter.
To overcome the disadvantages of both aerobic and anaerobic biofilm-based reactors, the use of anaerobic-aerobic systems presents several benefits and has been remarkably employed in industrial and municipal wastewater treatment in order to meet the effluent discharge standard (Frostell 1983; Cervantes et al. 2006).
The biofilm-based approaches of wastewater treatment are generally characterized by the ease and the safety of handling. In this section of the chapter, the most commonly used biofilm-based bioreactors are presented highlighting the advantages and disadvantages of each treatment system.
Types of Biofilm-Based Bioreactors
Biofilm-based wastewater treatment technology has been heralded as a promising cost-effective clean-up technology. Biofilm bioreactors are playing an extremely important role in environmental biotechnology. Despite the fact that many aspects of their design and technical operations remain poorly understood, a variety of these latter are installed worldwide while the researchers are still conducting intensive investigations for a better control of these promising depollution strategies.
Biofilm reactors are essentially composed of five compartments, while some additional components may be typical of a type of reactor: (1) influent, referring to the wastewater containing a given concentration of the pollutant of concern; (2) containment structure; (3) biofilm carrier or substratum referring to the used material for the growth and attachment of microbial cells; (4) effluent water collection system; and (5) an aeration or a mixing system for agitation and carrier distribution.
In this section, some of the commercially available biofilm-based processes are discussed. This includes membrane biofilm reactors (MBR), moving-bed biofilm reactors (MBBR), fluidized-bed reactors (FBR), trickling filter (TF), and microbial fuel cells (MFCs).
Membrane Biofilm Reactors
Membrane biofilm reactors (MBR) have been claimed for a long time as a promising biotechnology for pollutant removal and/or recovery from aqueous solutions. It refers essentially to a combination of a biological degradation of waste compounds using a biofilm-based system and physical separation realized by a membrane unit replacing the secondary settler. Presenting distinct advantages compared to other treatment technologies, it serves for the treatment of various industrial and urban wastewaters, proving an effective removal capability of both organic and inorganic matter (Di Fabio et al. 2013). Indeed, it provides an excellent quality of the effluent, reduced sludge production. It also provides a great flexibility toward influent variability, high volumetric loading, and good disinfection efficiency. It can be implemented within two different configurations (Lin et al. 2012). The first configuration is called submerged or immersed configuration and the second is the external or sidestream configuration. The submerged configuration came for the first time in 1989 with the idea of Yamamoto et al. (1989) that consists of the direct immersion of the membrane module inside the reactor. The driving force in this configuration is created by a negative pressure on the permeate side or pressurizing the bioreactor. This configuration showed distinct advantages such as the lower energy consumption and thus the lower operating cost and less cleaning procedures. These advantages have encouraged the development of this system. Regarding the external configuration, the membrane is placed outside the bioreactor allowing the recirculation of the mixed liquor. This configuration allows an easy control and membrane replacement. The driving force in this configuration is related to the high cross-flow velocity (CFV) through the bioreactor (Le-Clech et al. 2006; Liao et al. 2006). To date, both MBR configurations have been successfully utilized for various industrial effluents for their several distinct advantages, mainly for the lower energy consumption. Recently, an innovative configuration has appeared consisting of the development of air-lift sidestream MBRs (Lin et al. 2012). The main idea in this configuration is to exploit all advantages of MBRs above mentioned, with the application of the sidestream airlift principle (Chen and Liu 2006; Shariati et al. 2010). This concept showed its great efficiency in the treatment of industrial and municipal wastewater (Futselaar et al. 2007) and many efforts are still made for its development for a better application.
Biofilm processes in MBR could be done by the addition of media in moving- or fixed-bed configurations, or aerated membranes in the bioreactor as a support for biofilm growth. Numerous materials may be employed for biofilm support. To date, cord media, RBC media, sponge, plastic media, and GAC are commercially applied in full-scale systems (Ivanovic and Leiknes 2012). This technology has showed its efficiency in the treatment of various pollutants. For instance, it allowed an overall efficiency of degradation over 90% (Chang et al. 2003, 2004). It also showed an excellent removal of both COD and inorganic nitrogen in a further work (Semmens et al. 2003).
The membrane biofilm bioreactors have become an option of choice and efficient alternative for the treatment of domestic and industrial effluents. However, its widespread application suffers from major limitation due to the membrane fouling and clogging layers and their consequences in terms of plant maintenance and operating costs (Le-Clech et al. 2006). Its wider application is also limited by the high energy demand related to the air scouring demand and the high price of membranes.
Membrane fouling is a common phenomenon in membrane applications, including MBR systems (Ngo et al. 2006). However, the fraction mostly contributing to this problem remains unclear; it may be caused by colloidal and soluble organic content such as biopolymers or EPS, suspended solids, and physical properties (Ivanovic and Leiknes 2012).
Many works have dealt with these disadvantages by the control membrane fouling mechanisms and finding cheaper membrane materials. It also aimed the optimization of energy consumption and hence made this system more realistic and a reliable alternative to activated sludge processes and other conventional technologies (Ivanovic and Leiknes 2012).
Moving-Bed Biofilm Reactors
The moving-bed biofilm reactor (MBBR) is a wastewater biofilm-based technology presently implemented in more than 50 countries. It was developed in Norway in the late 1980s and early 1990s (Ødegaard et al. 1994).The MBBR plants were successfully used for municipal and various types of industrial wastewater treatment (Bassin and Dezotti 2018).
The principle behind this process is the combination of the best features of activated sludge process and those of biofilter. This type of reactor may be used for aerobic, anoxic, or anaerobic processes. The carriers that serve as housing for biofilm growth in this system move freely in the tank volume. In aerobic case, their movement is caused by the agitation set up by the air, while in anoxic and anaerobic processes the carriers are kept in movement by a mechanical mixing (Ødegaard 2006).
MBBRs are continuous-flow reactor units in which the most commonly used biofilm carrier is named K1. They present a cylindrical form constituted of high-density polyethylene (density 0.95 g/cm3) with a cross on the inside of the cylinder and “fins” on the outside. There is no filling fraction of the bioreactor with these carriers; it can vary from 25 to 70% of the total tank volume. However, it is recommended to be below 70% (corresponding to 350 m2.m−3 effective specific area in the case of K1) (Ødegaard 2006). Biofilms primarily grow in the inside of the plastic carriers, protected from external abrasion.
The implementation of moving beds rather than fixed ones presents the advantage of minimizing the clogging limitation and the ability to utilize the whole volume of the bioreactor.
As for other biofilm-based processes, the transport of substrates is of extreme importance. In this process, the turbulence in the reactor due to the shearing forces assures not only the appropriate diffusion of compounds with the biofilm, but also the low thickness of the formed biofilm.
Additionally, the use of this process minimizes or eliminates the need for biomass recirculation, which is a major problem of fixed-bed biofilm process and activated sludge systems. Only the excessive biomass has to be separated from the solution (Ivanovic and Leiknes 2012).
In comparison with fixed- or fluidized-bed biofilm reactors, moving-bed biofilm reactor provides a higher available surface for microbial growth and attachment due to the carrier materials. Moreover, it allows an efficient mixing condition inside the reactor, favoring hence the liberation of the biogas and the dispersing of volatile acids throughout the aqueous solution (Karadag et al. 2015). As for other treatment processes, the combination of moving-bed biofilm bioreactors to other treatment techniques was previously proposed. Indeed, the combination of this technology with coagulation and flotation process for high-rate secondary treatment was previously reported (Ødegaard 2006).
The MBBR has been demonstrated as a well-proven, compact, and robust reactor for wastewater treatment that served for carbon oxidation as well as for nitrification and denitrification goals as single stage or in combined systems (Gilbert et al. 2014; Malovanyy et al. 2015) and operational results were satisfying in both lab scale and larger scales. A noted disadvantage of these systems is that the carrier must be removed in order to benefit the reactor components (Butler and Boltz 2014).
Fluidized-Bed Biofilm Reactors
Fluidized-bed biofilm reactors (FBBR) are based on the use of small carriers, forming a bed inside a column kept in fluidized movement due to the flowing wastewaters and the bed hence expands. Within this system, a recycle line is used in order to maintain a fixed, vertical hydraulic flow of introduced wastewater.
The aeration is typically realized during recycle, where the influent wastewater mixes with the effluent recycled from the top of the bed. The air addition to the recycle stream is possible; however, it was found to cause a turbulence inside the reactor which may cleave the attached biofilm from the carriers.
Generally, media particles are distributed in FBBR within an increasing gradient of size from the top to the bottom of the bioreactor. Depending on the degree of particle expansion, the bed is classified as deemed expanded or fluidized. Many biofilm support materials have been typically used within this system, such as silica-based materials (Puhakka et al. 1995) zeolite, and GAC (Kida et al. 1990). However, in order to provide greater specific surface area, which is a key point of this technology, small materials (below 1 mm) have been used at pilot-scale experiments.
Once the driving force of this flowing inside the bioreactor exceeds the gravity (i.e., 30–50 m.h−1), the small particles become suspended and separated (Lewandowski and Boltz 2011).
Fluidized-bed biofilm reactor (FBBR) is widely recognized to present better mass transfer characteristics in comparison with fixed biofilm reactors. It showed its efficiency for tertiary denitrification in the case of the municipal wastewater treatment. For wastewater treatment, FBBR are used for the removal of oxidized contaminants (McCarty et al. 2005).
The fluidization of the media particles presents the advantage of maximizing the contact surface between microbial cells and effluents. It increases also the mass transfer and consequently the treatment efficiency. However, a low degree of bed expansion is recommended, as it decreases the flow velocity, consumes less energy, and increases the concentration of effective biomass.
The amount of attached microbial cells in FBBR is very important, exhibiting a high microbial diversity. This parameter permits a rapid recovery of the system after the variation of instability conditions (Malaspina et al. 1996; Borja et al. 2004). Nevertheless, it increased volumetric oxygen biomass because of the important biomass concentration.
Many successful applications of this system have been reported. For instance, Puhakka et al. (1995) used Pseudomonas sp. and Rhodococcus sp. in a laboratory-scale fluidized-bed biofilm reactor for the remediation of chlorophenol-contaminated groundwater. The treatment allowed an appreciable mineralization of chlorophenol efficiency (over 99.9% of 2,3,4,6-tetrachlorophenol, 2,4,6-trichlorophenol, and pentachlorophenol removal efficiency) at chlorophenol loading rates of 1000 mg.L−1.d−1 and hydraulic retention times of less than 1 h.
Trickling Filter
The trickling filter (TF) has been in use for more than 50 years. It is a three-phase biofilm reactor, including an influent recirculation pump station, the TF, and a clarification unit. It is generally composed of (1) an influent water distribution system, through which wastewaters are introduced into the reactor. The distribution may occur either by fixed-nozzle or rotary distributors; (2) a containment structure; (3) a support media; (4) an underdrain system; and (5) a ventilation system. Wastewater treatment using TFs requires a further liquid-solid separation for the elimination of suspended solids as the TF treatment results in the total suspended solid production. This step is typically carried out using circular or rectangular secondary clarifiers.
The rotatory distributor is advantageous for the influent distribution; it allows an intermittent wastewater application and an effective substratum wetting. These parameters avoid the odor emission and dry pockets and permit the biofilm to have resting periods serving primarily as a process aeration mechanism (Lewandowski and Boltz 2011).
The gradient of temperature between ambient air and air inside the trickling filter may provide a natural ventilation. When there is a difference between both temperatures, the provided dose of the oxygen is not suitable. In this case, the air supply may be achieved by the underdrain system that provides a space below the trickling filter for the collection of treated influent (Grady et al. 2011).
TFs have been proved for their capacity of meeting treatment objectives in terms of carbon oxidation and nitrification. The supported biofilm on the filter media uses oxygen in the form of air for the carbon oxidation. TFs are suitable for carbon oxidation and combined carbon oxidation and nitrification when a solid separation is included in the treatment train. Good results of nitrification have been observed with a combined oxidation and nitrification, where the concentration of ammonia was below 3 mg NH4+ N.L−1 and the reached BOD concentration is below 10 mg.L−1. The ammonia concentration is even lower when the nitrification is the main treatment goal (Metcalf and Eddy 2003).
A key element that should be taken into consideration in the design of TFs is the selection of filter media. The most commonly used material in this system was for a long time stones and gravel. However, these materials were restricting the air circulation in the filter and consequently the available oxygen quantity for the growth of microbial biofilm. This problem has limited the quantity of the treated wastewater and also reduced the specific surface area for microbial attachment that can accommodate the BOD loading for the reactor. Furthermore, stone bed trickling filters were limited by the clogging of the void spaces when treating high organic loads because of the excessive microbial cell growth. However, rock-media TFs were able to provide great treatment performance under low organic loading (i.e., <1 kg BOD5 d−1.m−3) (Grady et al. 2011).
Thus, other materials have been used to overcome these limitations including plastic rings, zeolite, ceramsite, sponge, etc. (Zhang et al. 2016). Compared to rock media, the use of these small particles enhanced oxygen transfer and biofilm thickness control. TFs using plastic modules with a specific surface area ranging from 89 to 102 m−2.m−3 are suitable for carbon oxidation and combined carbon oxidation and nitrification (Lewandowski and Boltz 2011). However, a noted disadvantage of this technology is that the trickling filter is not a volume-effective system.
Microbial Fuel Cells
The microbial fuel cell (MFC) is a recently developed biofilm-based system. This technology allows to overcome simultaneously two actual worldwide challenges, which are the depollution of wastewaters and the green energy production, by the conversion of the chemical energy stored in organic matter into electricity.
MFCs involve a specific type of microbial species exhibiting an ability to contribute to the generation of a current, by exchanging an electron network with an electrode. These microbial cells form a biofilm on the surface of the electrodes called electroactive biofilms “EAB.” Many papers have reported the successful use of MFCs with pure culture of various bacterial species. However, the performance of MFCs using mixed cultures has overcome the use of pure cultures, achieving substantially greater power densities (Ringeisen et al. 2007). Community analysis has revealed a wide diversity of microorganisms that could exist in MFCs; however bacteria and algae species are the most commonly used (Saba et al. 2017).
MFCs can be provided in different configurations. A typical MFC is basically constituted of two chambers linked by a conductive material containing a resistor, or operated under a load: anodic and cathodic. In these chambers separate biochemical and electrochemical reactions occur. In the anodic chamber (negative terminal), the organic matter is oxidated by the action of the microbial cells within the biofilm, resulting in the generation of electrons and protons. The electrons are then transferred to the anode and flow to the other part of the MFC containing the cathode (positive terminal), through an external circuit. Electrons can be transferred to the anode by electron mediators or shuttles, by direct membrane-associated electron transfer, or by so-called nanowires produced by the bacteria (Logan et al. 2006). In the cathodic chamber, oxygen plays the role of electron acceptor. These electrons, in combination with protons, diffuse from the anode through a separator and the oxygen provided from aerobic conditions results in the production of water molecules (Min and Logan 2004).
MFCs are currently used to produce electricity and treat organic and inorganic wastes. In fact, a great deal of attention is presently paid to optimize the key influencing factors such as design, materials of construction, and voltage generation mechanisms (Li and Sheng 2010; Huang et al. 2011; Ghasemi et al. 2012). Significant advancements have been carried out on the generated power density by MFC devices (Santoro et al. 2017). It has markedly increased after many research investigations, from a very low value of less than 0.1 mW.m−2 (Kim et al. 1999) to nearly 7 W.m−2 (Fan et al. 2008).
MFC technology has proved its performance in the treatment of many pollutants. Indeed, many industrial wastewaters have been used as inoculums in anode chamber such as food-processing effluents (Blanchet et al. 2015) and refinery wastewater (Zhang et al. 2014). Indeed, wastewaters rich in heavy metals (with high reduction potential) can be potentially used as an alternative electron acceptor. Hence, the contained inorganic matters are reduced into non- or less toxic forms. It also showed its great ability of denitrification without the need for exogenous electron donor, as the electrons generated from organic matter oxidation are released to external circuit and transferred to cathodic chamber facilitating hence nitrate reduction (Clauwaert et al. 2007). Additionally, the anaerobic conditions in the anode chamber make the MFC technology suitable for the treatment of high-strength wastewaters (COD over 8000 mg.L−1).
MFCs have been claimed as a promising biotechnology for pollutant removal and electricity generation, mainly because they are energy efficient, producing lower biomass, and the COD removal is achieved without additional oxygenation. However, significant challenges are still facing its practical application. Aiming the improvement of MFC performance, the increase in reactor volume was proposed; however, unsatisfactory results were obtained with larger MFCs.
These limitations are essentially observed with the expansion of laboratory-scale experiments to pilot scale. In fact, the generated power density is difficult to be maintained within larger systems. The volumetric power density showed generally a tendency to decrease with the increase in MFC size, while a minimum threshold volumetric power density of 1 kW.m−3 should be maintained (Zhang et al. 2013). This may be ascribed to the ohmic losses and the low conductivity of wastewaters (Butler and Boltz 2014). However, some exceptional results were obtained. Indeed, the work of Fan et al. (2012) reported that the increased reactor size provided a doubled volumetric power density due to the important cathode specific area (Fan et al. 2012). These results showed the possible maintenance of power density in MFC scale-up with a good optimization of the key factors influencing the system performance. Indeed, the increase of cathode specific area by the use of electrodes with three-dimensional structure showed its capability of enhancing MFC performance and the ability to overcome this limitation. This strategy suggests the enlargement of available surface area for microbial attachment without increasing the MFC volume (Aelterman et al. 2008).
For scale-up in MFCs, the parallel connection of MFCs in series is generally used. This traditional way proved its disadvantages such as the voltage reversal and operation unstability (Shin et al. 2006; Oh and Logan 2007).
Moreover, the voltage loss due to the substrate cross-conduction effect may be seriously limiting the full-scale use. In fact this phenomenon was found when two individual stacks were serially connected with both electrical and hydraulic connections (Zhuang and Zhou 2009).
Conclusion
Biofilm-based systems for wastewater treatment is a rapidly expanding research area that was intensively discussed in the literature. Their implementation is claimed beneficial thanks to the ease and simplicity of the operation compared to activated sludge processes. It has also showed high resistance to toxic substances and shock loads. However, the applications of this technology at industrial scale are limited due to the gap of techno-economic information on system performance, sustainability, reliability, and life cycle between laboratory- and/or pilot-scale to field-scale operations. This requires further works in large-scale wastewater operations.
Furthermore, biofilm-based systems involve living biomass that are influenced by the effect of surrounding conditions resulting from pH changes, metabolic activities, etc. Consequently, they are complicated to describe and to eventually model for practical use. Continued efforts are still needed to address biofilm control that can hinder these technologies such as membrane fouling.
References
Abdolali A, Guo W, Ngo H et al (2014) Typical lignocellulosic wastes and by-products for biosorption process in water and wastewater treatment: a critical review. Bioresour Technol 160:57–66. https://doi.org/10.1016/j.biortech.2013.12.037
Abzazou T, Araujo RM, Auset M et al (2016) Tracking and quantification of nitrifying bacteria in biofilm and mixed liquor of a partial nitrification MBBR pilot plant using fluorescence in situ hybridization. Sci Total Environ 541:1115–1123. https://doi.org/10.1016/j.scitotenv.2015.10.007
Aelterman P, Versichele M, Marzorati M et al (2008) Loading rate and external resistance control the electricity generation of microbial fuel cells with different three-dimensional anodes. Bioresour Technol 99:8895–8902. https://doi.org/10.1016/j.biortech.2008.04.061
Alatraktchi FA, Zhang Y, Angelidaki I (2014) Nanomodification of the electrodes in microbial fuel cell: impact of nanoparticle density on electricity production and microbial community. Appl Energy 116:216–222. https://doi.org/10.1016/j.apenergy.2013.11.058
Asri M, Elabed A, El Ghachtouli N et al (2017) Theoretical and experimental adhesion of yeast strains with high chromium removal potential. Environ Eng Sci. https://doi.org/10.1089/ees.2016.0515
Babel S, Kurniawan TA (2004) Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere 54:951–967. https://doi.org/10.1016/j.chemosphere.2003.10.001
Badia-Fabregat M, Lucas D, Tuomivirta T et al (2017) Study of the effect of the bacterial and fungal communities present in real wastewater effluents on the performance of fungal treatments. Sci Total Environ 579:366–377. https://doi.org/10.1016/j.scitotenv.2016.11.088
Bahafid W, Joutey NT, Sayel H et al (2013) Chromium adsorption by three yeast strains isolated from sediments in Morocco. Geomicrobiol J 30:422–429. https://doi.org/10.1080/01490451.2012.705228
Bassin JP, Dezotti M (2018, forthcoming) Moving Bed Biofilm Reactor (MBBR). In: Advanced biological processes for wastewater treatment, pp 37–74
Blanchet E, Desmond E, Erable B et al (2015) Comparison of synthetic medium and wastewater used as dilution medium to design scalable microbial anodes: application to food waste treatment. Bioresour Technol 185:106–115. https://doi.org/10.1016/j.biortech.2015.02.097
Borja R, Rincón B, Raposo F et al (2004) Mesophilic anaerobic digestion in a fluidised-bed reactor of wastewater from the production of protein isolates from chickpea flour. Process Biochem 39:1913–1921. https://doi.org/10.1016/j.procbio.2003.09.022
Butler CS, Boltz JP (2014) Biofilm processes and control in water and wastewater treatment. Compr Water Qual Purif 3:90–107. https://doi.org/10.1016/B978-0-12-382182-9.00083-9
Cakir FY, Stenstrom MK (2005) Greenhouse gas production: a comparison between aerobic and anaerobic wastewater treatment technology. Water Res 39:4197–4203. https://doi.org/10.1016/j.watres.2005.07.042
Cervantes F, Pavlostathis S, Van Haandel AC (2006) Advanced biological treatment processes for industrial wastewaters: principles and applications. IWA Publishing, London
Chan YJ, Chong MF, Law CL et al (2009) A review on anaerobic–aerobic treatment of industrial and municipal wastewater. Chem Eng J 155:1–18. https://doi.org/10.1016/j.cej.2009.06.041
Chang CC, Tseng SK, Chang CC et al (2003) Reductive dechlorination of 2-chlorophenol in a hydrogenotrophic, gas-permeable, silicone membrane bioreactor. Bioresour Technol 90:323–328. https://doi.org/10.1016/S0960-8524(03)00149-4
Chang CC, Tseng SK, Chang CC et al (2004) Degradation of 2-chlorophenol via a hydrogenotrophic biofilm under different reductive conditions. Chemosphere 56:989–997. https://doi.org/10.1016/j.chemosphere.2004.04.051
Chen S, Liu J (2006) Landfill leachate treatment by MBR: performance and molecular weight distribution of organic contaminant. Chin Sci Bull 51:2831–2838. https://doi.org/10.1007/s11434-006-2177-y
Chen S, Sun D, Chung J-S (2008) Simultaneous removal of COD and ammonium from landfill leachate using an anaerobic-aerobic moving-bed biofilm reactor system. Waste Manag 28:339–346. https://doi.org/10.1016/j.wasman.2007.01.004
Clauwaert P, Rabaey K, Aelterman P et al (2007) Biological denitrification in microbial fuel cells. Environ Sci Technol 41:3354–3360. https://doi.org/10.1021/es062580r
Cong LTN, Mai CTN, Thanh VT et al (2014) Application of a biofilm formed by a mixture of yeasts isolated in Vietnam to degrade aromatic hydrocarbon polluted wastewater collected from petroleum storage. Water Sci Technol 70:329–336. https://doi.org/10.2166/wst.2014.233
Costerton JW, Geesey GG, Cheng KJ (1978) How bacteria stick. Sci Am 238:86–95. https://doi.org/10.1038/scientificamerican0178-86
Das N, Basak LVG, Salam JA et al (2012) Application of biofilms on remediation of pollutants – an overview. J Microbiol Biotechnol Res 2:783–790
Davey ME, O’toole GA (2000) Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev 64:847–867. https://doi.org/10.1128/MMBR.64.4.847-867.2000
Day SM (1993) US environmental regulations and policies – their impact on the commercial development of bioremediation. Trends Biotechnol 11:324–328
Di Fabio S, Lampis S, Zanetti L et al (2013) Role and characteristics of problematic biofilms within the removal and mobility of trace metals in a pilot-scale membrane bioreactor. Process Biochem 48:1757–1766. https://doi.org/10.1016/j.procbio.2013.08.005
Edwards KJ, Bond PL, Gihring TM et al (2000) An archaeal iron-oxidizing extreme acidophile important in acid mine drainage. Science 287:10–13
El Abed S, Ibnsouda KS, Latrache H et al (2012) Theoretical effect of cedar wood surface roughness on the adhesion of conidia from Penicillium expansum. Ann Microbiol 62:1361–1366. https://doi.org/10.1007/s13213-011-0384-5
Fan Y, Sharbrough E, Liu H (2008) Quantification of the internal resistance distribution of microbial fuel cells. Environ Sci Technol 42:8101–8107. https://doi.org/10.1021/es801229j
Fan Y, Han S-K, Liu H (2012) Improved performance of CEA microbial fuel cells with increased reactor size. Energy Environ Sci 5:8273. https://doi.org/10.1039/c2ee21964f
Ferronato C, Silva B, Costa F et al (2016) Vermiculite bio-barriers for Cu and Zn remediation: an eco-friendly approach for freshwater and sediments protection. Int J Environ Sci Technol 13:1219–1228. https://doi.org/10.1007/s13762-016-0957-8
Fomina M, Gadd GM (2014) Biosorption: current perspectives on concept, definition and application. Bioresour Technol 160:3–14. https://doi.org/10.1016/j.biortech.2013.12.102
Frostell B (1983) Anaerobic-aerobic biological treatment of starch industry waste waters. Starch -Stärke 35:185–189. https://doi.org/10.1002/star.19830350602
Futselaar H, Schonewille H, De Vente D et al (2007) NORIT AirLift MBR: side-stream system for municipal waste water treatment. Desalination 204:1–7. https://doi.org/10.1016/j.desal.2006.02.027
Ghasemi M, Shahgaldi S, Ismail M et al (2012) New generation of carbon nanocomposite proton exchange membranes in microbial fuel cell systems. Chem Eng J 184:82–89. https://doi.org/10.1016/j.cej.2012.01.001
Gieg LM, Fowler SJ, Berdugo-Clavijo C (2013) Syntrophic biodegradation of hydrocarbon contaminants. Curr Opin Biotechnol 27:21–29. https://doi.org/10.1016/j.copbio.2013.09.002
Gilbert EM, Agrawal S, Karst SM et al (2014) Low temperature partial nitritation/anammox in a moving bed biofilm reactor treating low strength wastewater. Environ Sci Technol 48:8784–8792. https://doi.org/10.1021/es501649m
Grady L, Daigger G, Lim H (2011) Biological wastewater treatment. CRC Press, Boca Raton
Hai R, He Y, Wang X et al (2015) Simultaneous removal of nitrogen and phosphorus from swine wastewater in a sequencing batch biofilm reactor. Chin J Chem Eng 23:303–308. https://doi.org/10.1016/j.cjche.2014.09.036
Hamadi F, Latrache H, Mabrrouki M et al (2005) Effect of pH on distribution and adhesion of Staphylococcus aureus to glass. J Adhes Sci Technol 19:73–85. https://doi.org/10.1163/1568561053066891
Hoh D, Watson S, Kan E (2016) Algal biofilm reactors for integrated wastewater treatment and biofuel production: a review. Chem Eng J 287:466–473. https://doi.org/10.1016/j.cej.2015.11.062
Horemans B, Breugelmans P, Hofkens J et al (2013) Environmental dissolved organic matter governs biofilm formation and subsequent linuron degradation activity of a linuron-degrading bacterial consortium. Appl Environ Microbiol 79:4534–4542. https://doi.org/10.1128/AEM.03730-12
Huang L, Chai X, Cheng S et al (2011) Evaluation of carbon-based materials in tubular biocathode microbial fuel cells in terms of hexavalent chromium reduction and electricity generation. Chem Eng J 166:652–661. https://doi.org/10.1016/j.cej.2010.11.042
Irving TE, Allen DG (2011) Species and material considerations in the formation and development of microalgal biofilms. Appl Microbiol Biotechnol 92:283–294. https://doi.org/10.1007/s00253-011-3341-0
Ivanovic I, Leiknes TO (2012) The biofilm membrane bioreactor (BF-MBR) – a review. Desalin Water Treat 37:288–295. https://doi.org/10.1080/19443994.2012.661283
Jung J, Choi N, Lee S (2013) Biofilm formation and exopolysaccharide (EPS) production by Cronobacter sakazakii depending on environmental conditions. Food Microbiol 34:70–80. https://doi.org/10.1016/j.fm.2012.11.008
Karadag D, Koroglu OE, Ozkaya B et al (2015) Anaerobic granular reactors for the treatment of dairy wastewater: a review. Int J Dairy Technol 68:459–470. https://doi.org/10.1111/1471-0307.12252
Kesaano M, Sims RC (2014) Algal biofilm based technology for wastewater treatment. Algal Res 5:231–240. https://doi.org/10.1016/j.algal.2014.02.003
Kida K, Morimura S, Sonoda Y et al (1990) Support media for microbial adhesion in an anaerobic fluidized-bed reactor. J Ferment Bioeng 69:354–359. https://doi.org/10.1016/0922-338X(90)90243-P
Kim BH et al (1999) Mediator-less biofuel cell. US Patent 5,976,719
Kreft JU, Wimpenny JW (2001) Effect of EPS on biofilm structure and function as revealed by an individual-based model of biofilm growth. Water Sci Technol 43:135–141
Le-Clech P, Chen V, Fane TAG (2006) Fouling in membrane bioreactors used in wastewater treatment. J Membr Sci 284:17–53. https://doi.org/10.1016/j.memsci.2006.08.019
Lee WN, Kang IJ, Lee CH (2006) Factors affecting filtration characteristics in membrane-coupled moving bed biofilm reactor. Water Res 40:1827–1835. https://doi.org/10.1016/j.watres.2006.03.007
Lefebvre O, Moletta R (2006) Treatment of organic pollution in industrial saline wastewater: a literature review. Water Res 40:3671–3682. https://doi.org/10.1016/j.watres.2006.08.027
Lettinga G, Van Velsen AFM, Hobma SW et al (1980) Use of the upflow sludge blanket (USB) reactor concept for biological wastewater treatment, especially for anaerobic treatment. Biotechnol Bioeng 22:699–734. https://doi.org/10.1002/bit.260220402
Lewandowski Z, Boltz J (2011) Biofilms in water and wastewater treatment. In: Treatise on water science. Academic, Oxford, pp 529–570
Leyva-Ramos R, Jacobo-Azuara A, Diaz-Flores PE et al (2008) Adsorption of chromium(VI) from an aqueous solution on a surfactant-modified zeolite. Colloids Surf A Physicochem Eng Asp 330:35–41. https://doi.org/10.1016/j.colsurfa.2008.07.025
Li W-W, Sheng G-P (2010) Microbial fuel cell in power generation and extended applications. Adv Biochem Eng Biotechnol 123:127–141. https://doi.org/10.1007/10_2011_125
Liao B-Q, Kraemer JT, Bagley DM (2006) Anaerobic membrane bioreactors: applications and research directions. Crit Rev Environ Sci Technol. https://doi.org/10.1080/10643380600678146
Lin H, Gao W, Meng F et al (2012) Membrane bioreactors for industrial wastewater treatment: a critical review. Crit Rev Environ Sci Technol 42:677–740. https://doi.org/10.1080/10643389.2010.526494
Liu H, Ramnarayanan R, Logan BE (2004) Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ Sci Technol 38:2281–2285
Liu C, Li J, Zhu X et al (2013a) Effects of brush lengths and fiber loadings on the performance of microbial fuel cells using graphite fiber brush anodes. Int J Hydrog Energy 38:15646–15652. https://doi.org/10.1016/j.ijhydene.2013.03.144
Liu T, Yang X, Wang ZL et al (2013b) Enhanced chitosan beads-supported Fe0-nanoparticles for removal of heavy metals from electroplating wastewater in permeable reactive barriers. Water Res 47:6691–6700. https://doi.org/10.1016/j.watres.2013.09.006
Logan BE, Hamelers B, Rozendal R et al (2006) Microbial fuel cells: methodology and technology. Environ Sci Technol 40:5181–5192. https://doi.org/10.1021/es0605016
Lohmeyer GT (1957) Trickling filters and operation tips. Sewage Ind Waste 29:89–98
Malaspina F, Cellamare CM, Stante L et al (1996) Anaerobic treatment of cheese whey with a downflow-upflow hybrid reactor. Bioresour Technol 55:131–139. https://doi.org/10.1016/0960-8524(95)00187-5
Malovanyy A, Yang J, Trela J et al (2015) Combination of upflow anaerobic sludge blanket (UASB) reactor and partial nitritation/anammox moving bed biofilm reactor (MBBR) for municipal wastewater treatment. Bioresour Technol 180:144–153. https://doi.org/10.1016/j.biortech.2014.12.101
Matz C, Kjelleberg S (2005) Off the hook – how bacteria survive protozoan grazing. Trends Microbiol 13:302–307. https://doi.org/10.1016/j.tim.2005.05.009
McCarty PL, Meyer TE, Maccarty PL (2005) Numerical model for biological fluidized-bed reactor treatment of groundwater. Environ Sci Technol 39:850–858
Memon JR, Memon SQ, Bhanger MI et al (2009) Banana peel: a green and economical sorbent for the selective removal of Cr(VI) from industrial wastewater. Colloids Surf B Biointerfaces 70:232–237. https://doi.org/10.1016/j.colsurfb.2008.12.032
Metcalf W, Eddy C (2003) Wastewater engineering: treatment and reuse. McGraw Hill, New York, p 384
Min B, Logan BE (2004) Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ Sci Technol 38:5809–5814. https://doi.org/10.1021/es0491026
Miqueleto AP, Dolosic CC, Pozzi E et al (2010) Bioresource technology influence of carbon sources and C/N ratio on EPS production in anaerobic sequencing batch biofilm reactors for wastewater treatment. Bioresour Technol 101:1324–1330. https://doi.org/10.1016/j.biortech.2009.09.026
Mitra A, Mukhopadhyay S (2016) Biofilm mediated decontamination of pollutants from the environment. AIMS Bioeng 3:44–59. https://doi.org/10.3934/bioeng.2016.1.44
Muhamad MH, Sheikh Abdullah SR, Mohamad AB et al (2013) Application of response surface methodology (RSM) for optimisation of COD, NH3-N and 2,4-DCP removal from recycled paper wastewater in a pilot-scale granular activated carbon sequencing batch biofilm reactor (GAC-SBBR). J Environ Manag 121:179–190. https://doi.org/10.1016/j.jenvman.2013.02.016
Ngo HH, Nguyen MC, Sangvikar NG et al (2006) Simple approaches towards the design of an attached-growth sponge bioreactor (AGSB) for wastewater treatment and reuse. Water Sci Technol 54:191–197. https://doi.org/10.2166/wst.2006.727
Nicolella C (2000) Wastewater treatment with particulate biofilm reactors. J Biotechnol 80:1–33. https://doi.org/10.1016/S0168-1656(00)00229-7
Ødegaard H (2006) Innovations in wastewater treatment: the moving bed biofilm process. Water Sci Technol 53:17–33. https://doi.org/10.2166/wst.2006.284
Ødegaard H, Rusten B, Westrum T (1994) A new moving bed biofilm reactor – applications and results. Water Sci Technol 29:157–165
Oh SE, Logan BE (2007) Voltage reversal during microbial fuel cell stack operation. J Power Sources 167:11–17. https://doi.org/10.1016/j.jpowsour.2007.02.016
Oh KH, Tuovinen OH (1994) Biodegradation of the phenoxy herbicides MCPP and 2, 4-D in fixed-film column reactors. Int Biodeterior Biodegrad 33:93–99
Pan X, Liu Z, Chen Z et al (2014) Investigation of Cr(VI) reduction and Cr(III) immobilization mechanism by planktonic cells and biofilms of Bacillus subtilis ATCC-6633. Water Res 55:21–29. https://doi.org/10.1016/j.watres.2014.01.066
Parvatiyar MG, Govind R, Bishop DF (1996) Biodegradation of toluene in a membrane biofilter. J Membr Sci 119:17–24
Puhakka JA, Melin ES, Järvinen KT et al (1995) Fluidized-bed biofilms for chlorophenol mineralization. Water Sci Technol 31:227–235. https://doi.org/10.1016/0273-1223(95)00170-R
Qu YY, Zhou JT, Wang J et al (2009) Population dynamics in bioaugmented membrane bioreactor for treatment of bromoamine acid wastewater. Bioresour Technol 100:244–248
Quintelas C, Fernandes B, Castro J et al (2008) Biosorption of Cr(VI) by a Bacillus coagulans biofilm supported on granular activated carbon (GAC). Chem Eng J 136:195–203. https://doi.org/10.1016/j.cej.2007.03.082
Quintelas C, Silva B, Figueiredo H et al (2010) Removal of organic compounds by a biofilm supported on GAC: modelling of batch and column data. Biodegradation 21:379–392. https://doi.org/10.1007/s10532-009-9308-5
Quintelas C, da Silva VB, Silva B et al (2011) Optimization of production of extracellular polymeric substances by Arthrobacter viscosus and their interaction with a 13X zeolite for the biosorption of Cr(VI). Environ Technol 32:1541–1549. https://doi.org/10.1080/09593330.2010.543930
Quintelas C, Pereira R, Kaplan E et al (2013) Removal of Ni(II) from aqueous solutions by an Arthrobacter viscosus biofilm supported on zeolite: from laboratory to pilot scale. Bioresour Technol 142:368–374. https://doi.org/10.1016/j.biortech.2013.05.059
Reysenbach A, Cady SL (2001) Microbiology of ancient and modern hydrothermal systems. Trends Microbiol 9:79–86
Ringeisen BR, Ray R, Little B (2007) A miniature microbial fuel cell operating with an aerobic anode chamber. J Power Sources 165:591–597. https://doi.org/10.1016/j.jpowsour.2006.10.026
Saba B, Christy AD, Yu Z et al (2017) Bioelectrochemistry characterization and performance of anodic mixed culture bio films in submersed microbial fuel cells. Bioelectrochemistry 113:79–84. https://doi.org/10.1016/j.bioelechem.2016.10.003
Santoro C, Arbizzani C, Erable B et al (2017) Microbial fuel cells: from fundamentals to applications. A review. J Power Sources 356:225–244. https://doi.org/10.1016/j.jpowsour.2017.03.109
Seghezzo L, Zeeman G, Van Lier JB et al (1998) A review: the anaerobic treatment of sewage in UASB and EGSB reactors. Bioresour Technol 65:175–190. https://doi.org/10.1016/S0960-8524(98)00046-7
Segura Y, Martínez F, Melero JA et al (2015) Zero valent iron (ZVI) mediated Fenton degradation of industrial wastewater: treatment performance and characterization of final composites. Chem Eng J 269:298–305. https://doi.org/10.1016/j.cej.2015.01.102
Semmens MJ, Dahm K, Shanahan J et al (2003) COD and nitrogen removal by biofilms growing on gas permeable membranes. Water Res 37:4343–4350. https://doi.org/10.1016/S0043-1354(03)00416-0
Shammas NK (2005) Coagulation and flocculation. In: Wang LK, Hung Y-T, Shammas NK (eds) Physicochemical treatment processes. Humana Press, Totowa, pp 103–139
Shariati FP, Mehrnia MR, Salmasi BM et al (2010) Membrane bioreactor for treatment of pharmaceutical wastewater containing acetaminophen. Desalination 250:798–800. https://doi.org/10.1016/j.desal.2008.11.044
Shin S, Choi Y, Na S et al (2006) Development of bipolar plate stack type microbial fuel cells. Bull Kor Chem Soc 27:281–285. https://doi.org/10.5012/bkcs.2006.27.2.281
Silva B, Figueiredo H, Quintelas C et al (2008) Zeolites as supports for the biorecovery of hexavalent and trivalent chromium. Microporous Mesoporous Mater 116:555–560. https://doi.org/10.1016/j.micromeso.2008.05.015
Singh R, Paul D, Jain RK (2006) Biofilms: implications in bioremediation. Trends Microbiol 14:389–397. https://doi.org/10.1016/j.tim.2006.07.001
Tarjányi-Szikora S, Oláh J, Makó M et al (2013) Comparison of different granular solids as biofilm carriers. Microchem J 107:101–107. https://doi.org/10.1016/j.microc.2012.05.027
Yamamoto K, Hiasa M, Mahmood T et al (1989) Direct solid-liquid separation using hollow fiber membrane in an activated sludge aeration tank. Water Sci Technol 21:43–54. https://doi.org/10.1016/B978-1-4832-8439-2.50009-2
Yang X-L, Jiang Q, Song H-L et al (2015) Selection and application of agricultural wastes as solid carbon sources and biofilm carriers in MBR. J Hazard Mater 283:186–192. https://doi.org/10.1016/j.jhazmat.2014.09.036
Zainul Akmar Z, Zainoha Z, Salmijah S et al (2007) Biological detoxification of Cr(VI) using wood-husk immobilized Acinetobacter haemolyticus. J Hazard Mater 148:164–171. https://doi.org/10.1016/j.jhazmat.2007.02.029
Zhang L, Li J, Zhu X et al (2013) Anodic current distribution in a liter-scale microbial fuel cell with electrode arrays. Chem Eng J 223:623–631. https://doi.org/10.1016/j.cej.2013.03.035
Zhang F, Ahn Y, Logan BE (2014) Treating refinery wastewaters in microbial fuel cells using separator electrode assembly or spaced electrode configurations. Bioresour Technol 152:46–52. https://doi.org/10.1016/j.biortech.2013.10.103
Zhang X, Li J, Yu Y et al (2016) Biofilm characteristics in natural ventilation trickling filters (NVTFs) for municipal wastewater treatment: comparison of three kinds of biofilm carriers. Biochem Eng J 106:87–96. https://doi.org/10.1016/j.bej.2015.11.009
Zhao F, Rahunen N, Varcoe JR et al (2008) Activated carbon cloth as anode for sulfate removal in a microbial fuel cell. Environ Sci Technol 42:4971–4976
Zhuang L, Zhou S (2009) Substrate cross-conduction effect on the performance of serially connected microbial fuel cell stack. Electrochem Commun 11:937–940. https://doi.org/10.1016/j.elecom.2009.02.027
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Section Editor information
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this entry
Cite this entry
Asri, M., Elabed, S., Ibnsouda Koraichi, S., El Ghachtouli, N. (2019). Biofilm-Based Systems for Industrial Wastewater Treatment. In: Hussain, C. (eds) Handbook of Environmental Materials Management. Springer, Cham. https://doi.org/10.1007/978-3-319-73645-7_137
Download citation
DOI: https://doi.org/10.1007/978-3-319-73645-7_137
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-73644-0
Online ISBN: 978-3-319-73645-7
eBook Packages: Chemistry and Materials ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics