Abstract
Pancreatic cancer is a highly lethal disease. Up to date, the only curative approach is surgical resection, which is only possible in a limited number of patients by the time of diagnosis. Thus, the development of new therapeutic options besides chemotherapy is extremely important for patients who do not qualify for surgery due to local irresectability or systemic tumor spread. During development and progression of pancreatic tumors, angiogenesis is an important mechanism to supply blood, oxygen, and nutrients for the growing tumor mass. Several angiogenic factors play a critical role during this process, including vascular endothelial growth factor (VEGF), as well as multiple factors involved in tyrosine kinase pathways, all of which are potential targets for systemic treatment approaches.
Pancreatic ductal adenocarcinoma represents the biggest proportion among all pancreatic tumor entities. It is histopathologically characterized by a hypovascular appearance and pronounced peritumoral desmoplastic tissue as well as extracellular matrix. In numerous experimental and clinical studies, anti-angiogenic therapy has been evaluated for pancreatic ductal adenocarcinoma with early promising results. However, in clinical phase III studies, only limited effects were achieved with targeted anti-angiogenic approaches.
In contrast, pancreatic neuroendocrine tumors, which are typically hypervascularized, are much more sensitive to anti-angiogenic substances. After a successful phase III study, sunitinib – a multi-targeted kinase inhibitor – has been approved for the treatment of this entity and is incorporated in current international guidelines as a second-line therapy recommendation.
The pathogenesis, diagnostic measures, as well as current experimental and clinical studies regarding angiogenesis and anti-angiogenic therapy of both pancreatic ductal adenocarcinoma and neuroendocrine tumors are summarized described in this chapter.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
Introduction
Pancreatic cancer (pancreatic ductal adenocarcinoma, PDAC) is one of the most aggressive solid tumor entities and a highly lethal malignancy. The only curative approach is surgical resection; radiotherapy, chemotherapy, or a combination of both can only act as palliative treatment or neo-/adjuvant therapy. Even with advances in surgery and conservative therapy, it remains fourth leading cause for cancer-associated mortality in Western countries with – in contrast to other malignancies – still increasing rates of incidence (Siegel et al. 2015). Symptoms including pain, jaundice, and weight loss show a late onset, and in only 15–20% of all patients, surgery is possible at the time of diagnosis. Thus, offering the chance of long-term survival only to a limited number of patients. When combined with adjuvant chemotherapy, 5-year survival rates of 20–25% can be achieved (Hackert and Büchler 2013). The importance of postoperative adjuvant chemotherapy has been demonstrated in large randomized studies during the last two decades (Valle et al. 2014; Oettle et al. 2013; Ryan et al. 2014) and represents the standard of care for all patients that are considered to be resectable by the time of diagnosis. In contrast, in a situation of systemic spread, especially peritoneal carcinomatosis or liver metastases, only palliative treatment is possible (Tempero et al. 2014; Seufferlein et al. 2012). For this purpose, standard chemotherapies include gemcitabine which can be supplemented by nab-paclitaxel as another cytotoxic substance or erlotinib, a tyrosine kinase inhibitor (see below) (Moore et al. 2007). An alternative treatment in PDAC stage 4 is the application of Folfirinox – a combination of 5-fluorouracil, folinic acid, irinotecan, and oxaliplatin – which seems to be the most effective substance today (Conroy et al. 2011). With the introduction of this regimen, median survival times in the palliative setting have increased from 6.8 months (gemcitabine) to 11.1 months (Conroy et al. 2011). As these outcomes are still unsatisfactory and a high proportion of patients suffer from early progressive disease under a palliative chemotherapy, there is urgent need for other approaches to improve the prognosis. During the last 20 years, the concept of anti-angiogenic therapy as a supplement or alternative to classical chemotherapy has been examined in a large number of tumor entities and numerous experimental as well as clinical studies have been conducted for PDAC as well as for pancreatic neuroendocrine tumors (pNETs).
Angiogenesis describes the formation of new blood vessels from preexisting vessels and – besides its physiological function, i.e., during wound healing or normal tissue growth – has been recognized as a pathophysiological phenomenon in many solid tumor entities (Craven et al. 2016).
Consequently, the pathophysiology and the factors involved in the de novo generation of vessels by tumors have been examined in many experimental models and translational studies with the aim to target these events and develop selective and potentially effective therapeutic approaches [see below]. Especially vascular endothelial growth factor (VEGF) pathway inhibitors represent the currently most promising substances. However, the clinical results with regard to oncological outcome in terms of progression-free and overall survival strongly depend on the nature of the tumor itself, as some tumors show sensitivity to these approaches (i.e., renal cell or ovarian cancer), whereas other entities are mostly resistant (i.e., prostate cancer or malignant melanoma). Regarding pancreatic tumors, very heterogeneous effects of anti-angiogenic therapy are observed, especially with regard to the differentiation between PDAC and pNET .
Pathogenesis
Tumor angiogenesis is a crucial mechanism for the supply of oxygen, glucose, and other nutrients to sustain the growth of tumor cells after they have initially survived via the mechanism of diffusion or the physiological blood supply at their original location in the parenchyma of an organ. Consequently, two distinct phases can be described during the process of tumor angiogenesis of pancreatic tumors (Bergers 1999).
The first phase, also referred to as pre-vascular phase, is characterized by an increase in tumor cell proliferation with an adequate apoptotic counterbalance resulting in a plateau phase of tumor cell growth. This plateau can exist for years as a premalignant condition and leads to the histopathological appearance of an increasing grade of dysplasia and finally the transformation to carcinoma in situ.
The transition into the next phase is the so-called angiogenic switch and caused by the misbalance between a higher nutrient and oxygen demand due to the increasing cell number and volume with the consequence that the tumor cells cannot cover the needed supply by the existing mechanisms without additional blood vessels from their environment. This stage initiates the subsequent “vascular phase,” during which pancreatic tumor cells grow exponentially lacking the counterbalancing apoptotic factors of the normal cell cycle. At the same time an overexpression of pro-angiogenic factors and a loss in physiological angiogenesis inhibitors create an imbalance leading to a de novo chaotic vessel formation with high vascular leakage (Hanahan and Folkman 1996). These mechanisms result in an aggressive tumor expansion, invasion, and possibly distant spread of tumor cells.
As a large variety of cells and signaling molecules are involved in tumor angiogenesis, this complex mechanism has to be regarded as an ambivalent and two-sided event as on one hand it is not completely understood today but on the other hand offers the opportunity to interfere in this process very specifically at many points with the aim to block angiogenesis and develop a targeted therapeutic approach which may be specific and differential for each individual tumor entity.
These specific characteristics of pancreatic tumorigenesis of each histopathological type give an explanation for the ambivalent response to anti-angiogenic therapies. PDAC arises from the ductal epithelium and makes up to 85% of pancreatic cancers; its typical histological appearance consist of a very avascular and fibrotic microenvironment making it difficult for drug delivery (Feig et al. 2012; Erkan et al. 2012). Therefore, PDAC is a mostly hypovascular entity (Fig. 1) and indolent to antiangiogenics. In contrast, pNETs are characterized by a hypervascularized structure (Fig. 2) and show promising responses, to therapeutic approaches aiming at anti-angiogenic mechanisms.
Experimental and Clinical Diagnostic Modalities for Angiogenesis in PDAC and pNET
The visualization and quantification of tumor vascularization can be performed by direct or indirect examination. In the experimental setting, there have been several approaches for direct examination of vessel density and morphology including intravital microscopy and ultrasound flow measurement. The latter has also been introduced in the clinical setting but has – comparable to functional cross-sectional imaging (computed tomography (CT) and magnetic resonance tomography (MRI)) or tumor angiography – not reached the level of a routine examination in practice today.
Intravital microscopy (IVM) offers the possibility to directly visualize tumor vessels and blood cell flow by in vivo examination (Tsuzuki et al. 2001a). This experimental method is mostly based on fluorescence imaging of red blood cells, leukocytes, or staining of endothelial cells by respective fluorescence markers (Schmidt et al. 2000). In animal models of PDAC, the generation of abnormal vessels with an increased diameter, reflecting an expansion of endothelial cell surface, has been demonstrated by this method (Fig. 3, (Ryschich et al. 2004)). Furthermore, a pathological leukocyte-endothelium interaction on these altered cell surfaces could be shown, and the fact that the vessel density as investigated by IVM methods during PDAC development was not increased (Schmidt et al. 2000) supports the hypothesis that not the number of vessels itself increases but their abnormal architecture is the key finding during tumorigenesis of PDAC. Due to the need for toxic staining substances for blood cells and endothelium as well as the invasive character of this method, IVM has only been applied in the experimental setting and is not a suitable method for clinical use.
Ultrasound examination of tumor vasculature and perfusion is based on contrast-enhanced approaches that allow Doppler flow measurement. In a PDAC mouse model, microbubble-enhanced contrast ultrasound was utilized to evaluate perfusion intensity in small PDAC lesions (Pysz et al. 2015; Foygel et al. 2013; Deshpande et al. 2011). By targeting contrast bubbles against thymocyte differentiation antigen, integrin, endoglin, or VEGF receptor 2 as specific binding markers for PDAC, an increased signal intensity was demonstrated in particular small tumor lesions and correlated with the respective histological finding of a higher vessel density in these areas. This underlines the potential of these biomarkers to facilitate early detection of tumorous lesions based on the perfusion characteristics. Although promising, these approaches bear the disadvantage of the need for specific targeted bubbles, and their accuracy is based on an invasive ultrasound examination that has to be performed on the tumor pancreatic surface directly or with a very close contact of the ultrasound probe to the tumor (i.e., in a subcutaneous model), which is consequently not applicable in a clinical setting but represents the basis for further development of patients-directed diagnostics. Ultrasound Doppler flow measurement during transabdominal or endoscopic ultrasound has been established during the last 15 years, mainly based on the development of the air-based contrast agent Levovist which has been introduced in the clinical practice. This method of ultrasound Doppler flow measurement has become applicable in a number of studies (Nishida et al. 2009; Chen et al. 2004; Scialpi et al. 2005; Hocke and Dietrich 2012; Dyrla et al. 2016; Kobayashi et al. 2014; Gincul et al. 2014; Iordache et al. 2012; Figueiredo et al. 2012).
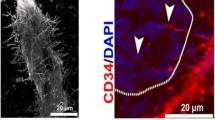
A 27-patient study on unresectable PDAC evaluated contrast-enhanced ultrasound examination in correlation with dynamic CT scan and VEGF as well as CD34 staining (Fig. 4) to intratumoral vessel density and diameter before and after chemoradiotherapy (Nishida et al. 2009). Ultrasound examination revealed a good correlation with vessel density, VEGF expression, and histological grading. Furthermore, it was also useful to determine therapy effects in terms of partial response or stable disease.
In another clinical study, contrast-enhanced power Doppler sonography was utilized to evaluate the enhancement characteristics of PDAC in correlation with the tumor vascularity observed on digital subtraction angiography (DSA) in 20 patients (Chen et al. 2004). Interestingly, this study showed a large heterogeneity in terms of hypo- (85% of the patients) and hypervascularity (15% of the patients) of the tumors but confirmed the high accuracy of ultrasound measurement compared with DSA as the gold standard. Besides the characterization of PDAC alone, contrast-enhanced power Doppler ultrasonography (US) has also been used to differentiate between PDAC and focal chronic pancreatitis as the most important clinical differential diagnosis (Scialpi et al. 2005). A high proportion of PDAC showed an increased number of vessels and irregular vessel characteristics whereas focal chronic pancreatitis presented mostly as avascular masses which confirmed the potential usefulness of this modality to further classify unclear pancreatic masses into malignant or benign lesions.
Regarding other perfusion-directed diagnostic clinical modalities for PDAC detection, functional cross-sectional imaging is currently a field of high interest. This includes MRI and CT imaging (Lemke et al. 2009; Fritz et al. 2016; Klauß et al. 2016; Klauss et al. 2012, 2013; Bangard et al. 2005) as noninvasive methods which can easily be applied as an addition to standard imaging, PDAC patients receive during their diagnostic workup. Both of these radiological modalities are especially helpful to differentiate PDAC versus chronic pancreatitis which can also be found to mimic cancerous lesions in conventional cross-sectional imaging (Klauß et al. 2016). Functional MRI has been established in experimental models of PDAC and angiogenetics by measurement of the endothelial transfer coefficient and fractional plasma volumes by dynamic contrast-enhanced MR imaging (Bangard et al. 2005). More recent clinical imaging studies have focused on functional MRI imaging using the perfusion fraction, and the intravoxel incoherent motion approach showed specific characteristics of PDAC patients, patients with any form of chronic pancreatitis, and healthy control participants (Klauss et al. 2012, 2013). A reduction of these parameters as a surrogate marker for reduced perfusion is observed in PDAC compared to healthy pancreatic tissue and can be used with a high sensitivity and specificity (96% and 100%, respectively) to differentiate between these two types of tissue.
Functional CT scan offers another comparable modality to determine vessel and perfusion characteristics of PDAC (Klauss et al. 2012). Tumor perfusion, blood volume, and permeability show specific changes in PDAC with a significant reduction of these parameters and can be used to differentiate tumor lesions from healthy tissue, especially when these cannot be sufficiently diagnosed in conventional cross-sectional imaging modalities.
As the mentioned findings of a reduced tumor perfusion contradict the proposed impact of tumor neo-angiogenesis, it has to be taken into account that PDAC are – especially in advanced stages – accompanied by a massive peritumoral desmoplastic reaction , which explains the macro-imaging observations of PDAC as rather low-perfused lesions as the reactive peritumoral fibrosis is known to be a bradytrophic tissue with low vessel density.
To summarize the current state of the abovementioned perfusion-based diagnostic modalities for PDAC detection or differentiation, Doppler sonography is useful in experienced hands as it is always depending on the expertise of the examiner and can only be reproduced individually. Functional cross-sectional imaging is an objective method which is gaining importance in clinical practice. However, it is not regarded as a standard yet, as its availability is still limited in centers around the world and the available study results are still based on small patient numbers. Future studies will help to gain more evidence and increase the routine application of functional MRI and CT in clinical practice.
Tumorigenesis and Angiogenesis in Pancreatic Adenocarcinoma
Pathophysiology
The underlying genetic mutations of pancreatic tumor development can be sporadic but also part of a familial gene mutation. In more than 90% of tumors, an activating mutation in the KRAS gene which is part of the mitogenic signaling is found, additional common mutations include the p53 pathway as well as the TGF-ß pathway (Bardeesy et al. 2002; Ahmed et al. 2002; Jonson et al. 2001). Some of these pathways and corresponding factors also contribute to angiogenesis (Table 1). Similar to the tumor sequence model in colorectal cancer, a related adenocarcinoma sequence can be observed in PDAC. The corresponding precursor lesions are classified by their increasing malignant characteristics and defined as “pancreatic intraepithelial neoplasias” (PanIN) . The higher the PanIN grade becomes (1–3), the higher the number of mutations that are observed. These sets of mutations correlate with the severity of dysplastic features found in the histological morphology (Brosens et al. 2015). Once the threshold of invasive growth has been reached at the end of the malignant transformation process, the cells display an interaction with the surrounding tissue. This is crucially important for the understanding of the features this tumor entity shows in terms of angiogenesis induction considering the fact that PDAC is – despite this vascularization process – still found to be a hypovascular lesion compared to healthy pancreatic exocrine tissue (Fig. 1).
PDAC cells release growth factors such as VEGF that bind to nearby endothelial cells and induce a response that stimulates endothelial cells to divide and form new blood vessels. These signaling pathways consequently play a crucial role in the possibility of PDAC to create a de novo vasculature which is required for growth and nutritional support of the growing tumor mass. VEGF has two functions in these processes. First, it shows a paracrine angiogenic activity and secondly a mitogenic autocrine activity observed. This is important for the understanding of its central function in PDAC growth promotion. The quantity of angiogenesis can be measured by microvascular density (MVD). Based on these considerations, it is not surprising that both high VEGF expression and high MVD count as a surrogate parameter for VEGF release and are clinically correlated with advanced tumor stages, a higher incidence of lymph node or distant metastases, and a high risk of early tumor recurrence, all of which are factors for poor prognosis and impaired survival in PDAC patients (Seo 2000; Itakura et al. 1997; Fujimoto et al. 1998; Khan et al. 2002; Linder et al. 2001; Karademir 2000; Niedergethmann et al. 2002).
As a part of the complex of contributing factors to the development and maintenance of pancreatic tumors, the PDAC microenvironment also plays a central role. The hypovascular structure observed in computed tomography (Megeibow 1992), and the results of intratumoral oxygen tension measurements (Koong et al. 2000) confirm the presence of hypoxia. One factor contributing to this constant hypoxic environment is the response of cancer-associated fibroblasts originating from pancreatic stellate cells and inflammatory cells (Nielsen et al. 2016; Masamune et al. 2008). These cells form a surrounding desmoplastic tissue which leads to gradually decreasing nourishment and oxygen saturation (Vasseur et al. 2010). Consequently, these changes serve as a stimulus for angiogenesis to overcome the shortage in blood and oxygen supply. Through transcription factors like the hypoxia-inducible factors, the hypoxic stimulus is transferred to the level of gene expression. In PDAC, upregulation of HIF-1α mRNA expression is found and is positively correlated to VEGF mRNA (Buchler et al. 2003). Furthermore, the fibrous stroma seems to cause not only a constant intratumoral hypoxia but also a high interstitial pressure interfering with drug delivery, thus resulting in a two-front effect regarding tumor progression and treatment (Provenzano et al. 2012).
Experimental Studies
Vascular endothelial growth factor (VEGF) represents the most important and most intensely described molecule involved in tumor angiogenesis of PDAC (Itakura et al. 1997). Its essential function in tumor growth has been recognized more than 20 years ago (Folkman 1995; McCulloch et al. 1995). In primary treatment-naive PDAC samples derived from surgical patients, Ikeda et al. (1999) could show a proportion of 68% of patients who exhibited VEGF expression and an even higher proportion of 75% for PD-ECGF expression. VEGF expression correlated with an increase in microvessel density, and both of these parameters were significant prognostic factors and showed an association with poorer survival (Karayiannakis et al. 2003).
The effect of VEGF is promoted via vascular endothelial growth factor receptor-2 (VEGFR2) expressed by PDAC cells. In an experimental setting, inhibition of mRNA for this receptor results in a downregulation of receptor expression and can be targeted by small mRNAs interfering with transcription factors including Sp1, Sp3, and Sp4 that are essential for the transactivation of mRNA that express VEGFR2 (Higgins et al. 2006).
The abovementioned second important function of VEGF as a mitogenic promoter has been demonstrated in human PDAC cell lines by an overexpression not only of VEGF and VEGFR but also of their corresponding kinase mediators KDR, MAPK, and flt-1 (Itakura et al. 1997; von Marschall et al. 2000; Korc 2003). These effects were found in both tumor cells as well as endothelial cells and induced an uncontrolled growth stimulation explained by this so-called autocrine/paracrine mitogenic loop, which shows the features of a positive feedback regulation induced by VEGF.
Another promoter of VEGF effects is NRP-1, originally described as a ligand for neuronal guiding. The expression of this cofactor is highly pronounced in PDAC. NRP-1 enhances the effects of VEGF on its corresponding kinases (i.e., MAPK) (Parikh et al. 2003). The upregulation of NPR-1 is mediated by EGF and can therefore be targeted by blocking EGF receptors which results in a decrease in VEGF-induced kinase activation. NPR-1 seems to be expressed in PDAC tumor cells alone and not by endothelial cells. It shows a positive feedback with VEGF, also mainly produced by tumor cells, whereas the corresponding VEGFR expression does not seem to be located in the tumor cells, but is focused in endothelial cells, which shows the close interaction between these molecules (Li et al. 2004).
The cell adhesion molecule CD146 (MUC-18, MCAM, Fig. 3) is a most recently identified target in PDAC potentially interfering with tumor angiogenesis. Especially the soluble form of CD146 has been demonstrated to have strong angiogenic effects by boosting endothelial progenitor cells (Stalin et al. 2016a). This effect has been transferred to experimental studies on PDAC and the increased tumor cell expression of CD146 correlated well with elevated soluble CD146. Via the binding protein angiomotin, there have been observed angiogenic as well mitogenic effects, suggesting a dual effect, comparable to that described for VEGF (Stalin et al. 2016b). Moreover, targeting the soluble form by monoclonal antibodies showed a high efficacy in terms of decreased vascularization as well as tumor growth.
Another novel mechanism of angiogenesis in PDAC is the identification of miRNA involved in tube formation and endothelial migration which are major prerequisites for de novo vascularization (Li et al. 2015). Specific miRNA involved in this process have been identified. These specific miRNAs can be inhibited by selective miRNA inhibitors, which might be utilized for therapeutic purposes in the future.
Besides the tumor cells – as described before – the tumor microenvironment is an essential factor in the understanding of PDAC growth, expansion, and dissemination. In an experimental mouse study for further examination, Tsuzuki et al. (2001a) showed that the expression of VEGF seems to be promoted by an orthotopic pancreatic microenvironment. VEGF-neutralizing antibodies seem to have the capacity to inhibit these interactions between the tumor and the microenvironment. Regarding the extracellular matrix, the impact of matrix metalloproteinase 9 (MMP-9) produced by inflammatory cells (stromal granulocytes (PMN)) in the tumor environment seems to play an important role (Bausch et al. 2011). MMP-9 has a direct angiogenic effect and furthermore shows the potential to enhance VEGF-mediated vessel growth. It has consequently been identified to be a possible therapeutic target in an experimental setting. A combined blockade of both, VEGF and MMP-9, in a rat model of PDAC showed a synergistic effect of these factors and underlines the interaction of tumor and surrounding tissue (Hotz et al. 2003). Though a monotherapy with either of these agents resulted in an antitumor effect, this effect was markedly enhanced when both substance were administered simultaneously. This approach shows that interfering with one single substance in the cascade of tumor angiogenesis may not be effective and only targeting a combination of angiogenesis promoters may be successful.
Tumorigenesis and Angiogenesis in pNET
Pathophysiology
PNET are comparatively rare with an overall percentage of 1–2% of all pancreatic tumor entities. The neoplasms arise from pancreatic endocrine tissue, which consists of various types of cells and subsequent endocrine function. In 55% of all cases, the tumors are hormonally active (Fischer et al. 2014) and thus classified by their hormonal function, i.e., gastrinoma, insulinoma, glucagonoma, VIPoma, and somatostatinoma, each of which have their very own specific clinical presentation (Table 1). In pNETs, an expression of hormones that are usually not produced by normal islet cells can be present. Some of these hormones are gastrin, vasoactive intestinal peptide (VIP), serotonin, growth hormone (GH), growth hormone-releasing hormone (GHRH), adrenocorticotropin (ACTH), corticotropin-releasing hormone (CRH), parathyroid hormone-related peptide (PTHrp), parathyroid hormone (PTH), calcitonin, ghrelin, human choriogonadotropin (hCG), or renin (Ro 2013). Consequently 45% of all pNETs present as nonfunctioning tumors. Symptoms in those cases depend solely on location and local tumor mass-related complications (i.e., jaundice in case of compression of the bile duct). More than half of all pNETs, with the exception of insulinomas, are malignant, many of which show a very aggressive presentation (Metz and Jensen 2008). PNETs can develop sporadically or – in approximately 10% of cases – can be part of a familial genetic disease. Syndromal disorders associated with pNET include MEN1, Von Hippel-Lindau syndrome, neurofibromatosis, and tuberculous sclerosis.
Experimental Studies
The most frequent genetic alterations in sporadic pNETs are found in the MEN1 gene, followed by mutations in the alpha thalassemia/mental retardation syndrome, X-linked (ATRX), and death domain-associated protein (DAXX) genes. The nuclear MENIN1 protein, which is encoded by the MEN1 gene, functions as a tumor suppressor in most settings by coordinating chromatin remodeling through transcription regulation. Among other functions, it regulates the expression of cell cycle progression inhibitors, interacts with DNA repair mechanisms, prevents the RAS-promoted activation of the MAPK pathway, might be linked to the Hedgehog pathway (Gurung et al. 2013), and in cases of pregnancy and obesity interestingly promotes proliferation of pancreatic endocrine cells (Karnik et al. 2005; Balogh et al. 2006).
Due to progress in DNA sequencing, recently an increasing amount of mutations in pNETs have been discovered. Right after MEN1, the most common mutations to be found were the alpha thalassemia/mental retardation syndrome, X-linked gene/ATRX), and the death domain-associated protein (DAXX) gene (De Wilde et al. 2012). Physiologically, these proteins are involved in determining histone deposition. Protein expression of these genes is less in pNET with this mutation which seems to play a role in tumor cells gaining “immortality” through the alternative lengthening of telomeres pathway (Heaphy et al. 2011; Capurso et al. 2015).
A higher expression and activity of the mammalian target of rapamycin (mTOR) which is part of a complex pathway called PI3K/Akt/mTOR pathway have been observed. About 14% of sporadic pNETs show mutations in PTEN, TSC2, and PI3K which act upstream of this pathway (Jiao et al. 2011). These mutations in the PI3K/Akt/mTOR pathway have been found to play a role in angiogenesis of pNETs and mTOR functions as a transduction factor that regulates protein translation being associated with cell metabolism, survival, proliferation, and motility (Missiaglia et al. 2010). A correlation of a higher expression of mTOR and p-mTOR with an increased mitotic count, tumor size, staging, vascular invasion, and metastasis has been established.
As endocrine glands need a well-established vascular network for hormone secretion, well-differentiated PNETs are highly vascularized tumors. Thus, they can be distinguished from PDAC by their vascularized appearance in radiological imaging. Interestingly, during tumor progression, a loss of vessel density is observed – a phenomenon called the “neuroendocrine paradox.”
With regard to tumor grading, these pNETs are mostly classified as “well- or moderately differentiated (G1-G2),” which explains the good clinical response to a treatment with angiogenesis inhibitors. Therefore, a high microvascular density is – in contrast to findings in many other cancers – associated with a favorable prognosis (Couvelard et al. 2005; Takahashi et al. 2007). In contrast, the low vascular density in poorly differentiated pNETs promotes tumor hypoxia and consequently an angiogenic switch characterized by upregulation of proangiogenic factors and increased endothelial cell proliferation. This results in abnormal vascular architecture similar to PDAC findings and therefore in a poor response to anti-angiogenics (Couvelard et al. 2008). To a degree comparable to PDAC tumorigenesis, factors involved in pNET angiogenesis are vascular endothelial growth factor (VEGF) and its receptor (VEGFR) as well as the platelet-derived endothelial growth factor (PDGF) and fibroblast growth factor (FGF)(Corbo et al. 2012). VEGF-A as one of the most potent factors for angiogenesis along with other VEGF family members plays a role not only in the highly vascularized character of pNET but also in the development of normal pancreatic endocrine cells. This factor is thought to be also part of the switch from normal pancreatic tissues to pNET and reversely can be blocked by anti-angiogenic agents (Bergers 1999; Hanahan et al. 1996).
Clinical Studies
Clinical studies on angiogenesis inhibition have been performed in recent years for PDAC (Table 2) as well as pNET (Table 3) with various observations regarding the efficacy of this approach in terms of tumor response and patient survival for both entities. In most of the studies for PDAC, these approaches have been combined with a standard chemotherapy regimen (i.e., gemcitabine) whereas in pNET, mainly single-drug approaches have been chosen as no established chemotherapy exists to date.
PDAC
Vaccination Studies
The mechanism of vaccination as an immunotherapy using an epitope peptide for VEGRF2 as an essential factor for tumor angiogenesis has been investigated in a phase 1 study combined with gemcitabine in a palliative patient collective (Miyazawa et al. 2010). Eighteenth patients were exposed to the vaccination peptide (subcutaneously) in a three-level dose-escalation protocol (n = 6 patients per group) to assess safety and immunological effects of this treatment. In 67% of the patients, a temporary disease control was observed resulting in 8.7 months of median overall survival. Under medium-level adverse events, all dosages were tolerated and a successful induction of specific cytotoxic T-lymphocytes was achieved in 61% of the study population. A similar phase I approach investigated an oral vaccine for VEGRF2 using a salmonella bacteria-based expression plasmid encoding VEGFR2 in advanced PDAC in combination with gemcitabine (Schmitz-Winnenthal et al. 2015). Although only 3-month observation data are available from this study, the high-dose vaccination resulted in a specific immune response on T effector cells and a consequent reduction of tumor perfusion as well an increase of the anti-angiogenic markers VEGF-A and collagen IV. No relevant adverse effects occurred which underlines the safety of this potentially effective vaccination approach.
The VEGFR2- derived epitope peptide elpamotide represented another approach to target tumor angiogenesis by vaccination. It was tested for advanced PDAC on the hypothesis that induction of cytotoxic T-lymphocytes directed against endothelial cells producing VEGFR leads to a reduction of angiogenesis (Wada et al. 2005). A phase I study combining elpamotide with gemcitabine showed a prolonged survival time of 8.7 months when compared to a gemcitabine monotherapy with 5.7 months (Miyazawa et al. 2010). This effect, however, could not be reproduced in a following RCT, where survival times remained unchanged (8.5. and 8.4 months, respectively) regardless of the addition of elpamotide (Yamaue et al. 2015).
Interferon Alpha
IFN-α as an immunomodulatory substance has been shown to have anti-angiogenic effects and a direct impairment of endothelial cell proliferation and migration (Zhu et al. 2008; Indraccolo 2010). The effects of IFN-α on the vasculature have been mainly attributed to inhibition of VEGF gene expression and downregulation of tumor-cell-derived fibroblast growth factor production as well as downregulation of IL-8. The gene expression profile induced by IFN-α in EC has recently been defined, and it was found that several genes encoding negative regulators of angiogenesis are upmodulated thus providing a potential amplification mechanism for this biological activity.
IFN-α has been clinically tested in an adjuvant setting after potentially curative resection of PDAC in several studies. An initial study on 17 resected PDAC patients who received a combination therapy of chemoradiation and IFN-α showed a striking 84% 2-year survival compared to 54% in a control group of 16 patients who received the protocol without IFN-α (Nukui et al. 2000). In the follow-up observation, a 5-year survival of 55% and an actual 10-year survival of 20.1% were observed (Picozzi et al. 2003; Rocha et al. 2016). Despite these promising observational data, the results of the protocol were not confirmed in a phase III RCT which showed similar survival for patients treated with or without IFN-α in an adjuvant setting (Knaebel et al. 2005; Märten et al. 2010).
Antibodies and Targeted Proteins
Bevacizumab is a monoclonal antibody-binding VEGF and approved for therapy in various solid tumors including colorectal, lung, breast, and renal cancer. In advanced PDAC, bevacizumab combined with gemcitabine was tested in an early phase II trial with 52 patients (Kindler et al. 2005). Based on the results of this study with a partial response or stable disease in 67% of the patients and a median survival of 8.8 months, further phase III studies were conducted. In a large RCT comparing bevacizumab and gemcitabine versus gemcitabine alone, 535 patients were included to confirm the results of the phase I trial (Kindler et al. 2010). Despite the encouraging results observed in the pilot setting, this RCT failed to confirm the efficacy of bevacizumab, and the addition of this antibody resulted in 5.8 months median survival compared to 5.9 months in the control arm and an increase rate of adverse events. In addition, bevacizumab was not beneficial when added to a combination therapy of gemcitabine and the tyrosine kinase inhibitor erlotinib in another RCT including 301 and 306 patients, respectively (Van Cutsem et al. 2009). Consequently, PADC therapy with bevacizumab has been omitted in recent years.
Aflibercept represents an anti-angiogenic fusion protein with antibody properties targeting and inactivating VEGF. Adopted from ocular vascular proliferative diseases, this protein was congruently tested in PDAC in a RCT with the inclusion of 546 patients (Rougier et al. 2013) as an addition to gemcitabine but failed to increase progression-free or overall survival and was in addition burdened by an increased rate of adverse events which finally led to a premature study termination and the omission of this approach.
Axitinib as an oral, potent, and selective VEGFR inhibitor (Hu-Lowe et al. 2008) had shown promising results in a randomized phase II study of 103 patients with locally advanced and metastatic PDAC with an improvement in median overall survival and a greater 1-year survival when combined with gemcitabine versus gemcitabine alone (Spano et al. 2008). Based on these results, it was tested in a larger phase III study on 632 patients in a RCT setting (Kindler et al. 2011). This study failed to confirm the benefit and showed similar survival for both patient groups and increased adverse events.
Kinase Inhibitors
The EGF receptor selective tyrosine kinase inhibitor erlotinib as another approach aiming at angiogenesis inhibition in PDAC was introduced in the palliative setting after a phase II RCT including 569 patients (Moore et al. 2007). However, though statistically significant, the combination of erlotinib with gemcitabine added 0.3 months to the median survival when compared to gemcitabine alone. Although the overall effect of erlotinib was disappointing, the clinical observation showed that a subgroup of patients had a much more pronounced survival benefit when a significant cutaneous rash occurred. To further elucidate this unexpected observation, an analysis of the KRAS and EGFR gene mutation status was performed under the hypothesis of specific genetic variants determining the response to erlotinib (da Cunha et al. 2010). No specific prognostically significant mutation could be identified in this study. A retrospective tissue analysis from another study showed that KRAS wild-type patients had the best prognosis when treated with erlotinib (Boeck et al. 2013). A valuable pre-therapeutic marker to define this subgroup has not yet been established, and the impact of erlotinib has rapidly decreased in the clinical setting today.
Sorafenib is a multi-targeted protein kinase inhibitor directed at VEGFR2 and 3 as well as PDGF and RAF kinase and shows anti-angiogenic properties in addition to antiproliferative effects (Wilhelm et al. 2004). Based on experimental data, a phase I study was conducted which showed stable disease in 57% of PDAC patients when combined with gemcitabine (Siu et al. 2006). A consecutive phase III study could not confirm these observations in a 104-patient collective (Gonçalves et al. 2012). In this RCT, neither response rates nor progression-free or overall survival (9.2 vs. 8 months, respectively) showed a superiority of sorafenib in comparison to gemcitabine monotherapy.
Sunitinib is another multi-targeted kinase inhibitor aiming at VEGFR, PDGFR, KIT, and Flt-3 receptors that are overexpressed in PDAC and therefore represent a therapeutic aim. An RCT investigated its effect as a maintenance therapy in 55 PDAC patients after 6 months of an initial chemotherapy followed by 3 months of sunitinib application (Reni et al. 2013). The anti-angiogenic therapy resulted in an improvement of progression-free survival (22.2% vs. 3.6%) and more patients with stable disease (51.9% vs. 21.4%). Although not statistically significant, overall 2-year survival showed promising outcomes as well (22.9% vs. 7.1%) which might qualify this approach of maintenance therapy for further clinical application in the future.
An important clinical aspect with regard to both, sorafenib and sunitinib, is the observation of drug-related mortality. A current meta-analysis on this adverse effect including more than 14,000 patients from 41 studies on tyrosine kinase inhibitors for various solid tumors could show a 1.9% risk of treatment-related death (Hong et al. 2014). Especially when combined with chemotherapy, the risk for cardiovascular failure or thromboembolic events may be increased by tyrosine kinase inhibitors which this must be carefully weighed against the benefit of these drugs.
Overall, the pathway of angiogenesis inhibition in clinical PDAC therapy has led to mainly disappointing results with regard to approaches using antibodies or targeted proteins except for tyrosine kinase inhibitors that seem to be useful for selected subgroups of patients. The approach of active vaccination may be promising but needs to be evaluated in further phase II and III studies.
PNET
Kinase Inhibitors
In contrast to PDAC, in pNET therapy, sunitinib therapy has gained a much more important significance during the last decade. In an initial phase I study including 28 patients with various malignancies, a potent antitumor activity under sunitinib therapy was shown, characterized by radiological response and especially the development of intratumoral necrosis which underlines the anti-angiogenic effect with a consecutive decrease of vascularization which could be confirmed by imaging modalities in this study (Faivre et al. 2006).
Based on the clinical benefit observed in this study, a consecutive phase II trial on pNET was conducted (Kulke et al. 2008). In 66 patients with advanced pNET, the objective response rate was 16.7% with 56.1% of patients showing stable disease for more than 6 months with a 1-year survival of 81.1% without relevant clinical side effects. To evaluate these observations in a phase III, a large international double-blind RCT compared sunitinib to placebo in patients with well-differentiated PNET who had radiological evidence of tumor progression (Raymond et al. 2011). The trial was designed to show a 50% improvement of progression-free survival, and 340 patients were intended for inclusion on this statistical basis. As a higher occurrence of deaths and serious adverse events in patients receiving placebo was observed, the trail was stopped earlier. At that point of time, progression-free survival in patients receiving sunitinib was more than double the progression-free survival in the placebo group. In addition, an improved overall survival as a secondary end point provided additional evidence of the efficacy of sunitinib in pNET therapy. Similar to the radiological observations in the phase 2 study, patients showed a high proportion of changes toward hypodense lesions in CT scans of the primary tumor as well as liver metastases which can be regarded as tumor necrosis and underlines the mechanisms of antitumor activity on the basis of anti-angiogenesis.
On the basis of these data, FDA and EMA approvals have been obtained for sunitinib in advanced pNET, and the current ENETS guidelines have included the recommendation for this therapy as a second- or third-line therapy and also allow the consideration of this approach in the first-line setting when an alternative treatment with somatostatin analogues, chemotherapy, and/or locoregional therapies are not feasible or promising (Falconi et al. 2016).
The efficacy of sunitinib in advanced pNET appears to be similar regardless of preceding chemotherapy or somatostatin analogue treatment which underlines the impact of anti-angiogenesis as a specific and successful therapeutic approach in the distinct tumor entity.
Future Directions
To improve the outcome of patients suffering from PDAC as a highly lethal disease, an interdisciplinary approach is necessary to improve screening tools and develop potentially new diagnostic methods for early detection as well as innovative systemic therapies, including approaches of targeted and personalized oncological therapy. Anti-angiogenic therapies as targeted approaches currently show a diverse range of results in PDAC depending on individual patient factors and supposed subtypes of cancer, which are not completely defined to date. Though promising experimental and phase I studies in PDAC suggest a potential role of anti-angiogenics, phase II/III study has failed to show a significant impact on disease control or survival for all patient, and only subcollectives of patients may benefit from such approaches. Thus, new directions, including vaccination, immunomodulation, or specific anti-angiogenic antibodies have to be adopted to meet individual patient characteristics. This adoption could be based on specific genetic profiling (i.e., as shown for the kinase inhibitor erlotinib (da Cunha et al. 2010) or for the efficacy of adjuvant chemotherapy in the ESPAC trials (Greenhalf et al. 2014)). However, these specific approaches are not introduced into clinical routine, and more research needs to be conducted to determine respective subgroups and thereby improve efficacy of individual screening and personalized cancer therapy in which future anti-angiogenic approaches could play an important role.
References
Ahmed MM, Alcock RA, Chendil D, Dey S, Das A, Venkatasubbarao K, Mohiuddin M, Sun L, Strodel WE, Freeman JW (2002) Restoration of transforming growth factor-beta signaling enhances radiosensitivity by altering the Bcl-2/Bax ratio in the p53 mutant pancreatic cancer cell line MIA PaCa-2. J Biol Chem 277(3):2234–2246
Balogh K, Rácz K, Patócs A, Hunyady L (2006) Menin and its interacting proteins: elucidation of menin function. Trends Endocrinol Metab 17:357–364
Bangard C, Gossmann A, Papyan A, Tawadros S, Hellmich M, Bruns CJ (2005) Magnetic resonance imaging in an orthotopic rat model: blockade of epidermal growth factor receptor with EMD72000 inhibits human pancreatic carcinoma growth. Int J Cancer 114(1):131–138
Bardeesy N, Morgan J, Sinha M, Signoretti S, Srivastava S, Loda M, Merlino G, DePinho RA (2002) Obligate roles for p16(Ink4a) and p19(Arf)-p53 in the suppression of murine pancreatic neoplasia. Mol Cell Biol 22(2):635–643
Bausch D, Pausch T, Krauss T, Hopt UT, Fernandez-del-Castillo C, Warshaw AL, Thayer SP, Keck T (2011) Neutrophil granulocyte derived MMP-9 is a VEGF independent functional component of the angiogenic switch in pancreatic ductal adenocarcinoma. Angiogenesis 14(3):235–243
Bergers G (1999) Effects of angiogenesis inhibitors on multistage carcinogenesis in mice. Science 284:808–812
Boeck S, Jung A, Laubender RP, Neumann J, Egg R, Goritschan C, Vehling-Kaiser U, Winkelmann C, Fischer von Weikersthal L, Clemens MR, Gauler TC, Märten A, Klein S, Kojouharoff G, Barner M, Geissler M, Greten TF, Mansmann U, Kirchner T, Heinemann V (2013) EGFR pathway biomarkers in erlotinib-treated patients with advanced pancreatic cancer: translational results from the randomised, crossover phase 3 trial AIO-PK0104. Br J Cancer 108(2):469–476
Brosens LA, Hackeng WM, Offerhaus GJ, Hruban RH, Wood LD (2015) Pancreatic adenocarcinoma pathology: changing “landscape”. J Gastrointest Oncol 6(4):358–374
Buchler P, Reber HA, Büchler M, Shrinkante S, Büchler MW, Friess H, Semenza GL, Hines OJ (2003) HIF-1 alpha and VEGF in pancreatic cancer. Pancreas 26:1–5
Capurso G, Archibugi L, Delle FG (2015) Molecular pathogenesis and targeted therapy of sporadic pancreatic neuroendocrine tumors. J Hepatobiliary Pancreat Sci 22:594–601
Chen CH, Yang CC, Yeh YH, Huang MH (2004) Contrast-enhanced power Doppler sonography of ductal pancreatic adenocarcinomas: correlation with digital subtraction angiography findings. J Clin Ultrasound 32(4):179–185
Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M, Groupe Tumeurs Digestives of Unicancer, PRODIGE Intergroup (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364(19):1817–1825
Corbo V, Beghelli S, Bersani S, Antonello D, Talamini G, Brunelli M, Capelli P, Falconi M, Scarpa A (2012) Pancreatic endocrine tumours: mutational and immunohistochemical survey of protein kinases reveals alterations in targetable kinases in cancer cell lines and rare primaries. Ann Oncol 23:127–134
Couvelard A, O’Toole D, Turley H, Leek R, Sauvanet A, Degott C, Ruszniewski P, Belghiti J, Harris AL, Gatter K, Pezzella F (2005) Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: negative correlation of microvascular density and VEGF expression with tumour progression. Br J Cancer 92:94–101
Couvelard A, Deschamps L, Rebours V, Sauvanet A, Gatter K, Pezzella F, Ruszniewski P, Bedossa P (2008) Overexpression of the oxygen sensors PHD-1, PHD-2, PHD-3, and FIH is associated with tumor aggressiveness in pancreatic endocrine tumors. Clin Cancer Res 14:6634–6639
Craven KE, Gore J, Korc M (2016) Overview of pre-clinical and clinical studies targeting angiogenesis in pancreatic ductal adenocarcinoma. Cancer Lett. pii: S0304-3835(15)007429 2016 Oct 10;381(1):201–10
da Cunha SG, Dhani N, Tu D, Chin K, Ludkovski O, Kamel-Reid S, Squire J, Parulekar W, Moore MJ, Tsao MS (2010) Molecular predictors of outcome in a phase 3 study of gemcitabine and erlotinib therapy in patients with advanced pancreatic cancer: National Cancer Institute of Canada Clinical Trials Group study PA.3. Cancer 116(24):5599–5607
De Wilde RF, Edil BH, Hruban RH, Maitra A (2012) Well-differentiated pancreatic neuroendocrine tumours: from genetics to therapy. Nat Rev Gastroenterol Hepatol 9:199–208
Deshpande N, Ren Y, Foygel K, Rosenberg J, Willmann JK (2011) Tumor angiogenic marker expression levels during tumor growth: longitudinal assessment with molecularly targeted microbubbles and US imaging. Radiology 258(3):804–811. https://doi.org/10.1148/radiol.10101079
Dyrla P, Lubas A, Gil J, Niemczyk S (2016) Doppler tissue perfusion parameters in recognizing pancreatic malignant tumors. J Gastroenterol Hepatol 31(3):691–695
Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, Friess H (2012) The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol 9(8):454–467
Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, Armand JP, Scigalla P, Raymond E (2006) Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 24:25–35
Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G, Klöppel G, Reed N, Kianmanesh R, Jensen RT (2016) All other Vienna consensus conference participants. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology 103(2):153–171
Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA (2012) The pancreas cancer microenvironment. Clin Cancer Res: Off J Am Assoc Cancer Res 18(16):4266–4276
Figueiredo FA, da Silva PM, Monges G, Bories E, Pesenti C, Caillol F, Delpero JR, Giovannini M (2012) Yield of contrast-enhanced power Doppler endoscopic ultrasonography and strain ratio obtained by EUS-elastography in the diagnosis of focal pancreatic solid lesions. Endosc Ultrasound 1(3):143–149
Fischer L, Bergmann F, Schimmack S, Hinz U, Prieß S, Müller-Stich BP, Werner J, Hackert T, Büchler MW (2014) Outcome of surgery for pancreatic neuroendocrine neoplasms. Br J Surg 101(11):1405–1412
Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1:27–31
Foygel K, Wang H, Machtaler S, Lutz AM, Chen R, Pysz M, Lowe AW, Tian L, Carrigan T, Brentnall TA, Willmann JK (2013) Detection of pancreatic ductal adenocarcinoma in mice by ultrasound imaging of thymocyte differentiation antigen 1. Gastroenterology 145(4):885–894.e3
Fritz F, Skornitzke S, Hackert T, Kauczor HU, Stiller W, Grenacher L, Klauss M (2016) Dual-energy perfusion-CT in recurrent pancreatic cancer – preliminary results. Röfo 188(6):559–565
Fujimoto K, Hosotani R, Wada M, Lee JU, Koshiba T, Miyamoto Y, Tsuji S, Nakajima S, Doi R, Imamura M (1998) Expression of two angiogenic factors, vascular endothelial growth factor and platelet-derived endothelial cell growth factor in human pancreatic cancer, and its relationship to angiogenesis. Eur J Cancer 34(9):1439–1447
Gincul R, Palazzo M, Pujol B, Tubach F, Palazzo L, Lefort C, Fumex F, Lombard A, Ribeiro D, Fabre M, Hervieu V, Labadie M, Ponchon T, Napoléon B (2014) Contrast-harmonic endoscopic ultrasound for the diagnosis of pancreatic adenocarcinoma: a prospective multicenter trial. Endoscopy 46(5):373–379
Gonçalves A, Gilabert M, François E, Dahan L, Perrier H, Lamy R, Re D, Largillier R, Gasmi M, Tchiknavorian X, Esterni B, Genre D, Moureau-Zabotto L, Giovannini M, Seitz JF, Delpero JR, Turrini O, Viens P, Raoul JL (2012) BAYPAN study: a double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Ann Oncol 23(11):2799–2805
Greenhalf W, Ghaneh P, Neoptolemos JP, Palmer DH, Cox TF, Lamb RF, Garner E, Campbell F, Mackey JR, Costello E, Moore MJ, Valle JW, AC MD, Carter R, Tebbutt NC, Goldstein D, Shannon J, Dervenis C, Glimelius B, Deakin M, Charnley RM, Lacaine F, Scarfe AG, Middleton MR, Anthoney A, Halloran CM, Mayerle J, Oláh A, Jackson R, Rawcliffe CL, Scarpa A, Bassi C, Büchler MW, European Study Group for Pancreatic Cancer (2014) Pancreatic cancer hENT1 expression and survival from gemcitabine in patients from the ESPAC-3 trial. J Natl Cancer Inst 106(1):djt347
Gurung B, Feng Z, Iwamoto DV, Thiel A, Jin G, Fan CM, Ng JM, Curran T, Hua X (2013) MENIN epigenetically represses hedgehog signaling in MEN1 tumour syndrome. Cancer Res 73:2650–2658
Hackert T, Büchler MW (2013) Pancreatic cancer: advances in treatment, results and limitations. Dig Dis 31(1):51–56
Hanahan D, Folkman J (1996) Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86(3):353–364
Hanahan D, Christofori G, Naik P, Arbeit J (1996) Transgenic mouse models of tumour angiogenesis: the angiogenic switch, its molecular controls, and prospects for preclinical therapeutic models. Eur J Cancer 86:2386–2393
Heaphy CM, de Wilde RF, Jiao Y, Klein AP, Edil BH, Shi C, Bettegowda C, Rodriguez FJ, Eberhart CG, Hebbar S, Offerhaus GJ, McLendon R, Rasheed BA, He Y, Yan H, Bigner DD, Oba-Shinjo SM, Marie SK, Riggins GJ, Kinzler KW, Vogelstein B, Hruban RH, Maitra A, Papadopoulos N, Meeker AK (2011) Altered telomeres in tumours with ATRX and DAXX mutations. Science 333:425
Higgins KJ, Abdelrahim M, Liu S, Yoon K, Safe S (2006) Regulation of vascular endothelial growth factor receptor-2 expression in pancreatic cancer cells by Sp proteins. Biochem Biophys Res Commun 345(1):292–301
Hocke M, Dietrich CF (2012) Vascularisation pattern of chronic pancreatitis compared with pancreatic carcinoma: results from contrast-enhanced endoscopic ultrasound. Int J Inflamm 2012:420787
Hong S, Fang W, Liang W, Yan Y, Zhou T, Qin T, Wu X, Ma Y, Zhao Y, Yang Y, Hu Z, Xue C, Hou X, Chen Y, Huang Y, Zhao H, Zhang L (2014) Risk of treatment-related deaths with vascular endothelial growth factor receptor tyrosine kinase inhibitors: a meta-analysis of 41 randomized controlled trials. Oncol Targets Ther 7:1851–1867
Hotz HG, Hines OJ, Hotz B, Foitzik T, Buhr HJ, Reber HA (2003) Evaluation of vascular endothelial growth factor blockade and matrix metalloproteinase inhibition as a combination therapy for experimental human pancreatic cancer. J Gastrointest Surg 7(2):220–227; discussion 227–228
Hu-Lowe DD, Zou HY, Grazzini ML et al (2008) Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res 14:7272–7283
Ikeda N, Adachi M, Taki T, Huang C, Hashida H, Takabayashi A, Sho M, Nakajima Y, Kanehiro H, Hisanaga M, Nakano H, Miyake M (1999) Prognostic significance of angiogenesis in human pancreatic cancer. Br J Cancer 79(9/10):1553–1563
Indraccolo S (2010) Interferon-alpha as angiogenesis inhibitor: learning from tumor models. Autoimmunity 43(3):244–247
Iordache S, Angelescu R, Filip MM, Costache MI, Popescu CF, Gheonea DI, Sãftoiu A (2012) Power Doppler endoscopic ultrasound for the assessment of pancreatic neuroendocrine tumors. Endosc Ultrasound 1(3):150–155
Itakura J, Ishiwata T, Friess H, Fujii H, Matsumoto, Büchler MW, Korc M (1997) Enhanced expression of vascular endothelial growth factor in human pancreatic cancer correlates with local disease progression. Clin Cancer Res 3:1309–1316
Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, Schulick RD, Tang LH, Wolfgang CL, Choti MA, Velculescu VE, Diaz LA Jr, Vogelstein B, Kinzler KW, Hruban RH, Papadopoulos N (2011) DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 331(6021):1199–1203
Jonson T, Albrechtsson E, Axelson J, Heidenblad M, Gorunova L, Johansson B, Höglund M (2001) Altered expression of TGFB receptors and mitogenic effects of TGFB in pancreatic carcinomas. Int J Oncol 19(1):71–81
Karademir S (2000) Tumour angiogenesis as a prognostic predictor in pancreatic cancer. J Hepatobiliary Pancreat Surg 7:489–495
Karayiannakis AJ, Bolanaki H, Syrigos KN, Asimakopoulos B, Polychronidis A, Anagnostoulis S, Simopoulos C (2003) Serum vascular endothelial growth factor levels in pancreatic cancer patients correlate with advanced and metastatic disease and poor prognosis. Cancer Lett 194(1):119–124
Karnik SK, Hughes CM, Gu X, Rozenblatt-Rosen O, McLean GW, Xiong Y, Meyerson M, Kim SK (2005) Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c. Proc Natl Acad Sci U S A 102:14659–14664
Khan AW, Dhillon AP, Hutchins R, Abraham A, Shah SR, Snooks S, Davidson BR (2002) Prognostic significance of intratumoural microvessel density (IMD) in resected pancreatic and ampullary cancers to standard histopathological variables and survival. Eur J Surg Oncol 28:637–644
Kindler HL, Friberg G, Singh DA, Locker G, Nattam S, Kozloff M, Taber DA, Karrison T, Dachman A, Stadler WM, Vokes EE (2005) Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol 23(31):8033–8040
Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O’Reilly E, Wozniak TF, Picus J, Bhargava P, Mayer RJ, Schilsky RL, Goldberg RM (2010) Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 28(22):3617–3622
Kindler HL, Ioka T, Richel DJ, Bennouna J, Létourneau R, Okusaka T, Funakoshi A, Furuse J, Park YS, Ohkawa S, Springett GM, Wasan HS, Trask PC, Bycott P, Ricart AD, Kim S, Van Cutsem E (2011) Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol 12(3):256–262
Klauss M, Stiller W, Fritz F, Kieser M, Werner J, Kauczor HU, Grenacher L (2012) Computed tomography perfusion analysis of pancreatic carcinoma. J Comput Assist Tomogr 36(2):237–242
Klauss M, Gaida MM, Lemke A, Grünberg K, Simon D, Wente MN, Delorme S, Kauczor HU, Grenacher L, Stieltjes B (2013) Fibrosis and pancreatic lesions: counterintuitive behavior of the diffusion imaging-derived structural diffusion coefficient d. Investig Radiol 48(3):129–133
Klauß M, Maier-Hein K, Tjaden C, Hackert T, Grenacher L, Stieltjes B (2016) IVIM DW-MRI of autoimmune pancreatitis: therapy monitoring and differentiation from pancreatic cancer. Eur Radiol 26(7):2099–2106
Knaebel HP, Märten A, Schmidt J, Hoffmann K, Seiler C, Lindel K, Schmitz-Winnenthal H, Fritz S, Herrmann T, Goldschmidt H, Krempien R, Mansmann U, Debus J, Diehl V, Büchler MW (2005) Phase III trial of postoperative cisplatin, interferon alpha-2b, and 5-FU combined with external radiation treatment versus 5-FU alone for patients with resected pancreatic adenocarcinoma – CapRI: study protocol [ISRCTN62866759]. BMC Cancer 5:37
Kobayashi G, Fujita N, Noda Y, Ito K, Horaguchi J, Koshida S, Kanno Y, Ogawa T, Masu K, Michikawa Y (2014) Vascular image in autoimmune pancreatitis by contrast-enhanced color-Doppler endoscopic ultrasonography: comparison with pancreatic cancer. Endosc Ultrasound 3(Suppl 1):S13
Koong AC, Mehta VK, Le QT et al (2000) Pancreatic tumours show high levels of hypoxia. Int J Radiat OncolBiol Phys 48:919–922
Korc M (2003) Pathways for aberrant angiogenesis in pancreatic cancer. Mol Cancer 2:8
Kulke MH, Lenz HJ, Meropol NJ, Posey J, Ryan DP, Picus J, Bergsland E, Stuart K, Tye L, Huang X, Li JZ, Baum CM, Fuchs CS (2008) Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol 26(20):3403–3410
Lemke A, Laun FB, Klauss M, Re TJ, Simon D, Delorme S, Schad LR, Stieltjes B (2009) Differentiation of pancreas carcinoma from healthy pancreatic tissue using multiple b-values: comparison of apparent diffusion coefficient and intravoxel incoherent motion derived parameters. Investig Radiol 44(12):769–775
Li M, Yang H, Chai H, Fisher WE, Wang X, Brunicardi FC, Yao Q, Chen C (2004) Pancreatic carcinoma cells express neuropilins and vascular endothelial growth factor, but not vascular endothelial growth factor receptors. Cancer 101(10):2341–2350
Li L, Li B, Chen D, Liu L, Huang C, Lu Z, Lun L, Wan X (2015) miR-139 and miR-200c regulate pancreatic cancer endothelial cell migration and angiogenesis. Oncol Rep 34(1):51–58
Linder S, Blåsjö M, von Rosen A, Parrado C, Falkmer UG, Falkmer S (2001) Pattern of distribution and prognostic value of angiogenesis in pancreatic duct carcinoma, a semiquantitive immunohistochemical study of 45 patients. Pancreas 22:240–247
Märten A, Schmidt J, Debus J, Harig S, Lindel K, Klein J, Bartsch DK, Capussotti L, Zülke C, Büchler MW (2010) CapRI: final results of the open-label, multicenter, randomized phase III trial of adjuvant chemoradiation plus interferon-2b (CRI) versus 5-FU alone for patients with resected pancreatic adenocarcinoma (PAC). J Clin Oncol 28:18s. (suppl; abstr LBA4012)
Masamune A, Kikuta K, Watanabe T, Satoh K, Hirota M, Shimosegawa T (2008) Hypoxia stimulates pancreatic stellate cells to induce fibrosis and angiogenesis in pancreatic cancer. Am J Physiol Gastrointest Liver Physiol 295(4):G709–G717
McCulloch P, Choy A, Martin L (1995) Association between tumor angiogenesis and tumor cell shedding into effluent venous blood during breast cancer surgery. Lancet 346:1334–1335
Megeibow AJ (1992) Pancreatic adenocarcinoma: designing the examination to evaluate the clinical questions. Radiology 183:297–303
Metz DC, Jensen RT (2008) Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology 135:1469–1492
Missiaglia E, Dalai I, Barbi S, Beghelli S, Falconi M, della Peruta M, Piemonti L, Capurso G, Di Florio A, delle Fave G, Pederzoli P, Croce CM, Scarpa A (2010) Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. J Clin Oncol 28(2):245–255
Miyazawa M, Ohsawa R, Tsunoda T, Hirono S, Kawai M, Tani M, Nakamura Y, Yamaue H (2010) Phase I clinical trial using peptide vaccine for human vascular endothelial growth factor receptor 2 in combination with gemcitabine for patients with advanced pancreatic cancer. Cancer Sci 101(2):433–439
Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W, National Cancer Institute of Canada Clinical Trials Group (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25(15):1960–1966
Niedergethmann M, Hildenbrand R, Wostbrock B, Hartel M, Sturm JW, Richter A, Post S (2002) High expression of vascular endothelial growth factor predicts early recurrence and poor prognosis after curative resection for ductal adenocarcinoma of the pancreas. Pancreas 25(2):122–129
Nielsen MF, Mortensen MB, Detlefsen S (2016) Key players in pancreatic cancer-stroma interaction: cancer-associated fibroblasts, endothelial and inflammatory cells. World J Gastroenterol 22(9):2678–2700
Nishida M, Koito K, Hirokawa N, Hori M, Satoh T, Hareyama M (2009) Does contrast-enhanced ultrasound reveal tumor angiogenesis in pancreatic ductal carcinoma? A prospective study. Ultrasound Med Biol 35(2):175–185
Nukui Y, Picozzi VJ, Traverso LW (2000) Interferon-based adjuvant chemoradiation therapy improves survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg 179(5):367–371
Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, Sinn M, Hinke A, Riess H (2013) Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 310(14):1473–1481
Parikh AA, Liu WB, Fan F, Stoeltzing O, Reinmuth N, Bruns CJ, Bucana CD, Evans DB, Ellis LM (2003) Expression and regulation of the novel vascular endothelial growth factor receptor neuropilin-1 by epidermal growth factor in human pancreatic carcinoma. Cancer 98(4):720–729
Picozzi VJ, Kozarek RA, Traverso LW (2003) Interferon-based adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg 185(5):476–480
Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR (2012) Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21:418–429
Pysz MA, Machtaler SB, Seeley ES, Lee JJ, Brentnall TA, Rosenberg J, Tranquart F, Willmann JK (2015) Vascular endothelial growth factor receptor type 2-targeted contrast-enhanced US of pancreatic cancer neovasculature in a genetically engineered mouse model: potential for earlier detection. Radiology 274(3):790–799
Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Hörsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P (2011) Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 364:501–513
Reni M, Cereda S, Milella M, Novarino A, Passardi A, Mambrini A, Di Lucca G, Aprile G, Belli C, Danova M, Bergamo F, Franceschi E, Fugazza C, Ceraulo D, Villa E (2013) Maintenance sunitinib or observation in metastatic pancreatic adenocarcinoma: a phase II randomised trial. Eur J Cancer 49(17):3609–3615
Ro C (2013) Pancreatic neuroendocrine tumors: biology, diagnosis and treatment. Chin J Cancer 32(6):312–324
Rocha FG, Hashimoto Y, Traverso LW, Dorer R, Kozarek R, Helton WS, Picozzi VJ (2016) Interferon-based adjuvant chemoradiation for resected pancreatic head cancer: long-term follow-up of the Virginia Mason protocol. Ann Surg 263(2):376–384
Rougier P, Riess H, Manges R, Karasek P, Humblet Y, Barone C, Santoro A, Assadourian S, Hatteville L, Philip PA (2013) Randomised, placebo-controlled, double-blind, parallel-group phase III study evaluating aflibercept in patients receiving first-line treatment with gemcitabine for metastatic pancreatic cancer. Eur J Cancer 49(12):2633–2642
Ryan DP, Hong TS, Bardeesy N (2014) Pancreatic adenocarcinoma. N Engl J Med 371(11):1039–1049
Ryschich E, Schmidt E, Maksan SM, Klar E, Schmidt J (2004) Expansion of endothelial surface by an increase of vessel diameter during tumor angiogenesis in experimental and hepatocellular and pancreatic cancer. World J Gastroenterol 10(21):3171–3174
Schmidt J, Ryschich E, Daniel V, Herzog L, Werner J, Herfarth C, Longnecker DS, Gebhard MM, Klar E (2000) Vascular structure and microcirculation of experimental pancreatic carcinoma in rats. Eur J Surg 166(4):328–335
Schmitz-Winnenthal FH, Hohmann N, Niethammer AG, Friedrich T, Lubenau H, Springer M, Breiner KM, Mikus G, Weitz J, Ulrich A, Buechler MW, Pianka F, Klaiber U, Diener M, Leowardi C, Schimmack S, Sisic L, Keller AV, Koc R, Springfeld C, Knebel P, Schmidt T, Ge Y, Bucur M, Stamova S, Podola L, Haefeli WE, Grenacher L, Beckhove P (2015) Anti-angiogenic activity of VXM01, an oral T-cell vaccine against VEGF receptor 2, in patients with advanced pancreatic cancer: a randomized, placebo-controlled, phase 1 trial. Oncoimmunology 4(4):e1001217. eCollection Apr 2015
Scialpi M, Midiri M, Bartolotta TV, Cazzolla MP, Rotondo A, Resta MC, Lagalla R, Cardinale AE (2005) Pancreatic carcinoma versus chronic focal pancreatitis: contrast-enhanced power Doppler ultrasonography findings. Abdom Imaging 30(2):222–227
Seo Y (2000) High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer 88(10):2239–2245
Seufferlein T, Bachet JB, Van Cutsem E, Rougier P, ESMO Guidelines Working Group (2012) Pancreatic adenocarcinoma: ESMO-ESDO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23(Suppl 7):vii33–vii40
Siegel RL, Miller KD, Jemal A (2015) Cancerstatistics, 2015. CA Cancer J Clin 65(1):5–29
Siu LL, Awada A, Takimoto CH et al (2006) Phase I trial of sorafenib and gemcitabine in advanced solid tumors with an expanded cohort in advanced pancreatic cancer. Clin Cancer Res 12:144–151
Spano JP, Chodkiewicz C, Maurel J et al (2008) Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomised phase II study. Lancet 371:2101–2108
Stalin J, Harhouri K, Hubert L, Garrigue P, Nollet M, Essaadi A, Muller A, Foucault-Bertaud A, Bachelier R, Sabatier F, Pisano P, Peiretti F, Leroyer AS, Guillet B, Bardin N, Dignat-George F, Blot-Chabaud M (2016a) Soluble CD146 boosts therapeutic effect of endothelial progenitors through proteolytic processing of short CD146 isoform. Cardiovasc Res. 11 May. pii: cvw096. [Epub ahead of print] 2016 Aug 1;111(3):240–51
Stalin J, Nollet M, Garigue P, Fernandez S, Vivancos L, Essaadi A, Muller A, Bachelier R, Foucault-Bertaud A, Fugazza L, Leroyer AS, Bardin N, Guillet B, Dignat-George F, Blot-Chabaud M (2016b) Targeting soluble CD146 with a neutralizing antibody inhibits vascularization, growth and survival of CD146-positive tumors. Oncogene. https://doi.org/10.1038/onc.2016.83. [Epub ahead of print]
Takahashi Y, Akishima-Fukasawa Y, Kobayashi N, Sano T, Kosuge T, Nimura Y, Kanai Y, Hiraoka N (2007) Prognostic value of tumor architecture, tumor-associated vascular characteristics, and expression of angiogenic molecules in pancreatic endocrine tumors. Clin Cancer Res 38:187–196
Tempero MA, Malafa MP, Behrman SW, Benson AB 3rd, Casper ES, Chiorean EG, Chung V, Cohen SJ, Czito B, Engebretson A, Feng M, Hawkins WG, Herman J, Hoffman JP, Ko A, Komanduri S, Koong A, Lowy AM, Ma WW, Merchant NB, Mulvihill SJ, Muscarella P 2nd, Nakakura EK, Obando J, Pitman MB, Reddy S, Sasson AR, Thayer SP, Weekes CD, Wolff RA, Wolpin BM, Burns JL, Freedman-Cass DA (2014) Pancreatic adenocarcinoma, version 2.2014: featured updates to the NCCN guidelines. J Natl Compr Cancer Netw 12(8):1083–1093
Tsuzuki Y, Mouta Carreira C, Bockhorn M, Xu L, Jain RK, Fukumura D (2001a) Pancreas microenvironment promotes VEGF expression and tumor growth: novel window models for pancreatic tumor angiogenesis and microcirculation. Lab Investig 81(10):1439–1451
Valle JW, Palmer D, Jackson R, Cox T, Neoptolemos JP, Ghaneh P, Rawcliffe CL, Bassi C, Stocken DD, Cunningham D, O’Reilly D, Goldstein D, Robinson BA, Karapetis C, Scarfe A, Lacaine F, Sand J, Izbicki JR, Mayerle J, Dervenis C, Oláh A, Butturini G, Lind PA, Middleton MR, Anthoney A, Sumpter K, Carter R, Büchler MW (2014) Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J Clin Oncol 32(6):504–512
Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, Verslype C, Scheithauer W, Shang A, Cosaert J, Moore MJ (2009) Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol 27(13):2231–2237
Vasseur S, Tomasini R, Tournaire R, Iovanna JL (2010) Hypoxia induced tumor metabolic switch contributes to pancreatic cancer aggressiveness. Cancers (Basel) 2(4):2138–2152
von Marschall Z, Cramer T, Höcker M, Burde R, Plath T, Schirner M, Heidenreich R, Breier G, Riecken EO, Wiedenmann B, Rosewicz S (2000) De novo expression of vascular endothelial growth factor in human pancreatic cancer: evidence for an autocrine mitogenic loop. Gastroenterology 119(5):1358–1372
Wada S, Tsunoda T, Baba T, Primus FJ, Kuwano H, Shibuya M, Tahara H (2005) Rationale for antiangiogenic cancer therapy with vaccination using epitope peptides derived from human vascular endothelial growth factor receptor 2. Cancer Res 65(11):4939–4946
Wilhelm SM, Carter C, Tang L et al (2004) BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64:7099–7109
Yamaue H, Tsunoda T, Tani M, Miyazawa M, Yamao K, Mizuno N, Okusaka T, Ueno H, Boku N, Fukutomi A, Ishii H, Ohkawa S, Furukawa M, Maguchi H, Ikeda M, Togashi Y, Nishio K, Ohashi Y (2015) Randomized phase II/III clinical trial of elpamotide for patients with advanced pancreatic cancer: PEGASUS-PC study. Cancer Sci 106(7):883–890
Zhu Y, Tibensky I, Schmidt J, Hackert T, Ryschich E, Jäger D, Büchler MW, Märten A (2008) Interferon-alpha in combination with chemotherapy has potent antiangiogenic properties in an orthotopic mouse model for pancreatic adenocarcinoma. J Immunother 31(1):28–33
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this entry
Cite this entry
Hackert, T., Wüsten, L., Büchler, M.W. (2019). Anti-angiogenics in Pancreatic Cancer Therapy. In: Marmé, D. (eds) Tumor Angiogenesis. Springer, Cham. https://doi.org/10.1007/978-3-319-33673-2_18
Download citation
DOI: https://doi.org/10.1007/978-3-319-33673-2_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-33671-8
Online ISBN: 978-3-319-33673-2
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences