Abstract
The opalinids (Opalinidae: genera Opalina, Cepedea, Protoopalina, Zelleriella, and Protozelleriella) are highly unusual protists with large cells, multiple flagella, and two to hundreds of nuclei. The name Opalina is derived from the iridescent appearance when light reflects on the delicately folded surface of the cells. Opalinids are found exclusively in the intestines of frogs and some other hosts. They form the group Slopalinida together with two related genera of intestinal flagellates, Karotomorpha and Proteromonas. The former is a tetrakont flagellate that inhabits the intestines of certain amphibians, while the latter possesses only two flagella and is found in a wider spectrum of vertebrate hosts. Both morphology and molecular data suggest that Karotomorpha is phylogenetically closer to the opalinids, although both flagellates were traditionally classified in a single family, Proteromonadidae. Molecular data have shown that yet another unusual gut protist is closely related to Slopalinida: the genus Blastocystis. Unlike its relatives, it bears no flagella and is usually observed in the form of spherical cells with huge vacuoles. It is quite common in the intestines of many vertebrates (including humans) and invertebrates. Together, these organisms form Opalinata, a diverse assemblage of variously modified unicellular eukaryotes.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
Summary Classification
-
●Opalinata
-
●●Slopalinida
-
●●●Proteromonadidae (Proteromonas)
-
●●●Karotomorphidae (Karotomorpha)
-
●●●Opalinidae (Protozelleriella, Zelleriella, Protoopalina, Cepedea, Opalina)
-
●●Blastocystea (Blastocystis)
Introduction
General Characteristics
Opalinata Wenyon, 1926, comprises several types of protists morphologically so distinct that their relationship was recognized only relatively recently. The “core” of Opalinata is formed by opalinids, members of the family Opalinidae Claus, 1874. They are a lineage of unusual unicellular eukaryotes with several conspicuous morphological characteristics. They are quite large (some of them may reach nearly three millimeters) and have multiple flagella and two to many nuclei. The surface of an opalinid cell is arranged in parallel folds. Light interference that occurs on these delicate structures leads to the beautiful opalescence of opalinids when they are observed in reflected light (hence their name). Opalinid genera can be distinguished on the basis of two features: number of nuclei (two vs. numerous) and cell form (cylindrical vs. flattened). Multinucleate genera are Opalina Purkinje and Valentin, 1835 (flattened, Fig. 1a) and Cepedea Metcalf 1920 (cylindrical, Figs. 1b and 6a), whereas Zelleriella Metcalf 1920 (flattened, Fig. 1c) and Protoopalina Metcalf, 1918 (cylindrical, Figs. 1d and 6b) have two nuclei. The most recently erected genus, Protozelleriella Delvinquier, Markus, and Passmore, 1991, is similar to Zelleriella in appearance but is unique in having a hyaline margin without flagella (Delvinquier et al. 1991b). Two additional genera, uninucleate Hegneriella Earl, 1971 and Bezzenbergia Earl, 1973 with four nuclei are generally not considered valid. The number of described opalinid species reaches several hundred, but a critical revision of the family would probably lead to a reduction of the number (Sandon 1976).
Schematic drawings of four opalinid genera. Circles within the cells represent nuclei; the lines represent kineties (rows of flagella). Metachronal waves of beating flagella are symbolized by the waves at the periphery of the cells. The anterior part of cells with falx (bold line) points to the right. Opalina (a) is multinucleate, and its cell body is flat. The kineties run to the cell margin from where they continue on the other side (dotted lines). Cepedea (b) is also multinucleate, but its cell body is circular in cross section. Zelleriella (c) is binucleate with flat body, either caudate, as seen in the figure, or rounded posteriorly. Protoopalina (d) is a binucleate genus with cylindrical cells (Figure from Corliss (1989))
Based on ultrastructural observations, proteromonad flagellates were recognized as the closest relatives of opalinids. The two genera of this paraphyletic group, Proteromonas Künstler, 1883 (Fig. 2a) and Karotomorpha Travis, 1934 (Fig. 2b) are represented by several species of rather thin, pointed intestinal flagellates with two or four flagella, respectively. Their Golgi apparatus, nucleus, and single mitochondrion are located in the anterior part of the cell near the kinetosomes. Grassé (1952) included two incertae sedis genera among proteromonads, Dimoerium Przesmycki, 1901 and Dimoeriopsis Hollande & Pesson, 1945. The latter is a parasite of freshwater snail eggs. There are no recent studies of these organisms and their biology and phylogenetic affinities should be rechecked.
Schematic drawings of Proteromonas and Karotomorpha. The cell of Proteromonas (a) bears one long, thick anterior flagellum (aU) and a trailing one (rU). The rhizoplast (Rh) passes through the Golgi apparatus (G) to the nucleus (N), behind which lies the mitochondrion (M). Karotomorpha bufonis (b) has two pairs of flagella, a short rhizoplast (Rh) running near the Golgi complex (G); the nucleus (N) is closely associated with a single mitochondrion (M). Sinistral surface striation (pellicular folds) is sometimes apparent (Figure from Brugerolle and Mignot (1989))
The last, quite surprising addition to the group Opalinata was the genus Blastocystis Aléxéieff, 1911 (Fig. 3). Its members lack flagella completely and are best known as spherical cells with a large central vacuole and several nuclei since this is how they usually appear in culture. Blastocystis is morphologically very different from other members of Opalinata, and its recognition as their sister group was based primarily on phylogenetic analyses of molecular data (SSU rRNA gene sequences). Proteromonas, Karotomorpha, opalinids, and Blastocystis constitute a very interesting monophyletic group of intestinal protists that display extreme morphological disparity, ranging from “normal” flagellates to the complex multiflagellated opalinids, on one hand, and to the morphologically reduced Blastocystis, on the other.
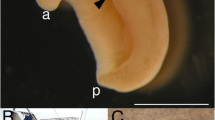
Microphotographs of protargol-stained vacuolar forms of two cultured Blastocystis strains. Both were isolated from chelonians: strain GEEL (a) from Geochelone elegans and strain PYX (b) from Pyxidea mouhotii. The scale bar (10 μm) applies to both images. Note the differences in size and number of nuclei. The preparations were stained by Ivan Čepička
Occurrence
All members of Opalinata occur in the intestines of various animals. Opalinids are common inhabitants of the large intestines of frogs of all continents. Some other poikilotherm vertebrates can also be their hosts. Karotomorpha is common in some amphibians, while species of the genus Proteromonas are commensal in reptiles, urodelan amphibians, and in the caecum of certain rodents. Blastocystis has the widest host range: it is found in various mammals (including humans), birds, reptiles, and amphibians but also in some invertebrates such as cockroaches.
No cultures of opalinids are available. However, some frogs maintained in labs (e.g., Xenopus laevis) are infected with them, usually with Protoopalina. A single axenic culture of Proteromonas was established by Kulda (1973) and is available from the Dept. of Parasitology, Faculty of Science, Charles University in Prague, Czech Republic. It is also deposited in the American Type Culture Collection (ATCC PRA-286). Blastocystis is quite easily cultured xenically and may be axenized. There are many cultures available in laboratories studying Blastocystis; ATCC provides a number of isolates.
Literature
One of the most important early authorities on opalinids was Metcalf, who, among other studies, published two major works (Metcalf 1923, 1940). Later important works include Grassé (1952) and Corliss (1955). Opalinid life cycles and morphogenesis are discussed in Wessenberg (1961). Three very useful modern reviews are Wessenberg (1978), Corliss (1989), and Delvinquier and Patterson (1993). Delvinquier et al. (1991a, b, 1993, 1995a, b, 1998), Delvinquier and Desser (1996), and Delvinquier and Marinkelle (1996, 1997) published a series of papers on opalinid distribution in amphibians throughout the world and described a new genus (Protozelleriella).
Proteromonas and Karotomorpha are dealt with in detail in the works of Grassé (1952) and Kulda and Nohýnková (1978). Their ultrastructure was studied especially by Brugerolle and Joyon (1975). The phylogenetic affinities of the two genera and opalinids are discussed by Patterson (1985).
Older literature on Blastocystis is rather scarce. Extensive critical reviews appeared in the 1990s; the most notable were those published by Zierdt (1991), Boreham and Stenzel (1993), and Stenzel and Boreham (1996). Later, several reviews were published by Tan (2004, 2008). A detailed publication dedicated to Blastocystis is that of Mehlhorn et al. (2012). Most recent advances are summarized in Clark et al. (2013). As if to outweigh the low number of older publications, a tremendous (and still growing) number of papers on various aspects of Blastocystis biology have been published in the last few decades.
History of Knowledge
The first observations of opalinids date back to 1683, when Leeuwenhoek investigated frog feces and saw numerous cells of Cepedea dimidiata swimming in his preparation (Dobell 1932). The genus Opalina was established by Purkinje and Valentin (1835). The name reflects the iridescent appearance of living cells. Opalinids were studied in detail by several investigators during the nineteenth century, most notably by Zeller (1877). A prominent author of opalinid studies during the first half of the twentieth century was Metcalf, who defined three new genera and described many new species. He concluded that opalinids were primitive ciliates and created the subclass Protociliata within Ciliata to accommodate them apart from true ciliates or Euciliata (Metcalf 1918). This approach was later abandoned by the majority of authors, who regarded opalinids as a unique group related to flagellates, but not to ciliates (e.g., Corliss 1955; Grassé 1952). A number of later studies added to the knowledge of opalinids, for example, those of Wessenberg (1961) and Kaczanowski (1971, 1973). Ultrastructural studies (Noirot-Timothée 1959; Patterson 1985; Pitelka 1956; Wessenberg 1966) provided new data and led to the current hypothesis on opalinid relationships.
The phylogenetic affinities between Proteromonas and Karotomorpha remained unrecognized for a long time. They were originally classified in distinct lineages of flagellates (bodonids vs. Polymastigidae or Tetramitidae). The first author who suggested a possible relationship between them was Grassé (1929, 1952). Further studies were conducted by Kulda (1961, 1973). An ultrastructural study by Brugerolle and Joyon (1975) further confirmed the evolutionary link between Proteromonas and Karotomorpha and, together with other works, informed the search for more distant relatives of proteromonads and thus to the discovery of their association with opalinids.
Blastocystis was described from various hosts more than a century ago by Aléxéieff (1911) under the name of B. enterocola, although a junior synonym, B. hominis (Brumpt 1912), is widely used for human isolates. For some 50 years since its description, Blastocystis was mostly overlooked and little studied. Its nature was not well understood – it was usually considered a harmless yeast or even a remnant/cyst of other organisms. The interest in it was reawakened mainly by Zierdt and his collaborators (e.g., Zierdt et al. 1967 and many later publications). He recognized that Blastocystis is not a fungus and continued to study its morphology, physiology, biochemistry, etc. His studies initiated further research on Blastocystis and many laboratories throughout the world study this organism today. The molecular phylogenetic study of Silberman et al. (1996) established that Blastocystis was a relative of slopalinids (represented by Proteromonas in the study).
Practical Importance
Although usually abundant in the cloacae of frogs, opalinids do not seem to cause any harm to their hosts. As quite common, yet rather enigmatic organisms, they have played, and can still play, an important role in research in the fields of cell biology, physiology, life cycle, host-symbiont interactions, (co)evolution, etc. Proteromonas and Karotomorpha may be numerous in the intestines of amphibians and reptiles but also seem to have no harmful effect; they are of no known economic importance.
Blastocystis is common in various hosts and is also one of the most frequently occurring eukaryotes found in the human intestine. Its role in pathogenesis is uncertain. Blastocystis is often connected with irritable bowel syndrome and other gastrointestinal symptoms. Some studies suggest a correlation between the presence of Blastocystis and these kinds of problems, but other studies indicate there is none (see, e.g., Clark et al. 2013; Poirier et al. 2012; Tan et al. 2010 for reviews and references). Even if there were such a correlation, it is still unclear whether Blastocystis can actually cause intestinal disorders or is just more efficient in colonization of the altered environment of unhealthy intestine. The whole issue is complicated by the fact that Blastocystis in human beings (and in animals, too) is genetically very variable – it is probable that some genetic lineages (subtypes) are more pathogenic than other ones. In some cases, Blastocystis was also associated with skin problems such as urticaria (Tan et al. 2010).
Habitats and Ecology
The vast majority of opalinids inhabit posterior parts of the intestine of frogs, but they can also be found in some other amphibians (e.g., Salamandridae, Ambystomatidae). Several species of opalinids were observed in freshwater fish, such as Protoopalina symphysodontis in Symphysodon (Foissner et al. 1979). They seem to be quite often found in Siluriformes (Sandon 1949). There are also a few marine species of Protoopalina: P. saturnalis lives in the intestine of the marine fish Box boops (Mignot and Molina 1988), while P. polykineta occurs in surgeonfish (Grim and Clements 1996) and P. pomacantha is found in angelfishes (Grim et al. 2000). Opalinids are occasionally seen in reptiles that presumably acquired them after ingestion of an infected frog (Delvinquier and Patterson 1993).
Because opalinids are so tightly bound to their amphibian hosts, their geographical distribution is dependent on the distribution of frogs. They are thus most diverse in tropical and subtropical regions. There are some patterns in the zoogeography of opalinids – some genera are absent or very rare in some regions: Zelleriella in Palaearctic, Opalina and Cepedea in Australia; conversely, Protozelleriella is known only from Africa (Delvinquier and Patterson 1993).
Opalinids themselves can serve as hosts to other protists, namely, amoebae of the genus Entamoeba (Chen and Stabler 1936; Stabler and Chen 1936; spelled “Endamoeba” in their works). Some metazoan parasites of frogs are predators of opalinids: Hazard (1941) observed the trematode Diplodiscus temperatus feeding on opalines, possibly eliminating them from adult frogs.
Karotomorpha and Proteromonas also inhabit the intestines of various vertebrates, where they are usually found intermixed with other gut protists. Karotomorpha bufonis is common in certain amphibians, both urodelans and frogs (e.g., Triturus spp., Bufo bufo). Proteromonas lacertaeviridis is a commensal of a wide range of reptiles – not only European lizards of the genus Lacerta but also many other lizards, snakes, or even tortoises. Several other species of Proteromonas, some (or all) of which might be synonymous to P. lacertaeviridis, were described from various reptiles. Urodelan amphibians, for example, Salamandra salamandra, may harbor Proteromonas longifilla, and other species can be found in the caecum of some rodents, for example, P. brevifilia in guinea pigs. Interestingly, Maia et al. (2012) found Proteromonas in a few blood and tail tissue samples from reptiles.
Besides being frequently reported from humans, Blastocystis can be found in a vast number of hosts including insects (Zaman et al. 1993), amphibians (Yoshikawa et al. 2004), reptiles (Teow et al. 1992), birds, and many nonhuman mammals (summarized in Stensvold et al. 2009; see also Parkar et al. 2010 and Alfellani et al. 2013). It is rather unclear, however, how many Blastocystis species there actually are and what their host specificity is. Despite the relatively uniform appearance of vacuolar forms of Blastocystis strains isolated from different (or the same) host species, genetic markers (usually SSU rRNA gene sequences) suggest there are multiple lineages that are molecularly divergent and probably ancient. The initial recognition of this hidden diversity led to nomenclatural confusion that made the problem even more difficult. A consensus proposed by Stensvold et al. (2007) recognizes the lineages as subtypes and uses numbers to distinguish between them. Currently, there are 17 subtypes defined (Alfellani et al. 2013), but the number may grow. The host specificity and zoonotic potential of the subtypes is still little known, but an overall picture is slowly emerging as more hosts are screened. Some subtypes are probably more generalist, while others display at least some host specificity. There are possibly human-specific subtypes, as well as examples of human infections accidentally acquired from bird or mammalian hosts (Clark et al. 2013). A number of isolates from poikilotherm vertebrates and invertebrates do not belong to any subtype and form their own lineages.
Characterization and Recognition
Opalinidae
Identification of opalinids is usually not difficult. First, the host is significant. Their host is most often a frog and they are located in the posterior part of the digestive tract. Opalinid cells are medium sized to large and covered with multiple flagella that beat in metachronal rhythms. Metachronal waves of flagellar activity are initiated in the anterior region of living cells and can be seen traveling to the posterior end. The cells are opalescent in reflected light. Two or many nuclei are visible within the cells. Unlike ciliates, which they superficially resemble, opalinids lack any oral structures and their nuclei are not differentiated in micro- and macronuclei.
Morphology and Ultrastructure. The most studied genus of opalinids is Opalina. Its cells are characterized as flattened, flexible, elliptical to elongated and with multiple nuclei (Fig. 1a). The biggest specimens can be more than one millimeter long. The cell surface is organized in a complex manner. The flagella (cilia) are arranged in oblique rows (kineties) that run in parallel from the anterior to the posterior end, spiraling around the cell. Kineties arise at an important morphogenetic center, the falx. The falx is a structure composed of several rows of kinetosomes bearing flagella and is located along the anterior end. The falx plays a role in the initiation of flagellar beating. Between the neighboring kineties, the pellicle is heavily folded in several ridges that are parallel with the kineties and supported by ribbons of interconnected microtubules (Fig. 4). The folds themselves are also interconnected by external linkages that stabilize the cortex architecture and ensure regular spacing of cortical ridges (Wooley 2006).
Schematic representation of surface structure of an opalinid cell. A few flagella in two kineties and the folds between them are shown. The folds are supported by ribbons of microtubules (dots) (Figure from Corliss (1989))
The flagella have the usual 9 × 2 + 2 axoneme structure. The detailed ultrastructure of the transitional zone between kinetosomes and axonemes (Fig. 5) is of phylogenetic importance as it is very similar in Karotomorpha and Proteromonas (Patterson 1985). The bases of flagella are cupped by a membranous pocket. Neighboring kinetosomes within a kinety are connected by an electron-dense connective (“desmos”).
Schematic drawing of flagellum ultrastructure of opalinids. The flagellar transitional region contains several conspicuous features: double transitional helix (tH), transitional plate (tP), and nine curved arms (A). The advanced basal bodies are interconnected by the desmos (D) (Figure from Corliss (1989))
The kineties are underlain by bands of microfilaments that are interconnected by additional perpendicular bands arising in regular intervals. Numerous vesicles are located between these lateral microfilament bands, just under the bottom level of the pellicular folds. Interestingly, rows of two vesicular types, spherical and flattened, alternate regularly at this level (Wessenberg 1978). The two types are randomly intermixed a little deeper in the cell. The spherical vesicles are coated and are formed at the bottom of the folds via pinocytosis. The flattened vesicles are believed to be exocytic and to compensate for the membrane demand of endocytosis (Delvinquier and Patterson 1993; Grim and Clements 1996). Endocytic vesicles fuse a bit further into the center of the cell to form larger digestive vacuoles (up to 4 μm in diameter).
Golgi complexes occur among the vacuoles, with their concave (trans) face oriented to the surface. In the central part of the cell are numerous ribosomes, mitochondria (formerly known as “Zeller bodies”), and nuclei. The mitochondrial cristae are tubular, and the mitochondria are often accompanied by lipid droplets. The nuclei of Opalina are flattened and 5–7 μm in diameter. Prominent masses of nucleoli are apparent after staining.
The ultrastructure of other opalinid genera does not differ substantially from that of Opalina. The falx of the cylindrical genera (Cepedea and Protoopalina) is parallel rather than perpendicular to the axis of the cell and is shorter. The number of kineties, which twist helically around the cell, is thus lower. The nuclei of the binucleated genera can be much bigger than those of Opalina and Cepedea: up to 40 μm in Protoopalina (cf. Fig. 6a, b). Protoopalina and Cepedea have a complex, branched network of microfibrillar bundles within the cell (Mignot and Affa’a 1995). Grim and Clemens (1996) report abundant bacterial endocytobionts in P. polykineta. Protozelleriella is a morphologically unique opalinid. It has a broad, Zelleriella-like cell with a thin anterior falx. However, the kineties originating on the falx are very short, forming a central fan-like array surrounded by a hyalinous margin devoid of flagella (Delvinquier et al. 1991b).
Microphotographs of two protargol-stained opalinids. Cepedea sp. (a) was isolated from Kassina senegalensis. Note multiple nuclei and kineties originating in the anterior (upper) part of the cell. Protoopalina intestinalis (b) was isolated from Bombina bombina. Its two nuclei are much larger than in Cepedea and are visibly connected. Some flagella are faintly apparent around the cells. The scale bar (10 μm) applies to both cells. The preparations were stained by Ivan Čepička
Mitosis, Cell Division, and Life Cycle. The nuclear division, chromosomes, and ploidy in Zelleriella were extensively studied by Chen (1936a, b, 1948). The nuclear membrane remains intact during mitosis; the mitotic spindle is formed within the nucleus. After the chromosomes are separated near the poles of the dividing nucleus, its central area is constricted and elongated, but the two daughter nuclei can remain joined by a narrow link for a long time. In Zelleriella, cytokinesis often precedes karyokinesis, leading to mononucleated daughter cells whose nuclei will proceed to telophase. Mitosis in other genera is similar, nuclei of multinucleated opalinids divide asynchronously, and mitosis is not strictly dependent on cytokinesis, although the number of nuclei is increased prior to cell division.
Binary fission in Opalina remains controversial in some respects: it is unclear whether transverse division (i.e., that cleaves the cell perpendicularly to its longitudinal axis) is a mechanism of actual propagation. Wessenberg (1961, 1978) was one of those who proposed this. The kineties are interrupted in transverse division, however, and for division to be successful, the posterior daughter cell would have to regenerate its falx de novo, which some other authors find dubious (e.g., Delvinquiér and Patterson 1993). Longitudinal division, on the other hand, is common. It is preceded by falx elongation, the falx is then bisected by a cleavage notch, and the cell is divided from the anterior to the posterior end along the kineties, which untwist during the process.
The life cycle of opalinids (Fig. 7) was studied notably by Wessenberg (1961). It is quite complex and is synchronized with the life cycle of the frog host. The best known example is the life cycle of Opalina: for the most of the year, the trophonts described above are the only stage found in the rectum of frogs. As the breeding season of frogs draws near, the opalines start to divide without growth (palintomy) producing tomonts and finally small tear-like individuals (progamonts) with a few nuclei. These stages round up and encyst. The cyst are spherical and 20–45 μm in diameter and contain several (most often 4–8) nuclei. They are released with feces into the water, where they remain viable for approximately 3 weeks. Young tadpoles feeding on detritus ingest the cysts. After the excystation in the digestive tract of a tadpole, the released stages – gamonts – divide further to produce unicellular gametes. Meiosis occurs during this process (Kaczanowski 1971). Opalines are anisogamous, producing macro- and microgametes. Both types are slender cells, approximately 40 μm long, with 8–10 kineties. Microgametes are much thinner and a bit shorter than the macrogametes and have a narrow “tail” which may lack cilia and seems to be sticky. They often swim with this part pointed anteriorly to attach themselves to macrogametes. It is not known which point of the life cycle is the stage where the sex of the gametes is determined. After syngamy, zygocysts are formed. These leave the tadpole in its feces and infect other tadpoles feeding on detritus. Upon ingestion and excystation, the sexual processes can repeat. Only in older tadpoles nearing metamorphosis do the excysted stages cease to produce new gametes and instead grow while their nuclei divide without cytokinesis. The resulting cells are “protrophonts” with an axial row of several nuclei. As they grow further, they become wider and flatter and change into trophonts. Some of these early trophonts may switch to palintomy again and produce some new cysts. They have a last chance to infect new hosts, which at this time are becoming young frogs and are already leaving the water. The perfect synchronization of the life cycle of opalinids and their hosts is believed to be achieved in part by an ability of opalinids to properly react to hormonal changes in frogs during the breeding season (El Mofty and Smyth 1964).
Diagrammatic and abbreviated life cycle of Opalina. Rounded trophonts (a) reproduce by longitudinal division (b). During the rearing season of the host, they undergo palintomy (c) and infective cysts (d) are finally released. After excystation (e), the gamonts (f) divide further, and eventually uninucleate gametes are produced (g). They fuse to form a zygote that encysts as a zygocyst (h). Excysted stages metamorphose into young trophonts (i). Stages a–d are found in adult frogs, with the infective cysts (d) passing out into the water with fecal material. Stages e–i are found in tadpoles; zygocysts (h) are again released into water to be reingested (Figure from Corliss (1989))
Proteromonas and Karotomorpha
Assuming that one is studying the intestinal contents of an appropriate host, both Proteromonas and Karotomorph a are best recognized by their slender cells and agile movement. Their cells typically measure about 15–20 × 5 μm. After Giemsa staining, the number and arrangement of flagella can also help to distinguish them from other flagellates. Fine striations may be visible on stained Karotomorpha cells (Fig. 2b).
Morphology and Ultrastructure. Proteromonas (Figs. 2a and 8a) has two apical flagella of different lengths. The longer one (about 40–50 μm) points forward during swimming and is thickened – its axoneme (of the typical eukaryotic structure) is accompanied by a striated fiber attached to one of the microtubular doublets and by additional microfibrils. The second, recurrent flagellum is about 30 μm long. Membranes at the bases of the two flagella are in close contact, forming a gap junction. The two kinetosomes are perpendicular and are interconnected by a short striated fibril. Two additional bundles of microtubules attach to the kinetosome of the posterior flagellum and a dense fiber to the other one (Brugerolle and Joyon 1975). Together, they form the rhizoplast, a structure that runs in the posterior direction through the Golgi complex, passes the nucleus (making a groove in its surface), and ends on the mitochondrion. In Karotomorpha (Figs. 2b and 8b), its four flagella point laterally out of the cell and all beat anteroposteriorly. They are arranged in two pairs – each pair is homologous to the two flagella of Proteromonas. Kinetosomes of each flagellar pair are again perpendicular and lie in a plane perpendicular to the cell axis. One kinetosome of each pair (the one homologous to the kinetosome of the posterior flagellum of Proteromonas) is associated with the two microtubular bands of the rhizoplast. The rhizoplast of Karotomorpha is shorter and does not reach the mitochondrion – it ends near the cell surface at the level of the nucleus. Several delicate features of phylogenetic significance can be found within the transitional region of the flagella of both genera. They include double transitional helix or transitional disk between the peripheral microtubules and the cell membrane, both of which are also present in the proximal region of opalinid flagella.
Diagrammatic reconstruction of the ultrastructure of Proteromonas and Karotomorpha. Proteomonas (a) has two flagella; the anterior one (aU) is thicker than the posterior one (rU) because its axoneme is accompanied by a fiber and fibrils. Between the two flagella, the cell membrane forms a gap junction. The rhizoplast (Rh) is a cytoskeletal structure connecting the kinetosomes with the nucleus (N) and mitochondrion (M). The rhizoplast runs through the Golgi complex (G). The surface of the cell is highly folded, with the ridges supported by single microtubules (mt). In the posterior part, the cell is covered with fine hairs – somatonemes (Sn). Endoplasmic reticulum (ER) and endocytic vacuoles (EV) are also present in the cells. Karotomorpha (b) differs mainly in having four laterally pointing flagella and deeper folds supported by ribbons of microtubules (mt). Its rhizoplast (Rh) does not reach the mitochondrion (M) and does not run through the Golgi (G). Karotomorpha lacks somatonemes. The cell contains nucleus (N), reticulum, and endocytic vacuoles (EV) (Figure from Brugerolle and Mignot (1989))
The cell surface is formed into about 30 shallow ridges in Proteromonas and about 20–25 deeper folds in Karotomorpha that twist helically around the cell (Brugerolle and Joyon 1975). These surface structures are supported by a cortical cytoskeleton (Fig. 8) in the form of single microtubules, each associated with a microfibril (Proteromonas) or ribbons of about ten interconnected microtubules (Karotomorpha). In the posterior two thirds of the Proteromonas cell, the cortical ridges bear somatonemes that cover the cell surface. Somatonemes are tripartite fine hairs consisting of a bent base, a tubular rod, and a terminal filament. Pairs of somatonemes are anchored to the cortical microtubules at regular intervals. The somatonemes have a similar structure to the mastigonemes associated with the anterior flagellum of typical stramenopile flagellates; the two structures are apparently homologous. Cavalier-Smith (1998) suggests that somatonemes protect the cell surface from larger particles which could either directly damage it or block pinocytosis. The much deeper folds of Karotomorpha would then serve a similar role and compensate for the loss of somatonemes.
The anterior region of Proteromonas and Karotomorpha cells contains three important organelles: the Golgi apparatus, nucleus, and a single mitochondrion. Endoplasmic reticulum is also concentrated here. Cisternae of the Golgi apparatus of Proteromonas are ring shaped, since the rhizoplast passes through them. The Golgi plays an important role in the assembly and transport of somatonemes, which are synthesized in cisternae of endoplasmic reticulum, then are transferred to the Golgi, and finally migrate to the cell surface (Brugerolle and Bardele 1988; Brugerolle and Joyon 1975). The nucleus is oval and has a nucleolus and peripheral chromatin. The mitochondrion lies posterior or posterolateral to the nucleus. It is roughly the same size as the nucleus and is surrounded by glycogen particles. The mitochondrial cristae are tubular. The cytoplasm may contain symbiotic bacteria, often near the mitochondrion (Brugerolle and Joyon 1975). The flagellates of both genera feed by pinocytosis; pinocytic vesicles are formed in the posterior part of the cell, among the rows of somatonemes or at the bottom of the cortical folds. Inside the cell, the vesicles fuse to form larger digestive vacuoles.
Cell Division and Life Cycle. The trophozoites of Proteromonas and Karotomorpha divide longitudinally. Cell division is better understood in Proteromonas (Grassé 1926, 1952). It begins with duplication of the kinetosomes and flagella; the rhizoplast is also doubled. The two pairs of kinetosomes then migrate away from each other. Meanwhile, division of the nucleus begins: chromosomes become visible, the nucleus extends perpendicularly to the cell axis, and the spindle forms within it. The membrane of the nucleus remains intact during mitosis. Rhizoplasts attach to both poles of the dividing nucleus; after karyokinesis, the two daughter nuclei remain associated with the rhizoplasts and through them also with the respective kinetosome pair. The mitochondrion divides during the telophase. Cytokinesis continues from the anterior to the posterior end of the cell. The life cycles of Proteromonas and Karotomorpha include cysts, which permit transmission from one host to another. The cysts are spherical, have a distinct cyst wall, and contain a single nucleus and a single mitochondrion surrounded with abundant glycogen granules. The rhizoplast is retained near the nucleus. Neither subpellicular microtubules nor flagella (or kinetosomes) were observed in cysts, however, the cysts may contain bacteria (Brugerolle and Joyon 1975).
Blastocystis
Unlike other Opalinata, Blastocystis is easily overlooked in fecal samples and may be confused with other objects. It does not move and may be of variable size and morphology. Therefore, molecular methods or cultivation might be preferred in routine diagnostics. The most commonly observed (and most easily diagnosed) form is the vacuolar form (see below).
Morphology and Different Forms. The described variability of sizes and shapes of Blastocystis cells is somewhat confusing. It is important to bear in mind the unusual genetic variability among Blastocystis isolates, which may account for differences among reports. Moreover, some observed forms may represent culture artifacts or degrading cells (Vdovenko 2010). A single Blastocystis strain can alternate between several forms. The best known of them is the vacuolar form (Fig. 3). Cells of this morphotype are spherical and usually have a diameter of several to about 15 μm, although much larger cells (up to hundreds of micrometers in diameter) were also observed (Zierd 1991). Their central vacuole occupies the majority of the cell volume. The vacuole most probably serves a storage function. It is surrounded by a layer of cytoplasm with one or more nuclei, mitochondria, Golgi complexes, and other typical eukaryotic organelles (Stenzel and Boreham 1996). The surface of vacuolar form cells is often covered with a fibrillar layer (surface coat), especially in freshly isolated cells. Although several mechanisms of multiplication were described, binary fission seems to be the predominant (if not the only) reproductive process (Tan 2008). Under certain conditions, granular forms may appear in cultures: these are similar to the vacuolar forms, but contain granules in the vacuole and/or cytoplasm (Dunn et al. 1989). Rarely, other forms were also reported, often from fresh stool samples: avacuolar and multivacuolar forms, with no or multiple vacuoles, and the amoeboid form (Stenzel and Boreham 1996). The latter also appears if Blastocystis is cultured on solid agar. Amoeboid cells are irregular in shape, seemingly nonmotile (although producing pseudopodia-like appendages), may or may not contain the central vacuole, and may differ in their ultrastructure (cf. Dunn et al. 1989; Tan and Suresh 2006). Blastocystis infects new hosts via small (up to about 5 μm) spherical-to-ovoid cysts (Stenzel and Boreham 1991; Tan 2008; Zaman et al. 1995).
Genomic Data. The growing interest in Blastocystis has resulted in the sequencing of several genomes, both mitochondrial (Pérez-Brocal and Clark 2008; Wawrzyniak et al. 2008) and nuclear (Denoeud et al. 2011). Both genomes are relatively small and show considerable reduction in gene number compared to other stramenopiles (Clark et al. 2013). The mitochondrial genome of Proteromonas has also been sequenced (Pérez-Brocal et al. 2010). Although its gene content is very similar to that of the Blastocystis mitochondrial genome, the two genomes differ strikingly in structure: it is circular in Blastocystis, but linear in Proteromonas. Because these organisms are anaerobes and the metabolism of their mitochondria is highly modified (Stechman et al. 2008), the mitochondria are often called “mitochondrion-like organelles.”
Maintenance and Cultivation
Opalinids can be easily retrieved from the frog intestine in large numbers. They can survive for up to several weeks in various media based on buffered saline solutions that are commonly used for the culturing of intestinal protists (see Delvinquier and Patterson 1993). It seems, however, that long-term cultures of opalinids are quite hard to establish and maintain (e.g., Wessenberg 1978), although several reports of successful cultivation exist. Lwoff and Valentini (1948) established a bacteria-free culture of Cepedea in a complex medium containing (among other ingredients) boiled frog liver and autoclaved frog rectal content. Interestingly, during late spring, cysts appeared in their cultures. After the cultures were contaminated by Gram-negative cocci, the opalinids grew better. Cultivation was also achieved by Yang and Bamberger (1953) and Yang (1960), who initially used egg slants overlaid with buffered saline (pH 7.8) supplemented with inactivated serum and antibiotics and later substituted the slants with liver concentrate.
Kulda (1973) established an axenic culture of Proteromonas lacertaeviridis at room temperature on Diamond’s TYM medium (Diamond 1957) supplemented with inactivated horse serum and a trace of agar. Before reaching the axenic state, proteromonads were for some time (several weeks) cocultivated with a yeast (Candida sp.) from the lizard host. They were later separated from the yeasts by repeated migration. Bacteria were eliminated from the primary culture with the use of antibiotics. For short-term purposes, both Proteromonas and Karotomorpha can survive several hours or days in various saline solutions commonly used for isolation of intestinal flagellates, for example, Ringer’s frog solution: NaCl 6.5 g, KC1 0.14 g, CaCl2 0.12 g, and NaHCO3 0.20 g in 1000 ml H2O (Brugerolle and Mignot 1989).
Blastocystis grows well xenically in various media and may be axenized (Tan 2008). For xenic cultures, Jones’ medium (Jones 1946) is often used. Axenized strains may be cultured in commercially available Iscove’s modified Dulbecco’s medium + horse serum (Clark and Diamond 2002). The ability of Blastocystis to form colonies on agar plates may be exploited during axenization and cloning (Tan et al. 2000).
Evolutionary History
There is no direct (i.e., fossil) evidence of the evolutionary history of Opalinata. The close link of opalinids to frogs (and also the geographical distribution of opalinid genera) suggests that their main radiation dates back to the Mesozoic. Hypotheses on the evolution within the group are based on morphological data: the number of nuclei (two/many) and cell shape (flat/cylindrical). Because all four possible combinations of the character states are found among opalinids, every evolutionary scenario requires convergence and/or reversal of some of these characters. Generally, the binucleated state is considered primitive and the multinucleated genera are believed to form a monophyletic derived group (Opalininae). Relationships among the three binucleated genera and Opalininae are unclear, but it has been suggested that Protozelleriella might be the most primitive representative of Opalinidae, indicating that the cells of Zelleriella are primitively flattened. The monophyly of Opalininae is supported by their geographical distribution and several ultrastructural features (Patterson and Delvinquier 1990), as well as by molecular data (Nishi et al. 2005). However, both electron microscopy and PCR-based sequencing were applied to a very limited number of species (and none belonging to Protozelleriella!).
Relationships of opalinids to other protists were mysterious for a long time. Their superficial resemblance to ciliates had led nineteenth-century protistologists to place opalinids in this group, although some criticism of this concept appeared early on. Phylogenetic affinities of opalinids were discussed in detail by Metcalf (1918), who erected a new subphylum Protociliata to accommodate opalinids separately from “other,” true ciliates. This arrangement satisfied many authors as it reflected both the morphological uniqueness of opalinids and the possibility that they formed a phylogenetic connection between flagellates and ciliates: the presence of two (or many) nuclei, cilia arranged in kineties, and sexual processes in the life cycle of opalinids were long perceived as features that one would expect in a hypothetical ancestor of ciliates (Wessenberg 1978). The debate – whether or not opalinids represent an intermediate stage between flagellates and ciliates – went on for several decades, with the majority of authors deserting the idea of a close affinity between ciliates and opalinids. They thus remained orphaned as an isolated taxon among flagellates (Corliss 1955; Grassé 1952), which is, more or less to say, an isolated taxon among eukaryotes.
The situation changed when substantial electron microscopy data emerged. A key study was that of Patterson (1985), who highlighted that there are several ultrastructural characteristics shared by opalinids, Proteromonas and Karotomorpha , as already noticed by Brugerolle and Joyon (1975). The arrangement of kinetosomes and associated structures is very similar and another synapomorphy is the folded cell surface, with the folds supported by single microtubules (Proteromonas) or microtubular ribbons (Karotomorpha and Opalinidae). These and other similarities led Patterson to postulate a close relationship between opalinids and proteromonads and to establish a new order Slopalinida comprising the two groups. The paraphyly of proteromonads was also recognized; Karotomorpha, with its pellicular folds supported by ribbons of microtubules and more flagella, is more closely related to opalinids than to Proteromonas. Some of the details of the kinetosome ultrastructure link slopalinids to other stramenopiles, as do other features of Proteromonas in particular (e.g., somatonemes). The molecular phylogenetic study of Silberman et al. (1996) not only supported the monophyly of stramenopiles and the placement of slopalinids among them but also revealed an unexpected relationship between slopalinids and Blastocystis. The close relationship between Proteromonas, Karotomorpha, and Opalinidae was later confirmed by molecular studies based on SSU rDNA (Kostka et al. 2004, 2007, Nishi et al. 2005). Interestingly, the study of Nishi et al. (2005) did not refute a possible link between opalinids and ciliates: phylogenetic analyses of tubulin genes tend to connect the two groups, although probably artefactually. Opalinata relationships are further discussed by Cavalier-Smith (1997, 1998) and Cavalier-Smith and Chao (2006). In these works, Karotomorpha and opalinids were treated together in the group Opalinea to the exclusion of Proteromonas. In later papers, Proteromonas was included (Cavalier-Smith and Scoble 2013; Ruggiero et al. 2015) – the expanded Opalinea group has then exactly the same composition as Patterson’s Slopalinida; the group containing only Karotomorpha and opalinids was called Opalinida therein.
References
Aléxéieff, A. (1911). Sur la nature des formations dites “kystes de Trichomonas intestinalis”. Compte Rendu des Séances de la Société de Biologie Paris, 71, 296–298.
Alfellani, M. A., Taner-Mulla, D., Jacob, A. S., Atim Imeede, C., Yoshikawa, H., Stensvold, C. R., & Clark, C. G. (2013). Genetic diversity of Blastocystis in livestock and zoo animals. Protist, 164, 497–509.
Boreham, P. F. L., & Stenzel, D. J. (1993). Blastocystis in humans and animals: Morphology, biology, and epizootiology. Advances in Parasitology, 32, 1–70.
Brugerolle, G., & Bardele, C. F. (1988). Cortical cytoskeleton of the flagellate Proteromonas lacertae: Interrelation between microtubules, membrane and somatonemes. Protoplasma, 142, 46–54.
Brugerolle, G., & Joyon, L. (1975). Étude cytologique ultrastructurale des genres Proteromonas et Karotomorpha (Zoomastigophorea Proteromonadida Grassé 1952). Protistologica, 11, 531–546.
Brugerolle, G., & Mignot, J. P. (1989). Phylum Zoomastigina, class Proteromonadida. In L. Margulis, J. O. Corliss, M. Melkonian, & D. J. Chapman (Eds.), Handbook of Protoctista (pp. 246–251). Boston: Jones and Barlett Publishers.
Brumpt, E. (1912). Côlite à Tetramitus mesnili (Wenyon 1910) et côlite à Trichomonas intestinalis Leuchart 1879. Blastocystis hominis n. sp. et formes voisines. Bulletin de la Société de Pathologie Exotique, 5, 725–730.
Cavalier-Smith, T. (1997). Sagenista and Bigyra, two phyla of heterotrophic heterokont chromists. Archiv für Protistenkunde, 148, 253–267.
Cavalier-Smith, T. (1998). A revised six-kingdom system of life. Biological Reviews, 73, 203–266.
Cavalier-Smith, T., & Chao, E. E.-Y. (2006). Phylogeny and megasystematics of phagotrophic heterokonts (kingdom Chromista). Journal of Molecular Evolution, 62, 388–420.
Cavalier-Smith, T., & Scoble, J. M. (2013). Phylogeny of Heterokonta: Incisomonas marina, a uniciliate gliding opalozoan related to Solenicola (Nanomonadea), and evidence that Actinophryida evolved from raphidophytes. European Journal of Protistology, 49, 328–353.
Chen, T.-T. (1936a). Observations on mitosis in opalinids I. The behavior and individuality of chromosomes and nucleoli. Proceedings of the National Academy of Sciences of the United States of America, 22, 594–601.
Chen, T.-T. (1936b). Observations on mitosis in opalinids II. The association of chromosomes and nucleoli. Proceedings of the National Academy of Sciences of the United States of America, 22, 602–607.
Chen, T.-T. (1948). Chromosomes in Opalinidae (Protozoa, Ciliata). Journal of Morphology, 83, 281–357.
Chen, T.-T., & Stabler, R. M. (1936). Further studies on the endamoebae parasitizing opalinid ciliates. Biological Bulletin, 70, 72–77.
Clark, C. G., & Diamond, L. S. (2002). Methods for cultivation of luminal parasitic protists of clinical importance. Clinical Microbiology Reviews, 15, 329–341.
Clark, C. G., van der Giezen, M., Alfellani, M. A., & Stensvold, C. R. (2013). Recent developments in Blastocystis research. Advances in Parasitology, 82, 1–32.
Corliss, J. O. (1955). The opalinid infusorians: Flagellates or ciliates? Journal of Protozoology, 2, 107–114.
Corliss, J. O. (1989). Phylum Zoomastigina, class Opalinata. In L. Margulis, J. O. Corliss, M. Melkonian, & D. J. Chapman (Eds.), Handbook of Protoctista (pp. 239–245). Boston: Jones and Barlett Publishers.
Delvinquier, B. L. J., & Patterson, D. J. (1993). The opalines. In J. P. Kreier & J. R. Baker (Eds.), Parasitic protozoa (Vol. 3, pp. 247–325). San Diego: Academic.
Delvinquier, B. L. J., & Desser, S. S. (1996). Opalinidae (Sarcomastigophora) in North American Amphibia. Genus Opalina Purkinje & Valentin, 1835. Systematic Parasitology, 33, 33–51.
Delvinquier, B. L. J., & Marinkelle, C. J. (1996). Opalinidae (Slopalinida) in South American Amphibia. Genus Opalina Purkinje & Valentin, 1835 in Colombia. Systematic Parasitology, 34, 27–35.
Delvinquier, B. L. J., & Marinkelle, C. J. (1997). Opalinidae (Slopalinida) in South American Amphibia. Genus Zelleriella Metcalf, 1920 in Colombia. Systematic Parasitology, 38, 93–110.
Delvinquier, B. L. J., Markus, M. B., & Passmore, N. I. (1991a). Opalinidae in African Anura I. Genus Opalina. Systematic Parasitology, 19, 119–146.
Delvinquier, B. L. J., Markus, M. B., & Passmore, N. I. (1991b). Opalinidae in African Anura. II. Genera Protozelleriella n. g. and Zelleriella. Systematic Parasitology, 19, 159–185.
Delvinquier, B. L. J., Markus, M. B., & Passmore, N. I. (1993). Opalinidae in African Anura. III. Genus Cepedea. Systematic Parasitology, 24, 53–80.
Delvinquier, B. L. J., Desser, S. S., & Johnson, J. (1995a). Opalinidae (Sarcomastigophora) in North American Amphibia. Genus Protoopalina Metcalf, 1918. Systematic Parasitology, 32, 141–147.
Delvinquier, B. L. J., Markus, M. B., & Passmore, N. I. (1995b). Opalinidae in African Anura. IV. Genus Protoopalina. Systematic Parasitology, 30, 81–120.
Delvinquier, B. L. J., Glaw, F., Markus, M. B., & Passmore, N. I. (1998). Opalinidae (Slopalinida) in Madagascan Anura: Zelleriella Metcalf, 1920 and Protoopalina Metcalf, 1918. Systematic Parasitology, 41, 187–196.
Denoeud, F., Roussel, M., Noel, B., Wawrzyniak, I., Da Silva, C., Diogon, M., Viscogliosi, E., Brochier-Armanet, C., Couloux, A., Poulain, J., Segurens, B., Anthouard, V., Texier, C., Blot, N., Poirier, P., Ng, G. C., Tan, K. S. W., Artiguenave, F., Jaillon, O., Aury, J.-M., Delbac, F., Wincker, P., Vivarès, C. P., & El Alaoui, H. (2011). Genome sequence of the stramenopile Blastocystis, a human anaerobic parasite. Genome Biology, 12, R29.
Diamond, L. S. (1957). The establishment of various trichomonads of animals and man in axenic cultures. Journal of Parasitology, 43, 488–490.
Dobell, C. C. (1932). Antony van Leeuwenhoek and His “Little Animals.”. Amsterdam: Swets and Zeitlinger.
Dunn, L. A., Boreham, P. F. L., & Stenzel, D. J. (1989). Ultrastructural variation of Blastocystis hominis stocks in culture. International Journal for Parasitology, 19, 43–56.
El Mofty, M. M., & Smyth, J. D. (1964). Endocrine control of encystation in Opalina ranarum parasitic in Rana temporaria. Experimental Parasitology, 15, 185–199.
Foissner, W., Schubert, G., & Wilbert, N. (1979). Morphologie, Infraciliatur und Silberliniensystem von Protoopalina symphysodonis nov. spec. (Protozoa: Opalinata), einer Opalinidae aus dem Intestinum von Symphysodon aequifasciata Pellegrin (Percoidei: Cichlidae). Zoologischer Anzeiger, 202, 71–85.
Grassé, P. P. (1926). Contribution à l’étude des Flagellés parasites. Archives de Zoologie Expériméntale et Générale, 65, 345–602.
Grassé, P. P. (1929). Sur la cytologie du Flagellé parasite Proteromonas lacertae viridis Grassi. Comptes rendus de l’Association des anatomistes, 18, 267–275.
Grassé, P. P. (1952). Traité de Zoologie: Phylogénie. Protozoaries: Généralités. Flagellés (Vol. I). Paris: Masson and Cie.
Grim, J. N., & Clements, K. D. (1996). Description of a new species of opalinid by light microscopy, SEM and TEM: Protoopalina polykineta, n. sp. from the intestines of the surgeonfish, Acanthurus nigrofuscus: Surface features, kinetal organization, vesicles, and endocytobionts. European Journal of Protistology, 32, 81–89.
Grim, J. N., Pérez-España, H., & Martínez-Díaz, S. F. (2000). The morphology of Protoopalina pomacantha, n. sp., symbiont in the rectum of the angelfishes, Pomacanthus zonipectus and Holacanthus passer. A light, scanning electron and transmission electron microscopic study. European Journal of Protistology, 36, 343–350.
Hazard, F. O. (1941). The absence of opalinids from the adult green frog, Rana clamitans. Journal of Parasitology, 27, 513–516.
Jones, W. R. (1946). The experimental infection of rats with Entamoeba histolytica; with a method for evaluating the anti-amoebic properties of new compounds. Annals of Tropical Medicine and Parasitology, 40, 130.
Kaczanowski, A. (1971). Opalina ranarum Purkinje et Valentin: Meiosis and dimorphism of nuclear behavior during meiosis. Acta Protozoologica, 9, 105–106.
Kaczanowski, A. (1973). Morphological studies on opalinids. II Cortical patterns in Opalina ranarum. Acta Protozoologica, 12, 29–51.
Kostka, M., Hampl, V., Cepicka, I., & Flegr, J. (2004). Phylogenetic position of Protoopalina intestinalis based on SSU rRNA gene sequence. Molecular Phylogenetics and Evolution, 33, 220–224.
Kostka, M., Cepicka, I., Hampl, V., & Flegr, J. (2007). Phylogenetic position of Karotomorpha and paraphyly of Proteromonadidae. Molecular Phylogenetics and Evolution, 43, 1167–1170.
Kulda, J. (1961). Flagellates from the cloacae of Czechoslovak amphibians and reptiles. In: Proceedings of the First International Conference on Protozoology, Prague, pp. 582–588. Progress in Protozoology: Czechoslovak Academy of Sciences.
Kulda, J. (1973). Axenic cultivation of Proteromonas lacertae-viridis (Grassi 1879). Journal of Protozoology, 20, 536–537.
Kulda, J., & Nohýnková, E. (1978). Proteromonadidae. In J. P. Kreier (Ed.), Parasitic protozoa (pp. 118–129). New York: Academic.
Lwoff, A., & Valentini, S. (1948). Culture du flagellé opalinide Cepedea dimidiata. Annales de l’Institut Pasteur, 75, 1–7.
Maia, J. P. M. C., Gómez-Díaz, E., & Harris, D. J. (2012). Apicomplexa primers amplify Proteromonas (Stramenopiles, Slopalinida, Proteromonadidae) in tissue and blood samples from lizards. Acta Parasitologica, 57, 337–341.
Mehlhorn, H., Tan, K. S. W., & Yoshikawa, H. (Eds.). (2012). Blastocystis: Pathogen or passenger? An evaluation of 101 years of research. Heidelberg: Springer.
Metcalf, M. M. (1918). Opalina and the origin of the ciliate Infusoria. Journal of the Washington Academy of Sciences, 8, 427–431.
Metcalf, M. M. (1923). The opalinid ciliate infusorians. U.S. National Museum Bulletin, 120, 1–484.
Metcalf, M. M. (1940). Further studies on the opalinid ciliate infusorians and their hosts. Proceedings of the United States National Museum, 87, 465–634.
Mignot, J.-P., & Affa’a, F. M. (1995). Patterning in opalinids. III: The cytoskeleton of Cepedea sudafricana (Fantham, 1923) Affa‘a & Lynn 1994, an intermediate type between Opalina ranarum and Protoopalina pseudonutii. Archiv für Protistenkunde, 145, 241–249.
Mignot, J.-P., & Molina, A. (1988). Etude ultrastructurale de Protoopalina saturnalis (Léger et Duboscq 1904) Metcalf 1918, protiste parasite du poisson marin Box boops L. Archiv für Protistenkunde, 135, 255–270.
Nishi, A., Ishida, K., & Endoh, H. (2005). Reevaluation of the evolutionary position of opalinids based on 18S rDNA, and α- and β-tubulin gene phylogenies. Journal of Molecular Evolution, 60, 695–705.
Noirot-Timothée, C. (1959). Recherches sur l’ultrastructure d’Opalina ranarum. Annales des Sciences Naturelles Zoologie et Biologie Animale (sér. 12) 1, 265–281.
Parkar, U., Traub, R. J., Vitali, S., Elliot, A., Levecke, B., Robertson, I., Geurden, T., Steele, J., Drake, B., & Thompson, R. C. A. (2010). Molecular characterization of Blastocystis isolates from zoo animals and their animal-keepers. Veterinary Parasitology, 169, 8–17.
Patterson, D. J. (1985). The fine structure of Opalina ranarum (family Opalinidae): Opalinid phylogeny and classification. Protistologica, 21, 413–428.
Patterson, D. J., & Delvinquier, B. L. J. (1990). The fine structure of the cortex of the protist Protoopalina australis (Slopalinida, Opalinidae) from Litoria nasuta and Litoria inermis (Amphibia: Anura: Hylidae) in Queensland, Australia. Jornal of Protozoology, 37, 449–455.
Pérez-Brocal, V., & Clark, C. G. (2008). Analysis of two genomes from the mitochondrion-like organelle of the intestinal parasite Blastocystis: Complete sequences, gene content, and genome organization. Molecular Biology and Evolution, 25, 2475–2482.
Pérez-Brocal, V., Shahar-Golan, R., & Clark, C. G. (2010). A linear molecule with two large inverted repeats: The mitochondrial genome of the stramenopile Proteromonas lacertae. Genome Biology and Evolution, 2, 257–266.
Pitelka, D. R. (1956). An electron microscope study of cortical structures of Opalina obtrigonoidea. Journal of Biophysical and Biochemical Cytology, 2, 423–432.
Poirier, P., Wawrzyniak, I., Vivarès, C. P., Delbac, F., & El Alaoui, H. (2012). New insights into Blastocystis spp.: A potential link with irritable bowel syndrome. PLoS Pathogens, 8, e1002545.
Purkinje, J. E., & Valentin, G. G. (1835). De phaenomeno generali et fundamentali motus vibratorii continui in membranis cum externis tum internis animalium plurimorum obvii. Wratislavia.
Ruggiero, M. A., Gordon, D. P., Orrell, T. M., Bailly, N., Bourgoin, T., Brusca, R. C., Cavalier-Smith, T., Guiry, M. D., & Kirk, P. M. (2015). A higher level classification of all living organisms. PLoS ONE, 10, e0130114.
Sandon, H. (1949). Opalinids from Nile fish. Nature, 164, 410.
Sandon, H. (1976). The species problem in the opalinids (Protozoa, Opalinata), with special reference to Protoopalina. Transactions of the American Microscopical Society, 95, 357–366.
Silberman, J. D., Sogin, M. L., Leipe, D. D., & Clark, C. G. (1996). Human parasite finds taxonomic home. Nature, 380, 398.
Stabler, R. M., & Chen, T.-T. (1936). Observations on an endamoeba parasitizing opalinid ciliates. Biological Bulletin, 70, 56–71.
Stechmann, A., Hamblin, K., Pérez-Brocal, V., Gaston, D., Richmond, G. S., van der Giezen, M., Clark, C. G., & Roger, A. J. (2008). Organelles in Blastocystis that blur the distinction between mitochondria and hydrogenosomes. Current Biology, 18, 580–585.
Stensvold, C. R., Suresh, G. K., Tan, K. S. W., Thompson, R. C. A., Traub, R. J., Viscogliosi, E., Yoshikawa, H., & Clark, C. G. (2007). Terminology for Blastocystis subtypes – A consensus. Trends in Parasitology, 23, 93–96.
Stensvold, C. R., Alfellani, M. A., Nørskov-Lauritsen, S., Prip, K., Victory, E. L., Maddox, C., Nielsen, H. V., & Clark, C. G. (2009). Subtype distribution of Blastocystis isolates from synanthropic and zoo animals and identification of a new subtype. International Journal for Parasitology, 39, 473–479.
Stenzel, D. J., & Boreham, P. F. L. (1991). A cyst-like stage of Blastocystis hominis. International Journal for Parasitology, 21, 613–615.
Stenzel, D. J., & Boreham, P. F. L. (1996). Blastocystis hominis revisited. Clinical Microbiology Reviews, 9, 563–584.
Tan, K. S. W. (2004). Blastocystis in humans and animals: New insights using modern methodologies. Veterinary Parasitology, 126, 121–144.
Tan, K. S. W. (2008). New insights on classification, identification, and clinical relevance of Blastocystis spp. Clinical Microbiology Reviews, 21, 639–665.
Tan, T. C., & Suresh, K. G. (2006). Amoeboid form of Blastocystis hominis – A detailed ultrastructural insight. Parasitology Research, 99, 737–742.
Tan, K. S. W., Ng, G. C., Quek, E., Howe, J., Ramachandran, N. P., Yap, E. H., & Singh, M. (2000). Blastocystis hominis: A simplified, high-efficiency method for clonal growth on solid agar. Experimental Parasitology, 96, 9–15.
Tan, K. S. W., Mirza, H., Teo, J. D. W., Wu, B., & MacAry, P. A. (2010). Current views on the clinical relevance of Blastocystis spp. Current Infectious Disease Reports, 12, 28–35.
Teow, W. L., Ng, G. C., Chan, P. P., Chan, Y. C., Yap, E. H., Zaman, V., & Singh, M. (1992). A survey of Blastocystis in reptiles. Parasitology Research, 78, 453–455.
Vdovenko, A. A. (2010). Blastocystis hominis: Origin and significance of vacuolar and granular forms. Parasitology Research, 86, 8–10.
Wawrzyniak, I., Roussel, M., Diogon, M., Couloux, A., Texier, C., Tan, K. S. W., Vivarès, C. P., Delbac, F., Wincker, P., & El Alaoui, H. (2008). Complete circular DNA in the mitochondria-like organelles of Blastocystis hominis. International Journal for Parasitology, 38, 1377–1382.
Wessenberg, H. (1961). Studies on the life cycle and morphogenesis of Opalina. University of California Publications in Zoology, 61, 315–370.
Wessenberg, H. (1966). Observations on cortical ultrastructure in Opalina. Journal de Microscopie (Paris), 5, 471–492.
Wessenberg, H. (1978). Opalinata. In J. P. Kreier (Ed.), Parasitic protozoa, intestinal flagellates, histomonads, trichomonads, amoeba, opalinids, and ciliates (Vol. 2, pp. 551–581). New York/London: Academic.
Wooley, D. M. (2006). Newly discovered linkages between the cortical (pellicular) ridges of Opalina. European Journal of Protistology, 42, 309–311.
Yang, W. C. T. (1960). On the continuous culture of opalinids. Journal of Parasitology, 46, 32.
Yang, W. C. T., & Bamberger, J. W. (1953). A technique for culturing Opalina. Science, 118, 252–253.
Yoshikawa, H., Morimoto, K., Nagashima, M., & Miyamoto, N. (2004). A survey of Blastocystis infection in anuran and urodele amphibians. Veterinary Parasitology, 122, 91–102.
Zaman, V., Ng, G. C., Suresh, K., Yap, E. H., & Singh, M. (1993). Isolation of Blastocystis from the cockroach (Dictyoptera: Blattidae). Parasitology Research, 79, 73–74.
Zaman, V., Howe, J., & Ng, M. (1995). Ultrastructure of Blastocystis hominis cysts. Parasitology Research, 81, 465–469.
Zeller, E. (1877). Untersuchungen über die Fortpflanzung und die Entwicklung der in unseren Batrachiern schmarotzenden Opalinen. Zeitschrift für Wissenschaftliche Zoologie, 29, 352–379.
Zierdt, C. H. (1991). Blastocystis hominis – Past and future. Clinical Microbiology Reviews, 4, 61–79.
Zierdt, C. H., Rude, W. S., & Bull, B. S. (1967). Protozoan characteristics of Blastocystis hominis. American Journal of Clinical Pathology, 48, 495–501.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this entry
Cite this entry
Kostka, M. (2017). Opalinata. In: Archibald, J., Simpson, A., Slamovits, C. (eds) Handbook of the Protists. Springer, Cham. https://doi.org/10.1007/978-3-319-28149-0_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-28149-0_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28147-6
Online ISBN: 978-3-319-28149-0
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences