Abstract
The Straminipila are characterized by their anterior flagellum with tripartite hairs and form a well-supported monophyletic branch of the larger Straminipila/Alveolata/Rhizaria (SAR) superkingdom. This is an account of the molecular systematics and phylogeny of osmotrophic and phagotrophic lineages of the Straminipila, comprising the slime nets and their thraustochytrid allies, as well as some lesser known lineages. The phylum Labyrinthulomycota s. lat. contains two main clades, one of which approximates to holocarpic thraustochytrids and the other to the labyrinthulids and aplanochytrids. Together with the flagellate bicosoecids and the protermonads and opalinids, they form a monophyletic clade that is sister to the golden-brown algae and Oomycota. The systematics of the Labyrinthulomycota s. lat. is still in flux as recent studies employing environmental barcoding have revealed the presence of diverse lineages not branching within genera characterized in terms of their morphology. The current review deals primarily with the two major lineages of the Labyrinthulomycota s. lat. and discusses other lineages only briefly, due to the scarce knowledge about these organisms. Characteristics associated with zoosporogenesis and sexual reproduction are discussed in relation to other members of the Straminipila.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Amoebae
- Amphitremida
- Aplanochytrids
- Bothrosome
- DHA (docosahexaenoic acids)
- Diplophrys
- Eelgrass wasting disease
- Ecology
- Ectoplasmic net
- Labyrinthulida
- Marine decomposers
- Seagrass wasting disease
- Scale coats
- Schizochytrium
- Slime nets
- Thraustochytrida
- Stramenopiles
- Straminipila
- Zoospore ultrastructure
-
●Labyrinthulomycota/Labyrinthomorpha
-
●● Labyrinthulomycetes/Labyrinthulea
-
●●●Labyrinthulales/Labyrinthulida
-
●●●●Aplanochytriaceae/Aplanochytriidae (Aplanochytrium (including Labyrinthuloides))
-
●●●●“Stellarchytriaceae/Stellarchytriidae” (Stellarchytrium)
-
●●●●Labyrinthulaceae/Labyrinthulidae (Labyrinthula)
-
●●● Oblongichytridiales/Oblongichytriida
-
●●●●Oblongichytridiaceae/Oblongichytriidae (Oblongichytrium)
-
●●●Thraustochytriales/Thraustochytrida
-
●●●●Althornidiaceae/Althorniidae (Althornia)
-
●●●●Thraustochytriacae/Thraustochytriidae (Aurantiochytrium, Botryochytrium, Japanochytrium, Monorhizochytrium, Parietichytrium, Schizochytrium, Sicyoidochytrium, Thraustochytrium, Ulkenia)
-
●●●Amphitremida
-
●●●●Amphitremidae (Amphitrema, Archerella, Paramphitrema)
-
●●●●Diplophrydaceae/Diplophryidae (Diplophrys)
-
●●●Amphifilida
-
●●●●Amphifilaceae/Amphifilidae (Amphifila)
-
●●●Sorodiplophryidae (Fibrophrys, Sorodiplophrys)
Summary classification of major lineages adapted from Tice et al. (2016). It should be noted that the higher level classification needs to be considered provisionally, as the deeper splits within Labyrinthulomycota are largely unresolved (Pan et al. 2017).
Introduction
General Characteristics
The osmotrophic fungus-like members of the kingdom Straminipila are characterized by absorptive nutrition and heterokont biflagellate zoospores. The term stramenopile was first introduced by Patterson (1989) in reference to the “straw hairs” (mastigonemes) that decorate the anterior flagella of this group of organisms (Fig. 1b, e). Dick (2001) pointed out this was an incorrect derivation of the Latin for “straw hair” and that the correct form should be straminipilous. However, Adl et al. (2005) favored the continued use of “stramenopile”, the form of the name that is most widely used (Lévesque 2011). It is now apparent that the Straminipila have their evolutionary origins in the sea and that many of the fungal-like organisms seem to be ecologically important and widespread pathogens of algae, animals, and plants (Beakes et al. 2012, 2014; Thines 2014).
Morphological features of labyrinthulids and thraustochytrids, part 1. Schematic drawing of Labyrinthula showing (a) uninucleate spindle-shaped cell bodies, which are coated in scales, containing mitochondria and Golgi dictyosomes and enveloping ectoplasmic net membrane and (b) biflagellate zoospore, with mastigonate anterior flagellum (AF) and shorter posterior flagellum (PF) with tapering terminal acroneme. Both adapted from Porter (1990). (c) Schematic drawings of the thraustochytrid Schizochytrium aggregatum thallus showing uninucleate (N) vegetative thallus and associated Golgi dictyosome (G) and surrounding mitochondria (M) and other organelles. (d) Schematic illustration of the bothrosome showing electron-dense plug material (EDMB), ectoplasmic net (EN), feeding endoplasmic reticulum (ER), plasma membrane (PM), and thallus scales (S). (e) Biflagellate zoospore, Thraustochytrium zoospore showing cell body covered in scales and anterior (AF) and posterior flagella (PF). From Porter (1990). (f) Transmission electron micrograph (TEM) showing a longitudinal profile of S. aggregatum zoospore, showing central nucleus (N) associated Golgi body (G) and paranuclear body (PN). (g–i) Diagrams of an S. aggregatum zoospore. (g) Ventral view of the flagellar roots showing the orientation anterior (A) and posterior (P) kinetosomes and their associated roots R1–R4). (h) Ventral and (i) right views of zoospore body showing the orientation of organelles and the flagellar apparatus: anterior basal body (A); anterior flagellum (AF); Golgi body; M, mitochondria; N, nucleus; P, posterior basal body; PN, paranuclear body (G); posterior flagellum (PF); flagellar roots (R1–4) (c, d, f, g–i From Iwata et al. published in Protist http://dx.doi.org/10.1016/j.protis.2016.12.002 Figs. 1, 3, and 5 with permission. All other photographs courtesy of Professor Daiske Honda, Konan University http://syst.bio.konan-u.ac.jp/labybase/index_en.html)
Apart from the posteriorly uniflagellate chytrids all of the zoosporic organisms traditionally studied by mycologists can now be placed in the still contentious Straminipila/Alveolate/Rhizaria (SAR) superclade (Burki et al. 2008; Hackett et al. 2007; Reeb et al. 2009).
This account reviews one of the smaller groups within this lineage, the Labyrinthulomycota (predominantly labyrinthulids and thraustochytrids), and updates the pre-molecular account of the group published in the first edition of the Handbook of Protoctista by Porter (1990). Most members of the Labyrinthulomycota are heterotrophic colorless or yellowish protists that absorb nutrients in an absorptive (osmotrophic) or phagotrophic manner. They typically feed saprotrophically (but parasites are known, e.g., Schärer et al. 2007) and are key players in the detrital food web, helping to break down often intractable plant and animal remains and making these substrates more accessible to grazing amoebae and ciliates (Raghukumar 2002; Bongiorni 2012). Many thraustochytrids can also feed phagotrophically (Raghukumar 1992), and some genera such as Aurantiochytrium (Fig. 2d) and Ulkenia (Fig. 4) have a free-living amoeboid stage. The Labyrinthulomycota are often referred to as “slime nets, ” which relates to the feature shown by many of the crown genera, the formation of a network of fine, often branching and anastomosing, cytoplasmic threads (Figs. 1c, 2c, and 3a, b, d) that extend into the environment from the cell bodies and originating from a unique structure, now generally called the bothrosome (Figs. 1d and 2g, j; Porter 1990; Beakes et al. 2014). These threads provide adhesion to the substrate and absorb nutrients (as in thraustochytrids) or form trackways along which the cell bodies glide (as in the labyrinthulids; Figs. 1a and 2h, i).
Morphological features of labyrinthulids and thraustochytrids, part 2. (a–f) Micrographs of the thraustochytrid Aurantiochytrium limacinum. DIC phase (a) and fluorescent (b) images of a colony of showing cells packed with oil globules which fluoresce orange when stained with nile red. (c) Colony of vegetative cells growing on agar showing fine branching ectoplasmic net (arrowed) emanating from the body cells (scale bar = 10 μm). (d) Amoeboid cell showing granular inclusions (arrowed) (scale bar = 5 μm) (e) Biflagellate zoospore, showing typical ovoid morphology of the Thraustochytridiales (scale bar = 5 μm). (f) TEM of vegetative thallus, showing central nucleus (N), associated Golgi dictyosome (G) and surrounding lipid (L) globules and mitochondria (m). (Scale bar = 1 μm). (g) TEM of bothrosome at the surface of Aplanochytrium sp. SEK349 cell. Note the cisternae of endoplasmic reticulum feeding into the plaque of electron-dense plug material (asterisk). (h–k) Micrographs of Labyrinthula sp. AN-1565. (h) Branching “slime net” colony growing on surface of agar (scale bar = 0.5 mm). (i) DIC micrograph showing spindle-shaped colony cells which migrate along the enveloping ectoplasmic net (not visible) (Scale bar = 10 μm). (j–k) Transmission electron micrographs showing transverse (j) and longitudinal (k) sections of thallus cells. Nuclei (N) are associated with a single Golgi dictyosome (G), and cytoplasm contains lipid globules (L), mitochondria (m), and vacuoles (V). The enveloping ectoplasmic net (E) originates from the bothrosome (arrow). (Scale bars = 5 μm)
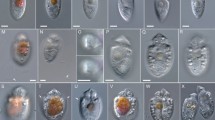
Morphology of the Amphitremida. (a–c) Micrographs of Diplophrys mutabilis from freshwater. (a) Elongated fusiform cell, showing terminal origin of ectoplasmic elements (white arrows) and contractile vacuole (black arrow). (Scale bar = 10 μm). (b) Whole mount transmission electron micrograph of cell body showing radiating branched ectoplasmic elements from cell poles. Bacteria are also shown (arrowheads) (Scale bar = 10 μm). (c) SEM image of a lyophilized cell showing circular overlapping cells and attached bacteria. (Scale bar = 1 μm) (From Takahashi et al. 2014 Protist 165: 50–65 Figs. 1b, 2a, and 3a http://dx.doi.org/10.1016/j.protis.2013.10.001 with permission). (d) DIC micrograph of an amoeba of coprophilic Sorodiplophrys stercorea showing anastomosing pseudopodia, with swellings (arrowed) (Scale bar = 10 μm) (From Tice et al. 2016, Fig. 1c Journal of Eukaryote Microbiology doi:10.1111/jeu.12311 with permission). (e) Brightfield micrograph of Amphitrema wrightianum, showing apical shell apertures (pseudostome), and green Trebouxiophyte endosymbionts. (Scale bar = 20 μm) (f) Brightfield micrograph of Archerella flavum, showing pigmented shell (test) with terminal pores. The protist cell is arrowed. (Scale bar = 20 μm) (From Gomaa et al. 2013 Fig. 1. PLoS ONE 8(1) http://dx.doi.org/10.1371/journal.pone.0053046 with permission)
The group also includes a number of unicellular colorless protist genera such as Amphifila, Amphitrema (Fig. 3e), Archerella (Fig. 3f), Diplophrys (Fig. 3a–c), and Sorodiplophrys (Fig. 3d) that produce fine rhizopodia-like structures (Anderson and Cavalier-Smith 2012; Gomaa et al. 2013; Takahashi et al. 2014). Some such as Ulkenia and related genera also have an amoeboid phase (Fig. 2d) in their life cycle (Beakes et al. 2014; Karling 1981; Porter 1990; Yokoyama et al. 2007).
The unusual set of characteristics associated with the Labyrinthulomycota has hampered their taxonomic assignment. As summarized recently (Beakes et al. 2014), labyrinthulids have been assigned to various unrelated groups, such as the Rhizopoda, Mycota, Amoebozoa, and different phyla of the Straminipila, such as Chrysophyta and Oomycota. Based on phylogenetic evidence, as summarized by Gomaa et al. (2013; Fig. 5), the labyrinthulids do not belong to any of these groups and are probably best treated as an independent phylum in the Straminipila, the Labyrinthulomycota, as proposed by Porter (1990).
The recent application of molecular phylogenetic techniques including extensive environmental sampling and sequencing of DNA has, similar to the fungi of the kingdom Mycota (Jones et al. 2011), revealed many, as yet mostly undescribed, and often probably uncultivatable, representatives of this group in diverse marine, freshwater, and terrestrial environments (Collado-Mercado et al. 2010; Diéz et al. 2001; Massana et al. 2002, 2006; Massana and Pedró-Alió 2008; Pan et al. 2017; Richards et al. 2012; Stoeck et al. 2003, 2006, 2007). This methodology has also revealed that several protist genera of previously unclear taxonomic affinity, such as Amphitrema and Archerella, which were formerly placed together with the filose testate amoebae, also belong in the Labyrinthulomycota (Gomaa et al. 2013; Pan et al. 2017; Tice et al. 2016).
Because of this phylogenetic uncertainty, as with many other protist groups, names in the Labyrinthulomycota have been published both according to zoological (ICZN) and botanical (ICBN/ICNfap) nomenclature. The majority of species within the traditional Labyrinthulomycota have been described by mycologists under the botanical code for nomenclature, whilst many recent changes were suggested under the code for zoological nomenclature and where possible both sets of nomenclature are given in this chapter.
Occurrence
The Labyrinthulomycota appear to be cosmopolitan and were considered to be saprotrophic or only weakly parasitic organisms, ubiquitous in marine and estuarine environments. The morphologically described part of the Labyrinthulomycota consists of a relatively small group of almost exclusively marine genera (Figs. 1b and 3) that typically feed saprotrophically and are an important part of the marine detrital food web (Raghukumar 2002; Bongiorni 2012). However, many thraustochytrids feed bacteriotropically (Raghukumar 2002), and some genera such as Ulkenia (Figs. 2 and 3h–j) have amoeboid stages engulfing their food. Thraustochytrids can be recovered in large numbers from marine sediments (Bongiorni 2012), including the deep sea (Raghukumar et al. 2001). Labyrinthulids are prevalent living on or within seaweeds and sea grasses, and there is an increasing evidence that they can live as parasites, commensals, or mutualists in plants (Bigelow et al. 2005; Bockelmann et al. 2012) and in other organisms, such as amoebae (Dykova et al. 2008) and mollusk tissues (e.g., Azevedo and Corral 1997).
However, most frequently they have been found associated with the surfaces of benthic algae, marine vascular plants, and detrital sediments (Porter 1990; Raghukumar 2002). Some, such as genus Althornia, are part of the free-floating eukaryotic plankton, and many others have been isolated from the marine water column (e.g., Collado-Mercado et al. 2010; Porter 1990), often in association with particulate “marine snow” (Naganuma et al. 2006; Raghukumar et al. 2001; Damare and Raghukumar 2008). Until the turn of the last millennium, the Labyrinthulomycota were considered to be exclusively marine organisms (Porter 1990), but about a decade ago, Labyrinthula terrestris has been described as a pathogen associated with turfgrass decline (Bigelow et al. 2005), and molecular studies have revealed an increasingly large number of freshwater members of this phylum (Anderson and Cavalier-Smith 2012; Gomaa et al. 2013; Richards et al. 2012). For instance, the testate protist genera Archerella and Amphitrema are common components of Sphagnum peatland, where they are often made visible by the endosymbiotic Trebouxiophyte algae (Fig. 3e, f) they contain (Gomaa et al. 2013). Many environmental sequences belonging to this clade have been isolated from anoxic sediments, which again suggests the habitats and roles occupied by these organisms is far more diverse than originally thought (Gomaa et al. 2013). Some members of the Labyrinthulomycota are genuine parasites which can have detrimental environmental impacts (such as on seagrass beds) and cause diseases of economical and ecological importance (Hatai 2012).
Literature and History of Knowledge
Because they are a small group, there are no dedicated taxonomic monographs on the Labyrinthulomycota, although illustrations of the main thraustochytrid taxa were included in the monograph of simple holocarpic biflagellate fungal-like organisms by Karling (1981). They are also included in the major systematic review of straminipilous fungi by Dick (2001). Labyrinthula was first observed by Cienkowski (1867) associated with intertidal algae in the Black Sea. The genus Thraustochytrium was first observed by Sparrow (1936), who described T. proliferum associated with benthic algae from Woods Hole, Massachusetts, and later monographed zoosporic fungi from various habitats (Sparrow 1960, 1973, 1976). Thraustochytrids were initially included in the oomycetes until the mid-1970s, when ultrastructural investigations revealed significant differences with between them and other biflagellate “zoosporic fungi” of the oomycetes (reviewed by Beakes et al. 2014; Perkins 1976; Moss 1985, 1986; Porter 1990). Physiological aspects of the thraustochytrids were reviewed by Goldstein (1973) and again showed important differences with other biflagellate “fungi.” The next significant advances in knowledge came with the advent of molecular systematics. Molecular phylogeny confirmed that labyrinthulids and thraustochytrids were part of the straminipilous lineage as suggested by their ultrastructure (Patterson 1989), although the precise branching order of the various straminipilous clades remained poorly resolved (Leipe et al. 1994). It was not until the advent of multigene analyses based on conserved protein genes that there was a clearer understanding of how the main lineages were related (Tsui et al. 2009; Tsui and Vrijmoed 2012 – see later sections). Only recently have environmental sequencing projects greatly expanded the knowledge on the diversity, habitats, and distribution of Labyrinthulomycota s.lat., as outlined by Pan et al. (2017) (Fig. 5).
Practical Importance
Thraustochytrids (Figs. 2a–g and 4) play important roles in nutrient cycling in marine ecosystems such as mangroves (Porter 1990; Raghukumar 2002), the open ocean, and sediments (Bongiorni 2012; Collado-Marcado et al. 2010; Kimura et al. 2001; Raghukumar et al. 2001). Although usually present in relatively low cell numbers, because of their large cell size compared with bacterioplankton, they nevertheless still make a significant contribution to the overall biomass of the oceans and probably play a significant role in the “microbial loop” by packaging and recycling nutrients in various communities of marine organisms (Raghukumar and Damare 2011).
Life cycles. Schematic drawings summarizing the variations in the life cycles of various thraustochytrid and labyrinthulid species (Adapted from http://syst.bio.konan-u.ac.jp/labybase/index_en.html)
Many labyrinthulomycetes (Fig. 2h–k) are parasites that can have a major impact on marine ecosystems or individual species. Muehlstein and Porter (1991) identified a new pathogenic species, Labyrinthula zosterae, as the causal agent of the eelgrass (Zostera marina) wasting disease . This disease of eelgrass first appeared in the 1930s and was responsible for the destruction of most of the vast subtidal stands of this vascular plant along the Atlantic coasts of North America and Northern Europe. Since larval stages of shellfish, such as oysters, scallops, and shrimp, all depend on eelgrass as a nursery bed, the loss of these stands has also detrimental effects on other ecosystems and the seafood industry. Since the discovery of Labyrinthula zosterae as a pathogen causing eelgrass disease, also the endophytic presence of Labyrinthula species in eelgrass has been documented, suggesting that various environmental factors might determine the virulence of Labyrinthula species (Bockelmann et al. 2012). However, also in terrestrial ecosystems, labyrinthulomycetes might play important, yet mostly unexplored, roles, as evidenced by the species Labyrinthula terrestris, the causal agent of a dieback disease of over-irrigated turfgrasses (Bigelow et al. 2005; Craven et al. 2005; Olsen 2007; Douhan et al. 2009) such as those found on coastal golf links and older reports of labyrinthulomycetes in inland habitats with high salinity (Amon 1978).
Thraustochytrid infections can cause serious economic losses to commercially reared shellfish. The most well-studied examples include the so-called “QPX thraustochytrid parasite” of the northern Quahog clam (Azevedo and Corral 1997; Lyons et al. 2005, 2007; Garcia-Vedrenne et al. 2013) and Aplanochytrium haliotidis infecting abalone (Bower 1987a, b; Bower et al. 1989). Other thraustochytrid species have also been shown to cause mass mortality amongst marine animals such as the nudibranch, Tritonia diomedea (McLean and Porter 1987), and cephalopods such as the lesser octopus, Eledone cirrhosa (Polglase 1980), and a squid, Illex illecebrosus (Jones and O’Dor 1983).
There has been much interest in exploiting marine thraustochytrids for a wide range of products they synthesize (particularly lipids; Fig. 2b), including the production of biodiesel, long-chain omega-3 fatty acids, and exopolysaccharides (Chang et al. 2012). In particular it is hope to culture them as an alternative to fish as a source of polyunsaturated fatty acids (PUFAs), such as docosahexaenoic acid (DHA) , which are important dietary supplements for both animals (Miller et al. 2007) and humans (Kabayashi et al. 2011; Ragukumar 2008). Recently genetic manipulation has been used to improve fatty acid production in thraustochytrids (Kobayashi et al. 2011), and this work has been the main driving force behind sequencing the genome of Aurantiochytrium (Liu et al. 2016). Currently, Schizochytrium species are used for the commercial production of DHA (Winwood 2013). Squalene, a compound reported to reduce the incidence of coronary heart disease and cancer, accumulates in the thraustochytrid Aurantiochytrium mangrovei grown in the presence of the terbinafine (Fan et al. 2010).
Habitats and Ecology
Methodology for Detection and Enumeration
Labyrinthulomycetes in the natural marine environment, including the water column, have been documented using various methods. Culture-based methods for determining and quantifying the prevalence of thraustochytrids in nature, using serial dilution and pine-pollen baiting, were pioneered by Gaertner (1968). The direct observation epifluorescence technique described by Raghukumar and Schaumann (1993) is another quantitative method that was considered to be a more sensitive and direct method for detecting and enumerating labyrinthulomycetes. More recently the introduction of molecular techniques involving sequencing of extracted environmental DNA and developing labyrinthulomycete-specific molecular probes has further extended the places where these organisms have been recorded from (Pan et al. 2017). Until now, they have been found in such extreme environments as arctic, subarctic, and antarctic habitats (Bahnweg and Sparrow 1974; Moro et al. 2003; Naganuma et al. 2006; Riemann and Schrage 1983; Stoeck et al. 2007), oceanic environments of the Indian Ocean (Damare and Raghukumar 2008, 2010), marine sediments (e.g., Bongiorni 2012), saline soils (Aschner 1958; Booth 1971; Bigelow et al. 2005), the deep sea (e.g., Amon 1978; Raghukumar et al. 2001), and shallow water hydrothermal vents (Colaco et al. 2006).
Environmental Tolerances of the Labyrinthulomycota
Apparently, labyrinthulomycetes seem to have a wide range of tolerance to different salinity conditions. Some Labyrinthula isolates have been found associated with the roots and root hairs of trees in sandy soils irrigated with low salinity (4.3‰) water (Aschner 1958). Labyrinthulids have also been isolated from inland saline soils (Amon 1978). Many thraustochytrids (including isolates of the genera Thraustochytrium, Schizochytriu, and Ulkenia) have been isolated from habitats reflecting a wide range of salinities from weakly brackish waters (3‰) to briny salt evaporation ponds (150‰) indicating they may be thought of as euryhaline organisms (Jones and Harrison 1976). However, none of the species of thraustochytrids that Bahnweg (1979a, b) studied would grow in pure culture above a salinity of 40‰, and Thraustochytrium pachyder um appears to be one of the few species so far described that shows growth and zoospore formation at salinities up to 60‰ (Schneider 1981). There are also species and isolates found in habitats of more or less constant salinity and thus might actually be stenohaline.
The ability to withstand other extreme or fluctuating environmental or culture conditions has been reported amongst thraustochytrids (e.g., Banweg 1979a, b; Kuznetsov 1981) – some can apparently resist repeated cycles of drying and freezing (anabiosis). There are reports that thraustochytrids from both frozen arctic coastal soil samples and dried 50-year-old herbarium sheets of marine algae were successfully isolated and grown in culture (Kuznetsov 1981). Isolates of thraustochytrids also survived drying for several days (Jain et al. 2005) or even years (Kuznetsov 1981).
Habitats of Labyrinthulales
Species of Labyrinthula are found in estuarine and near-shore marine habitats throughout the world associated with (or isolated from) organic detritus, macroalgae, diatoms, and particularly estuarine plants, such as mangroves and other marine vascular plants (Porter 1990). In hanging-drop or other laboratory cultures, the cells of Labyrinthula readily colonize a variety of vascular plant and algal tissues. They penetrate the cell walls and appear to decompose the cellular contents. In laboratory culture, labrinthulids are capable of decomposing many different microorganisms as a substrate, including bacteria, yeast, hyphal fungi, diatoms, filamentous algae, and other thraustochytrids (Perkins 1976; Porter 1990). Species of Labyrinthula are reliably isolated from submerged moribund or adrift leaves of marine vascular plants and pieces of filamentous or thalloid macroalgae. It has long been believed that healthy algae and marine grasses do not contain Labyrinthula cells within their tissues (Porter 1990), although these organisms can be regularly isolated from their tissues and Bockelmann et al. (2012) reported their endophytic presence. However, Labyrinthula is usually not necrotrophic but rather feeds on epibiotic microorganisms and decomposing plant and algal material (Porter 1990). Based on current knowledge, the genus Labyrinthula is primarily associated with coastal environments. The genus Stellarchytrium associated with starfish has recently been described (FioRito et al. 2016) and represents a case in which an actual organism was found for a group otherwise only known from environmental sequencing, in this case, the LAB1/6/8 clade (Pan et al. 2017).
Habitats of Thraustochytriales
Species of thraustochytrids (which includes species now classified in both the Thraustochytriaceae and Aplanochytriaceae) have also been isolated from estuarine and marine habitats throughout the world. Members of the thraustochytrids are able to grow in culture on a variety of plant- and animal-derived substrates (Perkins 1973). For instance, they have been observed growing on the spore cases of vesicular-arbuscular mycorrhizal fungi from barrier sand dunes (Koske 1981). They are generally isolated from decomposing algal and plant material, as well as from sediments, although they may also be found in plankton collected in offshore trails (Damare and Raghukumar 2010). In general, thraustochytrids seem to be mostly surface inhabitants of particulate organic material, primarily saprotrophic in their nutrition. In tropical and sub-tropical areas, mangrove plants (e.g., Avicennia, Bruguiera, Kandelia, and Rhizophora) are probably the most well-studied habitat in which to find labyrinthulomycetes (e.g., Fan and Chen 2006; Leaño 2001), where they appear to be primarily as saprobes colonizing the surface of organic detritus.
Thraustochytrids appear to be amongst the initial colonizers of fallen senescent mangrove leaves, alongside oomycetes (Thines 2014; Marano et al. 2016), and thus play an important role in nutrient cycling through exogenous production of their cellulase and xylanase degradation enzymes (Fan et al. 2002; Leaño 2001; Raghukumar 2002; Raghukumar et al. 1994). Thraustochytrids can be recovered in large numbers from marine sediments including from the deep sea (Bongiorni 2012). This group thereby contributes significantly to the biomass in the estuarine or marine environment.
In contrast to labyrinthulids, thraustochytrids appear to grow poorly on living algae and vascular plants. This has been primarily attributed to the presence of secondary metabolites with antimicrobial properties which limit the growth and propagation of these organisms (Raghukumar 2002). However, 7 days after leaf fall, thraustochytrids were found colonizing fallen leaves of Rhizophora apiculata (Raghukumar et al. 1995). Labyrinthulomycetes isolated from mangrove areas include Schizochytrium sp., Thraustochytrium sp., Ulkenia sp., and several unidentified strains of Labyrinthula sp. and Aplanochytrium sp. (Leaño 2001; Leander et al. 2004; Yokochi et al. 2001). The extensive colonization by thraustochytrid thalli on the surfaces of decomposing seaweeds has been noted (Miller and Jones 1983). Thraustochytrids, as epibionts, are probably feeding on other epibiotic microorganisms and decomposing plant and algal material. It is reported that the extent of colonization increased with the rate of decomposition; thus, they are probably saprotrophic followers of labyrinthulids, oomycetes, and zoosporic fungi. Yokochi et al. (2001) reported not only Labyrinthula sp. as a saprobe on Padina arborescens and Sargassum sp. but also Aplanochytrium sp. on Dictyota cervicornis, Chaetomorpha sp., and Cladophora sp. (Leander et al. 2004). Aplanochytrium minutum and Ulkenia visurgensis were found associated with decaying Sargassum cinereum (Sathe-Pathak et al. 1993).
Some thraustochytrids may also be capable of necrotrophic/parasitoid growth on marine invertebrates, particularly mollusks such as nudibranchs (McLean and Porter 1987), squid (Jones and O’Dor 1983), and octopus (Polglase 1980). Thraustochytrids are also regular components of the gut microbiota of certain echinoids (Wagner-Merner et al. 1980) and have been found in a variety of Mediterranean sponges (Höhnk and Ulken 1979) and on the surface mucus of hermatypic corals (Harel et al. 2008). Thraustochytrids themselves may host viruses (Perkins 1976), e.g., herpes-type DNA virus particles (Kazama and Schornstein 1973). As herpes-type viruses are present in some vertebrates and invertebrates, (Segarra et al. 2010; Evans et al. 2017), this raises the possibility that thraustochytrids may be virus vectors for other organisms.
In spite of the many reports of thraustochytrids isolated from numerous substrates and locations, there have been surprisingly few direct observations of thraustochytrids in nature. Schizochytrium-like thalli were observed parasitizing colonies of the diatom Thalassionema collected from the North Sea (Gaertner 1979). Thraustochytrid-like thalli in Antarctic sediments fixed immediately after collection have been described (Riemann and Schrage 1983). Although diagnostic features were not presented, the thraustochytrids in these samples most closely resemble the genus Aplanochytrium.
Parasitic and Symbiotic Relationships
Parasitism is another ecological strategy found in a few species of Labyrinthula. Labyrinthula spp. have been isolated from the marine algae Chaetomorpha, Lyngbya, Cladophora, Rhizoclonium (Raghukumar 1987a, b), and several marine vascular plants, such as Cymodocea, Posidonia, Spartina, Thalassia, and Zostera (Bockelmann et al. 2012; Garcias-Bonet et al. 2011; Stowell et al. 2005), although it is unlikely whether all these are parasitic associations. However, as mentioned previously, Labyrinthula zosterae has been identified as the cause of the wasting disease of eelgrasses (Zostera capricorni, and Zostera marina) resulting in the decline of eelgrass population (Armiger 1964; Muehlstein et al. 1988); and Labyrinthula terrestris has been identified as the cause of rapid blight on turfgrasses (Bigelow et al. 2005; Stowell et al. 2005; Craven et al. 2005; Olsen 2007). Garcias-Bonet et al. (2011) studied the occurrence and pathogenicity of Labyrinthula sp. in Mediterranean seagrass meadows. They found that their isolates could infect a number of different seagrass genera (Posidonia, Cymodocea, and Zostera) and indicates their isolate had a broader host range than found in most North American studies which indicated that pathogenicity was host genus-specific (Muehlstein et al. 1988; Short et al. 1993; Vergeer and den Hartog 1991, 1994). Labyrinthuloides (now classified as Aplanochytrium) schizochytrops was commonly isolated from living plants of the seagrass Halodule wrightii and was thought to be living as an endophyte, although may also have been responsible for a brownish discoloration on the host leaves (Quick 1974). The Schizochytrium-like thalli parasitizing the diatom Thalassionema (Gaertner 1979) were later followed by additional reports of thraustochytrids as diatom pathogens. For example, Ulkenia amoeboidea was found capable of infecting a number of diatoms, including Coscinodiscus sp., Grammatophora sp., Melosira sp., Navicula sp., and Nitzschia sp. (Raghukumar 2006). However, it remains unclear if diatom parasitism constitutes a major ecological niche of Labyrinthulomycota and if these infections have a significant ecological impact.
Many marine invertebrates (e.g., corals, clams, flatworms, sea stars, and sea urchin) have been reported to harbor labyrinthulomycetes, and in some, this relationship may be parasitic, as with Aplanochytrium haliotidis on abalone (Bower 1987b) and the QPX thraustochytrid parasite on Quahog clam (Azevedo and Corral 1997; Lyons et al. 2005, 2007). The latter has been most extensively studied as an animal pathogen, and its genome has recently been sequenced in order to try and understand the basis of virulence (Garcia-Verdrenne et al. 2013). Recently a newly recognized species, Thraustochytrium caudivorum, was shown to parasitize the marine free-living flatworm Macrostomum lignano (Schärer et al. 2007), causing lesions that can lead to the dissolution of the posterior part or even complete animal. Three newly described Labyrinthulales species, Stellarchytrium dubum, Oblongichytrium porteri, and Aplanochytrium blankum, were isolated from dermal tissues of ochre sea stars (Pisaster ochraceus) that were exhibiting symptoms of starfish wasting disease (FioRito et al. 2016), although a direct causal relationship with the disease has yet to be established. Thraustochytrids are also capable of necrotrophic growth (perhaps parasitic) on marine invertebrates, particularly mollusks such as nudibranchs (McLean and Porter 1987), octopus (Polglase 1980), and squid (Jones and O’Dor 1983).
Other Labyrinthulomycota appear to have commensal relationships with their hosts such as Labyrinthula and Oblongichytrium multirudimentale on the coral Fungia granulosa (Kramarsky-Winter et al. 2006; Harel et al. 2008) or are saprobic such as Aplanochytrium minuta on scleractinian coral mucus (Raghukumar and Balasubramanian 1991). Thraustochytrids are regular components of the gut microbiota of certain echinoids (Wagner-Merner et al. 1980) and have been found in a variety of Mediterranean sponges (Höhnk and Ulken 1979), although details of these relationships are still unknown. Interestingly, thraustochytrids may host viruses (Perkins 1976), and herpes-type DNA virus particles have been described in a Thraustochytrium sp. (Kazama and Schornstein 1973). This is the only herpes-type virus to have been found in a host that is not a vertebrate and raises the possibility that thraustochytrids may be virus vectors for other organisms (Porter 1990). However, Labyrinthulomycota also carry RNA viruses of unknown host spectrum (Takao et al. 2005).
Freshwater and Terrestrial Labyrinthulomycota
Until the advent of molecular systematics, it had been generally assumed that there were no genuinely freshwater or terrestrial members of the Labyrinthulomycota, although there were historic reports of Labyrinthula species infecting the freshwater alga Vaucheria (Zopf 1892). Recently it was shown that a number of phagotrophic freshwater protists (Fig. 3) such as the unicellular Diplophrys parva and D. mutabilis in the Thraustochytriales (Anderson and Cavalier-Smith 2012; Takahashi et al. 2014) and unicellular Archella flavum and Amphitrema wrightianum (Gomaa et al. 2013) and the sorocarpic Sorodiplophrys stercorea (Tice et al. 2016) in the Amphitremida all cluster in the Labyrinthulomycota clade. All of these heterotrophs are characterized by having fine filose pseudopodia. Diplophrys parva was isolated from the intestinal tract of a goldfish (Anderson and Cavalier-Smith 2012) and D. mutabilis from a freshwater lake (Takahashi et al. 2014), whereas Archerella and Amphitrema were both free-living protists isolated from wet Sphagnum moss (Gomaa et al. 2013). The coprophilic genus Sorodiplophrys was isolated from horse and cow dung (Tice et al. 2016). Environmental sampling has also revealed many more isolates belonging to the Amphitremida and Amphifilidae clades (Fig. 5), including isolates from various terrestrial soils, freshwater ecosystems, and anoxic sediments (Anderson and Cavalier-Smith 2012; Gomaa et al. 2013; Takahashi et al. 2014; Tice et al. 2016).
Phylogeny. Trees summarizing phylogenetic relationships within the Labyrinthulomycota and with other Straminipila. Molecular phylogenetic scheme based on small subunit (SSU) rRNA gene sequences showing phylogenetic position Archerella and Amphitrema within the Amphitremida. This tree also shows relationship of Labyrinthulomycota and other heterokont members of the Straminipila. The tree was adapted from Gomaa et al. 2013. PLoS One 8(1) http://dx.doi.org/10.1371/%20journal.pone.0053046 with permission. Phylogenetic scheme for Labyrinthulomycota based on partial 18S rRNA sequences, including also environmental sequences. Adapted from Pan et al. 2017.
Characterization and Classification
Thallus (Cell) Morphology and Ultrastructure
Members of the Labyrinthulaceae are characterized by forming colonies of spindle-shaped thalli (cells) that are ensheathed in a membranous ectoplasmic network which form a branched track system along which the cells freely migrate (Figs. 1a and 2h, i). Members of the Thraustochytriaceae on the other hand form ovoid or spherical thalli, which are associated with a fine ectoplasmic network of rhizoid (rhizopodia)-like threads (Fig. 3c–i) which act as anchoring and feeding structures (Perkins 1976; Bremer 1976; Moss 1985, 1986; Porter 1990). In terms of size and general appearance, this gives thraustochytrid thalli a superficial similarity to those of hyphochytrids and chytrid fungi (Karling 1981). The planktonic genus Althornia lacks rhizoids and absorbs nutrients directly from the environment (Karling 1981; Moss 1986; Porter 1990). The thalli of the Aplanochytridiaceae (now placed in the Labyrinthulales) superficially resemble thraustochytrids (Figs. 3g and 4) but are able to glide slowly along the surface of their ectoplasmic threads (Leander and Porter 2001). A number of previously enigmatic unicellular, sometimes colonial protists with fine-branching rhizopodia often arising bipolarly from the cells (Fig. 3a, b, d, f) have now been included in the Labyrinthulomycota, in a number of newly created families such as the Amphifilidae, Diplophryidae, and Sorodiplophryidae (Anderson and Cavalier-Smith 2012; Takahashi et al. 2014; Tice et al. 2016). In addition, the mixotrophic testate amoeba-like genera in the Amphitremidae, Amphitrema, Archerella, and Paramphitrema have cells protected in flask-shaped puncate shells (Fig. 3e, f) from which the rhizopodia emanate (Gomaa et al. 2013).
The Labyrinthulomycota have a typical straminipilous cytoplasmic ultrastructure with mitochondria with tubular-vesiculate cristae and prominent Golgi dictyosomes (Figs. 1f and 2f, g, j, k; Perkins 1976; Moss 1985, 1986; Porter 1990; Anderson and Cavalier-Smith 2012; Iwata et al. 2016). The cells usually contain cytoplasmic vacuoles and oil globules (Figs. 1c and 2a, b, f, j, k). However, an ultrastructural feature that defines the Labyrinthulales and Thraustochytriales is that their ectoplasmic nets originate from the thallus body from a unique endomembrane complex associated with an electron-dense plaque on the plasma membrane (now known as the bothrosome – Figs. 1d and 2g) from which cisternae of endoplasmic reticulum radiate (Perkins 1976; Moss 1985, 1986; Porter 1990; Iwata et al. 2016). Previously this body has also been variously referred to as the sagenogenetosome (Perkins 1976) or sagenogen (Dykstra and Porter 1984). The early stages of the bothrosome complex development and net formation following zoospore settlement have recently been described in Schizochytrium by Iwata et al. (2016). This study has shown that the bothrosome forms within minutes of zoospore settlement at the anterior-ventral pole of the cell close to the Golgi body. Immunofluorescence labelling revealed that actin co-localized with newly formed bothrosome co-localized, and that within 18 min of settlement, the ectoplasmic net system had formed, with net filaments rich in actin (Iwata et al. 2016). The ectoplasmic net, unlike the rhizoid system of hyphochytrids and chytrids, is not walled (Figs. 1c, d, and 2j, k) and as well as containing actin, only contains cisternae of endomembrane (Moss 1985, 1986; Porter 1990; Takahashi et al. 2014). However, a classical bothrosome structure does not appear to be associated with slender rhizoids/filopodia of the unicellular protist-like members of the phylum in the Ampifilaceae (e.g., Amphifila marina – Dykstra and Porter 1984).
Another major difference between the thalli of the Labyrinthulomycota compared to other straminipilous fungi is that the thallus is surrounded by Golgi-derived ovoid, round or hexagonal scales (Fig. 3c; Perkins 1976; Moss 1985, 1986; Porter 1990) which are not cellulosic but composed of sulfated polysaccharides containing fucose or galactose (Bahnweg and Jäckle 1986; Honda et al. 1999; Moss 1985, 1986). In older thalli layers, scales can form a consolidated wall (Fig. 2f) around the thallus but do not coat the tracks or rhizoids (Perkins 1976; Dykstra and Porter 1984; Porter 1990). Surface scales are also a feature of the planktonic unicellular genus Amphifila marina (formerly Diplophrys – see Anderson and Cavalier-Smith 2012), which led Dykstra and Porter (1984) to suggest this enigmatic protist had Labyrinthulomycete affiliations. Cells of the freshwater heterotrophs Diplophrys parva (Anderson and Cavalier-Smith 2012) and D. mutabilis (Takahashi et al. 2014) are also coated in small Golgi-derived capsule-shaped or ovoid scales (Fig. 3c). In contrast, the cells of species in the Amphitremidae are contained in a thick rigid lightly pigmented shell (Fig. 3e, f; Gomaa et al. 2013).
Zoospore Formation and Fine Structure
The ways in which these thalli differentiate into motile zoospores and proliferate have been the main defining characteristics (see Karling 1981) of the thraustochytrid genera (Fig. 4), although it now appears that this morphology is poorly correlated with underlying genetic relatedness (Yokohama and Honda 2007; Yokohama et al. 2007; Beakes et al. 2014). In some Thraustochytrium species, the whole thallus cytoplasm differentiates into biflagellate zoospores, which are released by the general splitting and disintegration of the thallus wall (Fig. 4; Karling 1981). In other Thraustochytrium spp., internal proliferation of new thalli occurs from cytoplasm cleaved from the basal portion of thallus, concomitantly with the main compartment cleaving into zoospores (Karling 1981; Beakes et al. 2014). Development of the thallus in the genus Aplanochytrium is similar except that only non-motile aplanospores are formed (Fig. 4). The genus Schizochytrium has thalli which divide by successive bipartitions to form progressively smaller units (Figs. 2c and 4), in which the zoospores ultimately differentiate (Karling 1981). Several genera (Botryochytrium, Parietichytrium, Sicyoidochytrium, and Ulkenia – Fig. 4) in the Thraustochytriales have a more complex life cycle in which a free-living amoeboid cell (Figs. 2d and 4) is released from the original parental thallus and which then settles and eventually differentiates into zoospores (Beakes et al. 2014).
Straminipilous zoospores range in size between 3 and 15 μm (Dick 2001) and, as in the Labyrinthulomycota, many are reniform with laterally inserted flagella (Figs. 1b, e, f, and 2e). The straminipilous zoospore has a remarkably conserved overall organization and structure supporting the origin of this clade from a common flagellate ancestor (Tsui et al. 2009; Beakes et al. 2014). The anterior flagellum in all members of the Straminipila is decorated with two parallel rows of tripartite tubular hairs (TTH) and usually four to five times the overall zoospore body length (Fig. 1b, e; Perkins 1976; Porter 1990). The TTH are made of proteins and serve to reverse the flagellum thrust, in effect pulling straminipilous zoospores through the water (Dick 2001). Thraustochytrid, but not labyrinthulid, zoospores are unusual in that the zoospore body is also coated in small scales (Fig. 1e, f; Perkins 1976; Kazama 1980; Porter 1990).
All straminipilous flagellate cells share the same underlying flagellar rootlet system (Fig. 1g–i) which shows a remarkable degree of conservation throughout the lineage (Barr and Désaulniers 1989; Andersen et al. 1991; Dick 2001; Iwata et al. 2016). Zoospores of biflagellate members of the Straminipila have four rootlets, two associated with each flagellum (Andersen et al. 1991; Barr and Allan 1985; Barr and Désaulniers 1987; Iwata et al. 2016). The R3 anterior rootlet is composed of three microtubules, and curves around the anterior end of the zoospore and from which on one side emanate a series of microtubular ribs (Fig. 1h, i; Beakes et al. 2014; Iwata et al. 2016). Labyrinthulomycete zoospores appear to lack the striated fan between the kinetosomes that are a feature of oomycete zoospores (Fig. 1g; Barr 1981; Barr and Allan 1985; Porter 1990; Iwata et al. 2016). Unusually for members of the Straminipila, the Labyrinthulomycota do not have a typical transitional helix (TH) structure associated above the flagellar plate but do have a similarly placed cone-like structure and electron-dense plug (Barr and Allan 1985; Beakes et al. 2014; Cavalier-Smith and Chao 2006). Nuclear division has been investigated by Perkins (1970) and Kazama (1974) and shows similarity to other members of the Straminipila (Beakes et al. 2014).
Sexual Cycle
Most members of the Straminipila appear to be diploid organisms that undergo gametic meiosis (Dick 2001; Sims et al. 2006). However, knowledge of the precise timing of meiosis and plasmogamy in the Labyrinthulomycota is still very uncertain (Porter 1990; Beakes et al. 2014). In labyrinthulids, evidence of meiosis has been found in thalli dividing up to produce flagellate zoospores (Perkins and Amon 1969; Porter 1990), but precisely where syngamy takes place has still not been established. However, epibiotic resting spores are produced by some species of thraustochytrids, although it has not been established if these are the result of sexual reproduction (Karling 1981; Porter 1990).
Classification and Systematics
Even though investigations of the past three decades have revealed new species, cytological details, and development cycles, the complete life cycle of any species of Labyrinthula remains to be worked out (Porter 1990; Beakes et al. 2014). However, it has now been established that Labyrinthulomycetes together with the human pathogen Blastocystis and the ciliate-like opalinids formed one major, early diverging branch of the Straminipila and that the Hyphochytriomycota, Oomycota, and golden-brown photosynthetic Ochrophyta formed another separate lineage, although both share a common ancestor (Fig. 5).
Prior the era of molecular systematics, the Labyrinthulomycota were divided into two families, the Labyrinthulaceae and the Thraustochytriaceae (Karling 1981; Porter 1990; Dick 2001) within a single order, the Labyrinthulales (or Labyrinthulida). The Labyrinthulaceae contained a single genus, Labyrinthula whereas the Thraustochytriaceae had around a dozen genera, mainly defined by thallus morphology and differentiation (Perkins 1976; Karling 1981; Moss 1985; Porter 1990; Dick 2001). The first in-depth molecular systematic study of the group was carried out by Honda et al. (1999) based on SSU rRNA gene sequence comparisons. Their isolates fell into two major clades, which they named the “labyrinthulid phylogenetic group” (LPG) and the “thraustochytrid phylogenetic group” (TPG) (Honda et al. 1999). The LPG clade included Labyrinthula and Aplanochytrium (syn. Labyrinthuloides) in one subclade and Schizochytrium minutum and Thraustochytrium multirudimentale in another. The TPG clade contained genera such as Schizochytrium , Ulkenia, as well as many Thraustochytrium spp. (Honda et al. 1999). Rather than the straightforward separation of the labyrinthulids and thraustochytrids, these studies revealed for the first time that the labyrinthulids in particular were part of a more diverse monophyletic assemblage that included a number of species that had traditionally been considered to be thraustochytrids. However, the LPG and TPG clades correlated well with the sugar composition of their thallus walls (Honda et al. 1999), with genera in the LPG clade predominantly having fucose and those in the TPG clade having galactose as their major cell wall constituents (Honda et al. 1999). A concurrent study by Leander and Porter (2001) however, suggested there were three major clades within the Labyrinthulomycota. There was an additional clade that included two Labyrinthuloides species, L. yorkensis, and L. minuta. These were subsequently transferred to the genus Aplanochytrium in a new family, the Aplanochytriaceae/Aplanochytriidae (Anderson and Cavalier-Smith 2012; Leander et al. 2004), which was sister to the Labyrinthulaceae.
The third clade represented the residual Thraustochytriaceae, containing many of the traditional thraustochytrid genera together with the enigmatic, bothrosome-lacking, planktonic protist Diplophrys (now Amphifila) marina and two isolates of the Quahog clam pathogen (so-called QPX isolates). What these molecular studies also highlighted was that many of the traditional thraustochytrid genera, such as Schizochytrium, Thraustochytrium, and Ulkenia, which were based on patterns of thallus development, were paraphyletic or polyphyletic (Honda et al. 1999; Leander and Porter 2001; Leander et al. 2004) showing that traditional morphological characters were not good indicators of genetic relatedness. Subsequent studies have led to a radical revision in thraustochytrid nomenclature, with the introduction of many new genera (Aurantiochytrium, Japanochytrium, Oblongichytrium, Parietichytrium, Sicyoidochytrium, and Stellarchytrium) based on combined molecular and biochemical characteristics (Yokoyama and Honda 2007; Yokoyama et al. 2007; FioRito et al. 2016). A recent taxonomic analysis of labyrinthulomycetes phylogenies is shown in Fig. 5 (adapted Gomaa et al. 2013; Pan et al. 2017). The order Labyrinthulales s. lat. includes a number of genera (Aplanochytrium, Stellarchytrium, and some Thraustochytrium spp.) that would have previously been placed in the Thraustochytriaceae. Some recent analyses have also separated another, morphologically unremarkable, thraustochytrid-like clade encompassing the genus Oblongichytrium, into their own separate family (Oblongichytriidae; Fig. 5; Pan et al. 2017).
The taxonomic subdivision of the Labyrinthulomycota is still in flux and has changed significantly in the last decade as a result of molecular phylogenetic investigations of both the core labryrinthulids and thraustochytrids, but also other groups of heterotrophic protists that are now known to be related. As a consequence of the above taxonomic studies and a series of more recent phylogenetic investigations (Colladao-Mercado et al. 2010; Anderson and Cavalier-Smith 2012; FioRito et al. 2016; Gomaa et al. 2013; Pan et al. 2017; Takahashi et al. 2014; Tice et al. 2016), there seem to be four or five higher-level clades within the phylum (excluding clades only known from environmental sequencing), namely, Labyrinthulales/Labyrinthulida, Thraustrochytriales/Thraustrochytrida, “Amphifilales/Amphifilida,” Amphitremidales/Amphitremida, and “Oblongichytriales/Oblongichytrida.” However, what is becoming increasingly clear from environmental sequencing is that they are a diverse group of which the vast majority of species still awaits discovery (Worden and Not 2008; Collado-Mercado et al. 2010; Richards et al. 2012; Gomaa et al. 2013; Ueda et al. 2015; Pan et al. 2017). As for most environmental lineages, only partial SSU sequences are available which has been proven to have an insufficient resolution for the deeper splits of the Labyrinthulomycota; it remains unclear, how many of the lineages known only from environmental sequencing can be assigned to the orders given above. In the most comprehensive analysis of environmental sequences currently available, Pan et al. (2017) recognized several additional lineages of the Labyrinthulomycota basal to the known orders or in unresolved positions, which group in four clades, mostly with low to moderate support. These clades, such as the LAB1/6/8 clade containing Stellarchytrium dubum, might deserve family- or order-level status once their members have been studied in more detail, e.g., in multigene phylogenies.
Class Labyrinthulomycetes/Labyrinthulomorpha Labyrinthulea? (Lister 1891) Olive ex Cavalier-Smith 1986
Order Labyrinthulales/Labyrinthulida E A Bessey 1950/Doffein 1901
Family Aplanochytriaceae/Aplanochytriidae Leander Ex Cavalier-Smith 2012
A monotypic family formerly included in thraustochytrids. They have typical ovoid to spherical thalli, attached to their substrate by a basal ectoplasmic net that only form non-motile aplanospores. However, unlike members of the Thraustochytriaceae, the thalli are able to slowly glide along the rhizoids. The genus, Aplanochytrium (which subsumes the genus Labyrinthuloides), contains around half a dozen described genera, but there are probably many undescribed species based on environmental sequencing.
Family Labyrinthulaceae/Labyrinthulidae Haeckel 1868/Cinekowksa 1867
This family contains the classic “ slime nets,” which form a colony of spindle-shaped thalli that are contained within a branching ectoplasmic network within which the cells migrate. The gliding motility of the cells, which at times is as fast as 100 μm/min, probably driven by a calcium-dependent contractile system of actin-like proteins in the ectoplasmic network (Nakatsuji and Bell 1980). Each cell has a single bothrosome connecting it to the ectoplasmic network. There are around a dozen or so species that have been recognized (Dick 2001). Most are saprotrophs associated with marine debris and decaying macroalgae and marine macrophytes. However, some species have been shown to be the causal agents responsible for the wasting disease of eelgrass (Zostera) beds (Muehlstein and Porter 1991) and the turfgrass dieback (Craven et al. 2005).
Family-Level Clade “Stellarchytriaceae/Stellarchytriidae” Undescribed, LAB 1/6/8
This clade, which possibly needs to be described as a new family or even order, is provisionally placed in the Labyrinthulales and contains various lineages known only from environmental sequencing (Pan et al. 2017) and the recently discovered species Stellarchytrium dubum (FioRito et al. 2016). Stellarchytrium dubum was isolated from diseased starfish, but its role in causing starfish wasting disease still needs to be investigated in detail.
Order Oblongichytriales/Oblongichytrida
Family Oblongichytriaceae/Oblongichytriidae Cavalier-Smith 2012
This monotypic family was first recognized as a result of molecular sequencing by Yokoyama and Honda (2007). It contains around a half-dozen species that were formerly included in the genus Schizochytrium based on thallus development. The family name is derived from their slender oblong zoospores they produce rather than the more ovoid zoospores typical of the Thraustochytriales. It appears to form an early diverging clade from the same root as the Labyrinthulales lineage (Fig. 5).
Order Thraustochytriales/Thraustochytrida Sparrow 1973
Even though it has now been split, this still is the largest and most diverse order in the Labyrinthulomycota with the most genera and species. They produce relatively small epibiontic thalli usually attached to substrate by a fine ectoplasmic network of fine-branched anastomosing filaments, which have role in both substrate attachment and feeding. Most are marine organisms that are saprotrophic epibiontic colonizers of a variety of marine detritus, but there are a number of pathogens, mostly of marine invertebrates.
Family Althornidiaceae/Althorniidae Jones and Alderman 1972
This monotypic and monospecies (A. crouchii) is the only truly planktonic thraustochytrid as it completely lacks the usual ectoplasmic network. It is also the only genus for which there is at present no sequence data, and therefore its taxonomic placement must be considered as provisional.
Family Thraustochytriacae/Thraustochytriidae Sparrow ex Cejp 1959
Typically thraustochytrids are not colonial but grow by enlargement of cells which develop either into single ovoid or globular thalli or clusters of thalli depending if proliferation takes place before spore formation (traditionally referred to as sori). Within these, either zoospores are differentiated or an amoeboid stage is formed, which are both released by the breakdown of the thallus wall. There are at present six to eight genera within the family, a number of which have been recently created as a result of molecular studies (Yohoyama and Honda 2007; Yokoyama et al. 2007). Genera included in this family are Aurantiochytrium, Botryochytrium, Japanochytrium, Monorhizochytrium, Parietichytrium, Schizochytrium, Thraustochytrium, and Ulkenia. Thraustochytrium is the largest genus with around 20 described species.
Order “Amphitremidales”/Amphitremida Gomaa et al. 2013
Family “Amphitremidiaceae”/Amphitremidae Poch 1913
These organisms were formerly grouped with the testate amoebae. The cells are enclosed with ovoid, cup-shaped, or rectangular punctate shells (Gomaa et al. 2013). Many contain green algal trebouxiophyte endosymbionts and have a mixotrophic nutrition (Gomaa et al. 2013). There are currently three recognized genera, Amphitrema, Archerella, and Paramphitrema. Named species have been isolated from freshwater habitats, such as freshwater wetlands. However, environmental sequencing has revealed many uncultured sequences in a sister group from anoxic and micro-oxic deep-sea sediments.
Family “Diplophrydaceae”/Diplophryidae Cavalier-Smith 2012
This was one of the first of the colorless protist groups that was found associated with the Labyrinthulid clade, although the initial species studied Diplophrys marina (Dykstra and Porter 1984) has now been moved to the Amphifilida. The Diplophryidae s. str. are small, largely freshwater heterotrophic protists with colorless spindle- to ovoid-shaped body cells from which a fine network of anastomosing filaments arises in bipolar fashion (Anderson and Cavalier-Smith 2012; Takahashi et al. 2014). The exact order placement of this family is not fully resolved, and it is placed with the Amphitremida on basis of recent phylogenetic investigations (Tice et al. 2016; Pan et al. 2017) although species also share morphological similarities with members of the next order.
Order “Amphifilales”/Amphifilida Cavalier Smith 2012
This is another order of colorless protists that have been phylogenetically elusive. They share many of the morphological characteristics of the genus Diplophrys described above, and the new genus was created by Anderson and Cavalier-Smith (2012) to contain the species Diplophrys marina which was in a separate clade from freshwater species of that genus. Another member of the family is the genus Sorodiplophrys, which had often been placed with dictyostelid amoebae (Tice et al. 2016).
Isolation Procedures
Thraustochytriales
Isolation procedures have been summarized by Porter (1990) and are briefly reviewed in this account. Thraustochytrids can be isolated by plating tissue sections on seawater agar, peptone-yeast-glucose seawater agar (PYGSA, approximately 50% seawater), modified Vishniac’s medium (KMV), or vegetable juice seawater agar, amended with penicillin and streptomycin to prevent bacterial growth. Small pieces (1 cm2 or less) of carefully rinsed (e.g., with sterile 50% seawater) tissue sections are placed on agar media and incubated at room temperature for 3 days or until thraustochytrid colonies are visible on the periphery of the tissue samples. Often the bottom surface of the tissue that is in direct contact with the agar is similarly colonized. Slide purification is the easiest method for obtaining an axenic culture of thraustochytrid. This is done by transferring a minute quantity of thraustochytrid cells, often with a fine glass needle, to a drop of sterile water on a slide then serially diluting until few cells or thalli are visible. Individual thalli can then be streaked to another agarised medium used in the isolation process. Baiting samples with pollen, especially from pines, is a method commonly used for isolating chytrids but similarly helpful when isolating thraustochytrids. For pollen baiting, the carefully rinsed substrate is placed in Petri dishes containing sterile-filtered seawater, onto which pollen grains (preferably sterilized) are dispersed. Colonization of pollen grains is usually evident within 2–10 days in the Petri dishes, but these may be held for several weeks if necessary. Thraustochytrid thalli can be observed on pollen grains with a dissecting microscope, ideally at high magnification (60–100×). Individual pollen grains can be transferred with a loop to agar plates or to small drops of sterile seawater from which zoospores, if released, can be picked up and streaked onto agar plates. Alternatively, colonized pollen grains can be transferred in mass to agar plates. Often, especially if a small initial inoculum is used, all of the colonized pollen grains will have colonies of the same species of thraustochytrid. It has been noted that not all thraustochytrids readily colonize pollen grains; thus, if a synoptic collection is desired, a variety of isolation procedures should be used. Maintenance of thraustochytrids can be achieved by regular subculturing or cryopreservation in 10% glycerol.
Labyrinthulales
Several methods for isolating members of the Labyrinthulales have been published (Amon 1978; FioRito et al. 2016; Garcias-Bonet et al. 2011; Yokochi et al. 2001). Moribund (discoloured) but not decomposed seagrass, marsh grass, mangrove litter, and algal fragments collected adrift or recently washed ashore are reliable sources for labyrinthulids. Organic sediments from marine and intertidal aerobic zones and tissues of invertebrate species may also yield labyrinthulids. Successful isolation has been reported with 1% serum seawater agar (SSA) but also with plain seawater agar. Half- to quarter-strength concentration of vegetable juice seawater agar and PYGSA amended with antibiotics (e.g., penicillin, streptomycin, or ampicillin) are similarly useful in isolating Labyrinthula. Often the thickness of agar media is minimized to ~2 mm (Yokochi et al. 2001) for ease in observing colonies with an inverted microscope. Similar to thraustochytrids, rinsed plant or algal materials are placed onto agar media and usually incubated at room temperature. Vividly swarming colonies radiating from tissue pieces are usually visible within 7 days of incubation. An agar block containing a swarm of labyrinthula can then be subcultured or cocultivated with marine yeast or bacteria (e.g., Vibrio, Psychrobacter). This method has been practiced often, since these microorganisms serve as host or food for labyrinthulids. However, maintaining a culture of labyrinthulids is challenging as isolate cessation after subculturing several times occurs, probably because the full life cycle is not concluded under these cultivation conditions.
Evolutionary History
In the absence of any fossil record for this group, all evolutionary speculation has to be based on the evidence of recent molecular phylogenetic studies. All of the osmotrophic fungal-like organisms studied by mycologists, except the plasmodiophorids, fall within the straminipilous branch of the chromalveolate assemblage (Fig. 1a adapted from Tsui et al. 2009; Fig. 1b from Moreira and Lόpez-Garcia 2002). The kingdom Straminipila defined by Dick (2001) was often seen as synonymous with the kingdom Chromista (Cavalier-Smith and Chao 2006) which is the name often favored by online taxonomic databases, even though the chromista, as originally defined also contain organisms not belonging to the Staminipila or the SAR supergroup (Burki et al. 2009). However, Dick (2001) argued that because of the non-photosynthetic osmotrophic groups in this lineage that the etymologically correct name Straminipila would be a more appropriate kingdom name, as this would highlight the synapomorphy of a monophyletic group. Nonetheless, a widely adopted form of spelling for this kingdom is Stramenopila (Adl et al. 2005; Lévesque 2011). Whether cryptophytes and haptophytes and their allies should also be considered as part of a wider supergroup is still debated (e.g., Reeb et al. 2009; Dorrell and Smith 2011).
Hyphochytrids and oomycetes are part of the lineage that shares a common ancestor with the photosynthetic ochrophytes (Tsui et al. 2009; Riisberg et al. 2009; Yubuki et al. 2010). It has recently been suggested that the stem origin of the Ochrophyta was around 571 millions of years ago, (a mean of estimates ranging from 735 to 434 million years ago: Brown and Sorhannus 2010). The Labyrinthulomycota are part of a sister clade, often collectively termed Bigyra which presumably evolved around the same time or only slightly earlier than the other osmotrophic Straminipila. The Labyrinthulomycota, Hyphochytriomycota, and Oomycota, as well as the ochrophyte straminipilous lineages share a common ancestor, which was most likely a photosynthetic mixotrophic marine flagellate (Tsui et al. 2009). The Labyrinthulomycota are part of one major straminipilous line and the Hyphochytriomycota and Oomycota of another (Fig. 5). This explains that whilst there are similarities between the Labyrinthulomycota and the other heterokont osmotrophs, they show much less in common than the other two groups.
The overall relationships between the major groups within the chromalveolate lineage, and the straminipilous groups in particular (see Beakes et al. 2014; Beakes and Thines, this volume), have been investigated using multiple protein-encoding genes (Tsui et al. 2009; Reeb et al. 2009; Riisberg et al. 2009). The statistically well-supported Alveolata kingdom, comprising Apicomplexa, Dinoflagellata, and Ciliata, forms the sister clade to the Straminipila (Keeling 2009). These can be divided into two main lineages: the first encompasses the bacteriotropic flagellate bicosoecids, the protistan gut-inhabiting opalinids (plus proteromonads and Blastocystis – equivalent to slopalinids defined by Patterson 1989) and the Labyrinthulomycota (Cavalier-Smith and Chao 2006; Tsui et al. 2009; Riisberg et al. 2009; Reeb et al. 2009), and the second straminipilous clade that includes the osmotrophic Hyphochytriomycota and Oomycota, the photosynthetic Ochrophyta, and a number of phagotrophic flagellates, such as Developayella and Pirsonia (Beakes et al. 2014). In this account, the Labyrinthulomycota is given phylum rank as in Porter (1990), and an emended Labyrinthulomycota s. lat. could be seen as containing other members of the phylum Bigyra (sensu Cavalier-Smith and Chao 2006), thereby replacing it.
The “chromalveolate hypothesis” proposes the red algal origin of the plastid in all chlorophyll c-containing algal groups (reviewed by Keeling 2009). However, recent comparative analyses of genomes of members of the Straminipila have led to the discovery of genes of green algal ancestry in both diatoms (Moustafa et al. 2011) and oomycetes (Richards et al. 2011; Jiang and Tyler 2012). It seems to cast doubt on such a simple “single-acquisition-multiple loss” interpretation (e.g., Maruyama et al. 2009; Stiller et al. 2009; Dorrell and Smith 2011). Theories involving multiple independent chloroplast acquisitions and horizontal gene transfer (HGT) have also been proposed as alternative explanations of the phylogenetic and genomic data (e.g., Martens et al. 2008; Stiller et al. 2009; Baurain et al. 2010). The eyespot in Labyrinthula zoospores (Perkins and Amon 1969) resembles those of photosynthetic Straminipila and may be indicative of the remains of an ancestral chloroplast. However, the fact that such structures have only been observed in the most derived group does perhaps cast doubt on this suggested origin (Tsui et al. 2009). Thraustochytrids produce omega-3 PUFA using a desaturase enzyme that in algae is usually found in chloroplasts (Sargent et al. 1995). If, as suggested by Tsui et al. (2009), it is assumed the ancestor to the straminipilous lineage was a mixotrophic photosynthetic flagellate, then at least two independent plastid losses must have occurred in the straminipilous line, one prior to diversification of the Labyrinthulid clade and the other after the divergence of the ochrophytes leading to the heterotrophic Oomycota and related lineages (Beakes et al. 2014). Alternatively, if plastid loss was deeply seated within the straminipilous line, then an independent reacquisition of a chromistan type plastid must have occurred to give rise to the Ochrophyta as suggested by Leipe et al. (1994). Plastid genes have been widely found in sequenced oomycete genomes (see Lévesque et al. 2010; Jiang and Tyler 2012) but were not reported in the compact 18.8 Mb genome of the anaerobic human gut parasite Blastocystis (Doenoeud et al. 2011). The preliminary genome sequence for the thraustochytrid Aurantiochytrium limacinum has been recently released (Collier 2012), but preliminary analysis has not so far revealed evidence of genes of plastid origin (Collier personal communication).
The earliest-diverging Labyrinthulomycota clades appear to contain thraustochytrid clades that have still retained the ability of phagotrophic nutrition, which is considered to be the ancestral state (Tsui et al. 2009; Gomaa et al. 2013). The presence of endosymbiotic Trebouxiphyte algae in the cells of members of the Amphitremida indicates this group has retained the ability to feed phagotrophically (Gomaa et al. 2013).
According to the analysis of Tsui et al. (2009), the key evolutionary event in the evolution of the labyrinthulomycetes within the straminipilous lineage was the evolution of the naked ectoplasmic net. The most derived groups are the Labyrinthulids and Aplanochytrids which have lost the ability to feed phagotrophically and rely entirely on osmotrophic nutrition. But also they have evolved gliding movement on the ectoplasmic net. The Aplanochytrids became separated from the Labyrinthulids by the loss of flagella (although it would be interesting to know if flagella genes can be found in their genome) and the acquisition of polygonal scales (Tsui et al. 2009).
What has become apparent in the past decade is that the Labyrinthulomycota are a more diverse assemblage than previously thought (Fig. 5), both in terms of ecological niches they occupy and their morphology than was believed at the time of the last Handbook review (Porter 1990). It is likely, that many more species and hidden genera are yet to be discovered that will provide new insights into the origins and evolutionary develeopment of this enigmatic group of protists.
References
Adl, S. M., Simpson, A. G., Farmer, M. A., Andersen, R. A., Andersen, O. R., Barta, J. R., Bowser, S. S., Brugerolle, G., Fensome, R. A., Frederico, S., James, T. Y., Karpov, S., Kurgens, P., Krug, J., Lane, C. E., Lewis, L. A., Lodge, J., Lynn, D. H., Mann, D. G., McCourt, R. M., Mendoza, L., Moestrup, Ø., Mozley-Standridge, S. E., Nerad, T. A., Shearer, C. A., Smirnov, A. V., Spiegel, F. W., & Taylor, M. F. J. R. (2005). The new higher level classification of the eukaryotes with emphasis on the taxonomy of protists. Journal of Eukaryotic Microbiology, 52, 399–451.
Amon, J. P. (1978). Thraustochytrids and labyrinthulids of terrestrial, aquatic and hypersaline environments of the great salt lake, USA. Mycologia, 70, 1299–1301.
Andersen, R. A., Barr, D. J. S., Lynn, D. H., Melkonian, M., Moestrup, O., & Sleigh, M. A. (1991). Terminology and nomenclature of the cytoskeletal elements associated with the flagellar/ciliary apparatus in protists. Protoplasma, 164, 1–8.
Anderson, O. R., & Cavalier-Smith, T. (2012). Ultrastructure of Diplophrys parva, a new small freshwater species, and a revised analysis of Labyrinthulea (Heterokonta). Acta Protozoologica, 51, 291–304.
Armiger, L. C. (1964). An occurrence of Labyrinthula in New Zealand Zostera. New Zealand Journal of Botany, 2, 3–9.
Aschner, M. (1958). Isolation of Labyrinthula macrocystis from soil. Bulletin of the Research Council of Israel, 6D, 174–179.
Azevedo, C., & Corral, L. (1997). Some ultrastructural observations of a thraustochytrid (Protoctista, Labyrinthulomycota) from clam Ruditapes descussatus (Mollusca, Bivalva). Diseases of Aquatic Organisms, 31, 73–78.
Bahnweg, G. (1979a). Studies on the physiology of Thraustochytriales. I. Carbon nutrition of Thraustochytrium spp., ecology of thraustochytrids and labyrinthulids 141 Schizochytrium sp., Japonochytrium sp., Ulkenia spp. and Labyrinthuloides spp. Veröffentlichungen des Instituts für Meeresforschung in Bremerhaven, 17, 269–273.
Bahnweg, G. (1979b). Studies on the physiology of Thraustochytriales. II. Growth requirements and nitrogen nutrition of Thraustochytrium spp., Schizochytrium sp., Japonochytrium sp., Ulkenia spp. and Labyrinthuloides spp. Veröffentlichungen des Instituts für Meeresforschung in Bremerhaven, 17, 245–268.
Bahnweg, G., & Jäckle, I. (1986). A new approach to taxonomy of the Thraustochytriales and Labyrinthulales. In S. T. Moss (Ed.), The biology of marine fungi (pp. 131–140). Cambridge: Cambridge University Press.
Bahnweg, G., & Sparrow, F. K. (1974). Occurrence, distribution and kinds of zoosporic fungi in subantartic and Antarctic waters. Veröffentlichungen des Instituts für Meeresforschung in Bremerhaven, 5, 149–157.
Barr, D. J. S. (1981). The phylogenetic and taxonomic implications of flagellar rootlet morphology among zoosporic fungi. Biosystems, 14, 359–370.
Barr, D. J. S., & Allan, P. M. E. (1985). A comparison of the flagellar apparatus in Phytophthora, Saprolegnia, Thraustochytrium, and Rhizidiomyces. Canadian Journal of Botany, 63, 138–154.
Barr, D. J. S., & Désaulniers, N. L. (1989). The flagellar apparatus of the oomycetes and hyphochytriomycetes. In J. C. Green, B. S. C. Leadbeater, & W. L. Diver (Eds.), The chromophyte algae, problems and perspectives (pp. 343–355). Oxford: Oxford University Press.
Baurain, D., Brinkman, H., Petersen, J., Rodriguez-Ezpeleta, N., Stechman, A., Demoulin, V., Roger, A. J., Burger, G., Lang, B. F., & Philippe, H. (2010). Phylogenomic evidence for separate acquisition of plastids in cryptophytes, haptophytes and stramenopiles. Molecular Biology and Evolution, 27, 1698–1709.
Beakes, G. W., Glockling, S. L., & Sekimoto, S. (2012). The evolutionary phylogeny of the oomycete “fungi”. Protoplasma, 249, 3–19.
Beakes, G. W., Honda, D., & Thines, M. (2014). Systematics of Straminipila, Labyrinthulomycota, Hyphochytriomycota, and Oomycota. In D. J. McLaughlin & J. W. Spatafora (Eds.), The Mycota – Systematics and evolution Part A VII (pp. 39–97). Heidelberg: Springer.
Bigelow, D. M., Olsen, M. W., & Gilbertson, R. L. (2005). Labyrinthula terrestris sp. nov., a new pathogen of turf grass. Mycologia, 97, 185–190.
Bockelmann, A. C., Beining, K., & Reusch, T. B. (2012). Widespread occurrence of endophytic Labyrinthula spp. in northern European eelgrass Zostera marina beds. Marine Ecology Progress Series, 445, 109–116.
Bongiorni, L. (2012). Thraustochytrids, a neglected component of oganic matter descompsition and food webs in marine sediments. In C. Raghukumar (Ed.), Biology of marine fungi, Progress in molecular and subcellular biology (Vol. 53, pp. 1–13).
Booth, T. (1971). Occurrence and distribution of some zoosporic fungi from soils of hibben and Moresby Islands, Queen Charlotte Islands. Canadian Journal of Botany, 49, 951–965.
Bower, S. M. (1987a). Labyrinthuloides haliotidis n. sp. (Protozoa, Layrinthulomorpha), a pathogenic parasite of of small juvenile abalone in a British Columbia mariculture facility. Canadian Journal of Zoology, 65, 2013–2020.
Bower, S. M. (1987b). Artificial culture of Labyrinthuloides haliotidis (Protozoa, Labyrinthomorpha), a pathogenic parasite of abalone. Canadian Journal of Botany, 65, 2013–2020.
Bower, S. M., McLean, N., & Whitaker, D. J. (1989). Mechanism of infection by Labyrinthuloides haliotidis (Protozoa, Labyrinthomorpha), a parasite of abalone (Haliotis kamtschatka) (Mollusca, Gastropoda). Journal of Invertebrate Pathology, 53, 401–409.
Bowler, C., Allen, A. E., Badger, J. H., Grimwood, J., Jabbari, K., Kuo, A., Maheswari, U., Martens, C., Maumus, F., Otillar, R. P., Rayko, E., Salamov, A., Vandepoele, K., Beszteri, B., Gruber, A., Heijde, M., Katinka, M., Mock, T., Valentin, K., Verret, F., Berges, J. A., Brownlee, C., Cadoret, J.-P., Chiovitti, A., Choi, C. J., Coesel, S., De Martino, A., Detter, J. C., Durkin, C., Falciatore, A., Fournet, J., Haruta, M., Huysman, M. J. J., Jenkins, B. D., Jiroutova, K., Jorgensen, R. E., Joubert, Y., Kaplan, A., Kröger, N., Kroth, P. G., La Roche, J., Lindquist, E., Lommer, M., Martin-Jézéque, V., Lopez, P. J., Lucas, S., Mangogna, M., McGinnis, K., Medlin, L. K., Montsant, A., Oudot-Le Secq, M.-P., Napoli, C., Obornik, M., Parker, M. S., Petit, J. L., Porcel, B. M., Poulsen, N., Robison, M., Rychlewski, L., Rynearson, T. A., Schmutz, J., Shapiro, H., Siaut, M., Stanley, M., Sussman, M. R., Taylor, A. R., Vardi, A., von Dassow, P., Vyverman, W., Willis, A., Wyrwicz, L. S., Rokhsar, D. S., Weissenbach, J., Armbrust, E. V., Green, B. R., de Peer, Y. V., & Grigoriev, I. V. (2008). The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature, 456, 239–244.
Bremer, G. B. (1976). The ecology of marine lower fungi. In E. B. G. Jones (Ed.), Advances in aquatic mycology (pp. 313–333). London: Elek Science.
Brown, J. W., & Sorhannus, U. (2010). A molecular genetic timescale for the diversification of autotrophic stramenopiles (Ochrophyta), substantive underestimation of putative fossil ages. PloS One, 5, e12759. doi:10.1371/journal.pone.0012759.
Burki, F., Shalchian-Tabrizi, K., & Pawlowski, J. (2008). Phylogenomics reveals a new ‘megagroup’ including most photosynthetic eukaryotes. Biology Letters, 4, 366–369.
Cavalier-Smith, T., & Chao, E. E. Y. (2006). Phylogeny and megasystematics of phagotrophic heterokonts (Kingdom Chromista). Journal of Molecular Evolution, 62, 388–420.
Chang, K. J. L., Dunstan, G. A., Abell, G. C., Clementson, L. A., Blackburn, S. I., Nichols, P. D., & Koutoulis, A. (2012). Biodiscovery of new Australian thraustochytrids for production of biodiesel and long-chain omega-3 oils. Applied Microbiology and Biotechnology, 93, 2215–2231.
Cienkowski, L. (1867). Ueber den Bau und die Entwickelung der Labyrinthuleen. Archiv für Mikroskopishce Anatomie, 3, 274–310.
Colaco, A., Raghukumar, C., Mohandass, C., Cardigos, F., & Santos, R. S. (2006). Effect of shallow water venting in Azores on a few marine biota. Cahiers de Biologie Marine, 47, 359–364.
Collado-Mercado, E., Radway, J. C., & Collier, J. L. (2010). Novel uncultivated labyrinthulomycetes reveales by 18S rDNA sequences from seawater and sediment samples. Aquatic Microbial Ecology, 58, 215–228.
Collier, J. (2012). Why sequence four Labrinthulomycete species? JGI DOE Joint Genome Institute. http://www.jgi.doe.gov/sequencing/why/labrinthulomycete.html. Accessed 23 Sept 2012.
Craven, K. D., Peterson, P. D., Windham, D. E., Mitchell, T. K., & Martin, S. B. (2005). Molecular identification of the turf grass rapid blight pathogen. Mycologia, 97, 160–166.
Damare, V., & Raghukumar, S. (2008). Abundance of thraustochytrids and bacteria in the equatorial Indian Ocean, in relation to transparent exopolymreic particles (TEPs). FEMS Microbiology Ecology, 65, 40–49.
Damare, V., & Raghukumar, S. (2010). Association of the stramenopilan proitst, the aplanochytrids, with zooplankton of the equatorial Indian Ocean. Marine Ecology Progress Series, 399, 53–68.
Dick, M. W. (2001). Straminipilous fungi (670 pp). Dordrecht: Kluwer.
Diéz, B., Pedrós-Alió, C., & Massana, R. (2001). Study of genetic diversity of eukaryotic picoplankton in different oceanic regions by small-subunit rRNA gene cloning and sequencing. Applied and Environmental Microbiology, 67, 2932–2941.
Doenoeud, F., Roussel, M., Noel, B., Wawrzyniak, I., Da Silva, C., Diogon, M., Viscogliosi, E., Brochier-Armanet, C., Couloux, A., Poulain, J., Segurens, B., Anthouard, V., Texier, C., Blot, N., Poirier, P., Ng, G. C., Tan, K. S., Artiguenave, F., Jaillon, O., Aury, J. M., Delbac, F., Wincker, P., Vivarès, C. P., & El Alaoui, H. (2011). Genome sequence of the stramenopile Blastocystis, a human anaerobic parasite. Genome Biology, 12, R29. doi:10.1186/gb-2011-12-3-r29.
Dorrell, R. G., & Smith, A. G. (2011). Do red and green make brown? Perspectives on plastid acquisitions within chromalveolates. Eukaryotic Cell, 10, 856–868. doi:10.1128/EC.00326-10.
Douhan, G. W., Olsen, M. W., Herrell, A., Winder, C., Wong, F., & Entwistle, K. (2009). Genetic diversity in Labyrinthula terrestris, a newly emergent plant pathogen, and the discovery of new Labyrinthulid organism. Mycological Research, 113, 1192–1199.
Dykova, I., Fiala, I., Dvorakova, H., & Peckova, H. (2008). Living together, the marine amoeba Thecamoeba hilla Shaeffer, 1926 and its endosymbiont Labyrinthula sp. European Journal of Protistology, 44, 308–316.
Dykstra, M. J., & Porter, D. (1984). Diplophrys marina, a new scale-forming marine protist with labyrinthulid affinities. Mycologia, 76, 626–632.
Evans, O., Paul-Pont, I., & Whittington, R. J. (2017). Detection of ostreid herpesvirus 1 microvariant DNA in aquatic invertebrate species, sediment and other samples collected from the Georges River estuary, New South Wales, Australia. Diseases of Aquatic Organisms, 122, 247–255.
Fan, K. W., & Chen, F. (2006). Production of high value products by marine microalgae thraustochytrids. In S. T. Yang (Ed.), Bioprocessing for value-added products from renewable resources (pp. 293–323). Amsterdam: Elsevier BV.
Fan, K. W., Vrijmoed, L. L. P., & Jones, E. B. G. (2002). Physiological studies of subtropical mangrove thraustochytrids. Botanica Marina, 45, 50–57.
Fan, K. W., Tsunchiro, A., Chen, F., & Jiang, Y. (2010). Enhanced production of squalene in the thraustochytrid Aurantiochytrium mangrovei by medium optimization and treatment with terbinafine. World Journal of Microbiology and Biotechnology, 26, 1303–1309.
FioRito, R., Leander, C., & Leander, B. (2016). Characterization of three novel species of Labyrinthulomycota isolated from ochre sea stars (Pisaster ochraceus). Marine Biology, 163, 170. doi:10.1007/s00227-016-2944-5.
Gaertner, A. (1968). Eine method des quantitativen Nachweises niederer mit pollen koderbarer pilze in meerwasser und im sediment. Veröffentlichungen des Instituts für Meeresforschung in Bremerhaven, 3, 75–92.
Gaertner, A. (1979). Some fungal parasites found in the diatom populations of the Rosfjord area (South Norway) during March 1979. Veröffentlichungen des Instituts für Meeresforschung in Bremerhaven, 18, 92–33.
Garcia-Verdrenne, A. E., Groner, M., Page-Karjian, A., Siegmund, G. F., Singhal, S., Sziklay, J., & Robert, S. (2013). Development of genomic resources for a thraustochytrid pathogen and investigation of temperature influences on gene expression. PloS One, 8, e74196. doi:10.1371/journal.pone.0074196.
Garcias-Bonet, N., Sherman, T. D., Duarte, C. M., & Marba, N. (2011). Distribution and pathogenicity of the protist Labyrinthula sp. in western Mediterranean seagrass meadows. Estauries and Coasts, 34, 1161. doi:10.1007/s12237-011-9416-4.
Goldstein, S. (1973). Zoosporic marine fungi (Thraustochytriaceae and Dermocystidiaceae). Annual Review of Microbiology, 27, 13–25.
Gomaa, F., Mitchell, E. A. D., & Lara, E. (2013). Amphitremida (Poche, 2013) is a new major, ubiquitous Labyrinthulomycete clade. PloS One, 8, e53046. doi:10.1371/journal.pone.0053046.
Hackett, J. D., Yoon, H. S., Li, S., Reyes-Prieto, A., Rümmele, S. E., & Bhattacharya, D. (2007). Phylogenomic analysis supports the monophyly of cryptophytes and haptophytes and the association of Rhizaria with chromalveolates. Molecular Biology and Evolution, 24, 1702–1713.
Harel, M., Ben-Dov, E., Rasoulouniriana, D., Siboni, N., Kramarsky-Winter, E., Loya, Y., Barak, Z., Wiesman, Z., & Kushmaro, A. (2008). A new thraustochytrid, strain Fng1, isolated from the surface mucus of the hermatypic coral Fungia granulosa. FEMS Microbiology Ecology, 64, 378–387.
Hatai, K. (2012). Diseases of fish and shellfish caused by marine fungi. In C. Raghukumar (Ed.), Biology of marine fungi, Progress in molecular and subcellular biology (Vol. 53, pp. 15–52). Berlin/Heidelberg: Springer.
Höhnk, W., & Ulken, A. (1979). Pilze aus marinen schwämmen. Veröffentlichungen des Instituts für Meeresforschung in Bremerhaven, 17, 199–204.
Honda, D., Yokochi, T., Nakahara, T., Ragukumar, S., Nakagiri, A., Schaimann, K., & Higashirhara, T. (1999). Molecular phylogeny of labyrinthulids and thraustochytrids based on the sequencing of the 18S ribosomal RNA gene. Journal of Eukaryotic Microbiology, 46, 637–647.
Iwata, I., Kimura, K., Tomaru, Y., Motomura, T., Koike, K., Koike, K., & Honda, D. (2016). Bothrosome formation in Schizochytrium aggregatum (Labyrinthulomycetes, stramenopiles) during zoospore settlement. Protist. doi:10.1016/j.protis.2016.12.002.
Jain, R., Raghukumar, S., Tharanathan, R., & Bhosle, N. B. (2005). Extracellular polysaccharide production by thraustochytrid protists. Marine Biotechnology, 7, 184–192.
Jiang, R. H. Y., & Tyler, B. M. (2012). Mechanisms and evolution of virulence in oomycetes. Annual Review of Phytopathology, 50, 295–318. doi:10.1146/annrev-phyto-081211-172912.
Jones, E. B. G., & Harrison, J. L. (1976). Physiology of marine phycomycetes. In E. B. G. Jones (Ed.), Recent advances in aquatic mycology (pp. 261–278). London: Elek Science.
Jones, G. M., & O’Dor, R. K. (1983). Ultrastructual observations on a thraustochytrid fungus parasitic in the gills of squid (Illex illecebrosus Lesueur). Journal of Parasitology, 69, 903–911.
Jones, M. D. M., Forn, I., Gadelha, C., Egan, M. J., Bass, D., Masana, R., & Richards, T. A. (2011). Discovery of novel intermediate forms redefines the fungal tree of life. Nature, 474, 200–203.
Karling, J. S. (1981). Predominantly holocarpic and eucarpic simple biflagellate phycomycetes. Vaduz: J. Cramer.
Kazama, F. Y. (1974). The ultrastructure of nuclear division in Thraustochytrium sp. Protoplasma, 82, 155–175.
Kazama, F. Y. (1980). The zoospore of Schizochytrium aggregatum. Canadian Journal of Botany, 58, 2434–2446.
Kazama, F. Y., & Schornstein, K. L. (1973). Ultrastructure of a fungus herpes-type virus. Virology, 52, 478–487.
Keeling, P. J. (2009). Chromalveolates and the evolution of plastids by secondary endosymbiosis. Journal of Eukaryotic Microbiology, 56, 1–8.
Kimura, H., Sato, M., Sugiyama, C., & Naganuma, T. (2001). Coupling of thraustochytrids and POM, and of bacterio- and phytoplankton in a semi-enclosed coastal area, implication for different substrate preference by the planktonic decomposers. Aquatic Microbial Ecology, 25, 293–300.
Kobayashi, T., Sakaguchi, K., Matsuda, T., Abe, E., Hama, Y., Hayashi, M., Honda, D., Okita, Y., Sugimoto, S., Okino, N., & Ito, M. (2011). Increase of eicosapentaenoic acid in thraustochytrids through thraustochytrids ubiquitin promoter-driven expression of a fatty acid Δ5 desaturase gene. Applied and Environmental Microbiology, 77, 3870–3876.
Koske, R. E. (1981). Labyrinthula inside the spores of a vesicular-arbuscular mycorrhizal fungus. Mycologia, 73, 1175–1180.
Kramarsky-Winter, E., Harel, M., Siboni, N., Ben Dov, E., & Brickner, I. (2006). Identification of a protist-coral association and its possible ecological role. Marine Ecology Progress Series, 317, 67–73.
Kuznetsov, E. A. (1981). Anabiosis in lower aquatic fungi. Mikologiya i Fitopatologiya, 15, 526–531.
Leander, C. A., & Porter, D. (2001). The Labyrinthulomycota is comprised of three distinct lineages. Mycologia, 93, 459–464.
Leander, C. A., Porter, D., & Leander, B. S. (2004). Comparative morphology and molecular phylogeny of aplanochytrids (Labryrinthulomycota). European Journal of Protistology, 40, 317–328.
Leaño, E. M. (2001). Straminipilous organisms from fallen mangrove leaves from Panay Island, Philippines. Fungal Diversity, 6, 75–81.
Leipe, D. D., Tong, S. M., Goggin, C. L., Slemenda, S. B., Pieniazek, N. J., & Sogin, M. L. (1994). 16S–like rDNA sequences from Developayella elegans, Labyrinthuloides haliotidis, and Proteromonas lacertae confirm that the stramenopiles are a primarily heterotrophic group. European Journal of Protistology, 33, 369–377.
Lévesque, C. A. (2011). Fifty years of oomycetes – From consolidation to evolutionary and genomic exploration. Fungal Diversity, 50, 35–46.
Lévesque, C. A., Brouwer, H., Cano, L., Hamilton, J. P., Holt, C., Huitema, E., Raffaele, S., Robideau, G. P., Thines, M., Win, J., Zerillo, M. M., Beakes, G. W., Boore, J. L., Busam, D., Dumas, B., Ferriera, S., Fuerstenberg, S. I., Gachon, C. M. M., Gaulin, E., Govers, F., Grenville-Briggs, L., Horner, N., Hostetler, J., Jiang, R. H. Y., Johnson, J., Krajaejun, T., Lin, H., Meijer, H. J. G., Moore, B., Morris, P., Phuntmart, V., Puiu, D., Shetty, J., Stajich, J. E., Tripathy, S., Wawra, S., van West, P., Whitty, B. R., Coutinho, P. M., Henrissat, B., Martin, F., Thomas, P. D., Tyler, B. M., De Vries, R. P., Kamoun, S., Yandell, M., Tisserat, N., & Buell, C. R. (2010). Genome sequence of the necrotrophic plant pathogen Pythium ultimum reveals original pathogenicity mechanisms and effector repertoire. Genome Biology, 11, R73. doi:10.1186/gb-2010-11-7-r73.
Liu, B., Ertesvåg, H., Aasen, I. M., Vadstein, O., Brautaset, T., & Heggeset, T. M. B. (2016). Draft genome sequence of the docosahexaenoic acid producing thraustochytrid Aurantiochytrium sp. T66. Genomics Data, 8, 115–116. doi:10.1016/j.gdata.2016.04.013.
Lyons, M. M., Ward, J. E., Smolowitz, R., Uhlinger, K. R., & Gast, R. J. (2005). Lethal marine snow, pathogen of bivalve mollusc conceales in marine aggregates. Limnology and Oceanography, 50, 1983–1988.
Lyons, M. M., Smolowitz, R., Gomez-Chiarri, M., & Ward, E. (2007). Epizootiology of Quahog parasite unknown (QPX) disease in northern quahogs ( = hard clams) Mercenaria mercenaria. Journal of Shellfish Research, 26, 371–381.
Marano, A. V., Jesus, A. L., De Souza, J. I., Jerônimo, G. H., Gonçalves, D. R., Boro, M. C., Rocha, S. C. O., & Pires-Zottarelli, C. L. A. (2016). Ecological roles of saprotrophic Peronosporales (Oomycetes, Straminipila) in natural environments. Fungal Ecology, 19, 77–88.
Martens, C., Vandepoele, K., & van de Peer, Y. (2008). Whole-genome analysis reveals molecular innovations and evolutionary transitions in chromalveolate species. Proceedings of the National Academy of Sciences, 105, 3427–3432.
Maruyama, S., Matsuzaki, M., Misawa, K., & Nozaki, H. (2009). Cyanobacterial contribution to the genomes of the plastid lacking protists. BMC Evolutionary Biology, 9, 197. doi:10.1186/1471-2148-9-197.
Massana, R., & Pedró-Alió, C. (2008). Unveiling new microbial eukaryotes in the surface ocean. Current Opinion in Microbiology, 11, 213–218.
Massana, R., Guillou, L., Diez, B., & Pedró-Alió, C. (2002). Unveiling the organisms behind novel eukaryotic ribosomal DNA sequences from the ocean. Applied and Environmental Microbiology, 68, 4554–4558.
Massana, R., Castresana, J., Balagué, V., Guillou, L., Romari, K., Groisillier, A., Valentin, K., & Pedró-Alió, C. (2004). Phylogenetic and ecological analysis of novel marine stramenopiles. Applied and Environmental Microbiology, 70, 3528–3534.
Massana, R., Terrado, R., Forn, I., Lovejoy, C., & Pedró-Alió, C. (2006). Distribution and abundance of uncultured heterotrophic flagellates in the world oceans. Environmental Microbiology, 8, 1515–1522.
McLean, I. N., & Porter, D. (1987). Lesions produced by a thraustochytrids in Tritonia diomedea (Mollusca, Gastropoda, Nudibranchia). Journal of Invertebrate Pathology, 49, 223–225.
Miller, J. D., & Jones, E. B. G. (1983). Observations on the association of thraustochytrids marine fungi with decaying seaweed. Botanica Marina, 26, 345–351.
Miller, M. R., Nichols, P. D., & Carter, C. G. (2007). Replacement of fish oil with thraustochytrid Schizochytrium sp. L oil in Atlantic salmon parr (Salmo salar L) diets. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 148, 382–392.
Moreira, D., & Lopez-Garcia, P. (2002). The molecular ecology of microbial eukaryotes unveils a hidden world. Trends in Microbiology, 10, 31–38.
Moro, I., Negrisolo, E., Callegaro, A., & Andreoli, C. (2003). Aplanochytrium stocchinoi, a new Labyrinthulomycota from the Southern Ocean (Ross Sea, Antarctica). Protist, 154, 331–340.
Moss, S. T. (1985). An ultrastructural study of taxonomically significant characters of the Thraustochytriales and Labyrinthulales. Botanical Journal of the Linnean Society, 91, 329–357.
Moss, S. T. (1986). Biology and phylogeny of the Labyrinthulales and Thraustochytriales. In S. T. Moss (Ed.), The biology of marine fungi (pp. 105–129). Cambridge: Cambridge University Press.
Moustafa, A., Beszteri, B., Maier, U. G., Bowler, C., Valentin, K., & Bhattacharya, D. (2011). Genomic footprints of a cryptic plastid endosymbiosis in diatoms. Science, 324, 1724–1726.
Muehlstein, L. K., & Porter, D. (1991). Labyrinthula zosterae sp. nov., the causative agent of wasting disease of eelgrass, Zostera marina. Mycologia, 83, 180–191.
Muehlstein, L. K., Porter, D., & Short, F. T. (1988). Labyrinthula sp., a marine slime mold producing the symptoms of wasting disease in eelgrass, Zostera marina. Marine Biology, 99, 465–472.
Naganuma, T., Kimura, H., Karimoto, R., & Pimenov, N. V. (2006). Abundance of planktonic thraustochytrids and bacteria and the concentration of particulate ATP in the Greenland and Norwegian seas. Polar Bioscience, 20, 37–45.
Nakatsuji, N., & Bell, E. (1980). Control by calcium of the contractility of Labyrinthula slimeways and of the translocation of Labyrinthula cells. Cell Motility, 1, 17–29.
Olsen, M. W. (2007). Labyrinthula terrestris, a new pathogen of cool-season turfgrasses. Molecular Plant Pathology, 8, 817–820.
Pan, J., del Campo, J., & Keeling, P. J. (2017). Reference tree and environmental sequence diversity of Labyrinthulomycetes. Journal of Eukaryotic Microbiology, 64, 88–96.
Patterson, D. J. (1989). Chromophytes from a protistan perspective. In J. P. Green, B. S. C. Leadbeater, & W. L. Diver (Eds.), The chromophyte algae, problems and perspectives (pp. 357–379). Oxford: Clarendon Press.
Perkins, F. O. (1970). Formation of centriole and centriole-like structures during meiosis and mitosis in Labyrinthula sp. (rhizopodea, Labyrinthulida). Journal of Cell Science, 6, 629–653.
Perkins, F. O. (1973). Observations of thraustochytriaceous (phycomycetes) and labyrinthulid (rhizopodea) ectoplasmic nets on natural and artificial substrates – An electron microscope study. Canadian Journal of Botany, 51, 485–491.
Perkins, F. O. (1976). Fine structure of lower marine and estuarine fungi. In E. B. G. Jones (Ed.), Recent advances in aquatic mycology (pp. 513–542). London: Elek Press.
Perkins, F. O., & Amon, J. P. (1969). Zoosporulation in Labyrinthula sp., an electron microscope study. Journal of Protozoology, 16, 235–256.
Polglase, J. L. (1980). A preliminary report on the thraustochytrid(s) and labyrinthulid(s) associated with a pathological condition in the lesser octopus Eledone cirrhosa. Botanica Marina, 23, 699–706.
Porter, D. (1990). Phylum Labyrinthulomycota. In L. Margulis, J. O. Corliss, M. Melkonian, & D. J. Chapman (Eds.), Handbook of Protoctista (pp. 388–398). Boston: Jones and Bartlett.
Quick, J. A. (1974). Labyrinthuloides schizochytrops n. sp., a new marine Labyrinthula with spheroid “spindle” cells. Transactions of the American Microscopical Society, 93, 344–365.
Raghukumar, C. (1987a). Fungal parasites of marine algae from Mandapam (South India). Diseases of Aquatic Organisms, 3, 137–145.
Raghukumar, C. (1987b). Fungal parasites of the marine alga, Cladophora and Rhizoclonium. Botanica Marina, 29, 289–297.
Raghukumar, S. (1992). Bacterivory, a novel dual role for thraustochytrids in the sea. Marine Biology, 113, 165–169.
Raghukumar, S. (2002). Ecology of marine protists, the Labrinthulomycetes (thraustochytrids and Labyrinthulids). European Journal of Protistology, 38, 127–145.
Raghukumar, C. (2006). Algal-fungal interactions in the marine ecosystem, symbiosis to parasitism. In A. Tewari (Ed.), Recent advances on applied aspects of Indian marine algae with reference to global scenario (Vol. 1, pp. 366–385). Bhavnagar: Central Salt and Marine Chemicals Research Institute.
Raghukumar, S. (2008). Thraustochytrid marine protists, production of PUFAs and other emerging technologies. Marine Biotechnology, 10, 631–640.
Raghukumar, S., & Balasubramanian, R. (1991). Occurrence of thraustochtrid fungi in corals and mucus. Indian Journal of Marine Science, 20, 176–181.
Raghukumar, S., & Damare, V. S. (2011). Increasing evidence for the important role of Labyrinthulomycetes in marine ecosystems. Botanica Marina, 54, 3–11.
Raghukumar, S., & Schaumann, K. (1993). An epifluorescence microscopy method for direct detection and enumeration of the fungi like marine protist, the thraustochytrids. Limnology and Oceanography, 38, 182–187.
Raghukumar, S., Sharma, S., Raghukumar, C., & Sathe-Pathak, V. (1994). Thraustochytrid and fungal component of marine detritus. IV. Laboratory studies on decomposition of leaves of Rhizophora apiculata Blume. Journal of Experimental Marine Biology and Ecology, 183, 113–131.
Raghukumar, S., Sathe-Pathak, V., Sharma, S., & Raghukumar, C. (1995). Thraustochytrid and fungal component of marine detritus. III, Field studies on decomposition of leaves of the mangrove Rhizophora apiculata Blume. Aquatic Microbial Ecology, 9, 117–125.
Raghukumar, S., Ramaiah, N., & Raghukumar, C. (2001). Dynamics of thraustochytrid protists in the water column of the Arabian Sea. Aquatic Microbial Ecology, 24, 175–186.
Reeb, V. C., Peglaer, M. T., Yoon, H. S., Bai, J. R., Wu, M., Shiu, P., Grafenberg, J. L., Reyes-Prieto, A., Rümmele, S. E., Gross, J., & Bhattacharya, D. (2009). Interrelationships of chromalveolates within a broadly sampled tree of photosynthetic protists. Molecular Phylogenetics and Evolution, 53, 202–211.
Richards, T. A., Soanes, D. M., Jones, M. D. M., Vasieva, O., Leonard, G., Paszkiewicz, K., Foster, P. G., Hall, N., & Talbot, N. J. (2011). Horizontal gene transfer facilitated the evolution of plant parasitic mechanisms in the oomycetes. Proceedings of the National Academy of Sciences, 108, 15258–15263.
Richards, T. A., Jones, M. D., Leonard, G., & Bass, G. (2012). Marine fungi, their ecology and molecular diversity. Annual Review of Marine Science, 4, 495–522.
Riemann, F., & Schrage, M. (1983). On a mass occurrence of a thraustochytrioid protist (fungi or rhizopodan protozoa) in an Antarctic anaerobic marine sediment. Veröffentlichungen des Instituts für Meeresforschung in Bremerhaven, 19, 191–202.
Riisberg, I., Orr, R. J., Kluge, R., Shalchian-Tabrizi, K., Bowers, H. A., Patil, V., Edvardsen, B., & Jakobsen, K. S. (2009). Seven gene phylogeny of heterokonts. Protist, 160, 191–204.
Sargent, J. R., Bell, M. V., Bell, J. G., Henderson, R. J., & Tocher, D. R. (1995). Origins and functions of (n-3) polyunsaturated fatty acids in marine oranisms. In G. Cevc & F. Paltauf (Eds.), Phospolipids, characterization, metabolism and novel biological applications (pp. 248–259). Champaign: AOCS Press.
Sathe-Pathak, V., Raghukumar, S., Raghukumar, C., & Sharma, S. (1993). Thraustochytrid and fungal component of marine detritus. I. Field studies on decomposition of the brown alga Sargassum cinereum J Ag. Indian Journal of Marine Science, 22, 159–167.
Schärer, L., Knoflach, D., Vizoso, D. B., Rieger, G., & Peintner, U. (2007). Thraustochytrids as novel parasitic protists of marine free-living flatworms, Thraustochytrium caudivorum sp. nov. parasitizes Macrostomum lignano. Marine Biology, 152, 1095–1104.
Schneider, J. (1981). Ein ökologischer vergleich aquatischer niederer pilze (Thraustochytrium sp.) von meeres- und binnenlandstandorten. Botanica Marina, 24, 475–484.
Segarra, A., Pépin, J. F., Arzul, I., Morga, B., Faury, N., & Renault, T. (2010). Detection and description of a particular ostreid herpesvirus 1 genotype associated with massive mortality outbreaks of Pacific oysters, Crassostrea gigas, in France in 2008. Virus Research, 153, 92–99.
Short, F. T., Porter, D., Iizumi, H., & Aioi, K. (1993). Occurrence of the eelgrass pathogen Labyrinthula zosterae in Japan. Diseases of Aquatic Organisms, 16, 73–77.
Sims, P. A., Mann, D. G., & Medlin, L. K. (2006). Evolution of the diatoms, insights from fossil, biological and molecular data. Phycologia, 45, 361–402.
Sparrow, F. K. (1936). Biological observation on the marine fungi of Woods Hole waters. Biological Bulletin of the Marine Biological Laboratory, 70, 236–263.
Sparrow, F. K. (1960). Aquatic Phycomycetes (2nd revised ed.). Ann Arbor: University of Michigan Press.
Sparrow, F. K. (1973). Mastigomycotina. In G. C. Ainsworth, F. K. Sparrow FK, & A. S. Sussman (Eds.), The fungi (Vol. 4b, pp. 61–73). New York/London: Academic Press.
Sparrow, F. K. (1976). The present status of classification in biflagellate fungi. In E. B. Gareth-Jones (Ed.), Recent advances in aquatic mycology (pp. 213–222). London: Elek Science.
Stiller, J. W., Huang, J., Ding, W., Trian, J., & Goodwillie, C. (2009). Are algal genes in nonphotosynthetic protists evidence of historical plastid endosymbioses. BMS Genomics, 10, 484. doi:10.1186/1471-2164-10-484.
Stoeck, T., Taylor, G., & Epstein, S. S. (2003). Novel eukaryotes frm a permanently anoxic Cariaco Basin (Carribean Sea). Applied and Environmental Microbiology, 69, 5656–5663.
Stoeck, T., Hayward, B., Taylor, G. T., Valera, R., & Epstein, S. S. (2006). A multiple PCR-primer approach to access the microeukaryotic diversity in the anoxic Cariaco Basin (Caribbean Sea). Protist, 157, 31–43.
Stoeck, T., Kasper, J., Bunge, J., Leslin, C., Ilyin, V., & Epstein, S. (2007). Protistan diversity in the arctic, a case of paleoclimate shaping modern biodiversity. PloS One, 8, e728. doi:10.1371/journal.pone.0000728.
Stowell, L. J., Martin, S. B., Olsen, M., Bigelow, D., Kohout, M., Peterson, P. D., Camberto, J., & Gelernter, W. D. (2005). Rapid blight, a new plant disease. APSnet Features. doi:10.1094/APSnetFeature/2005-0705.
Takahashi, Y., Yoshida, M., Inouye, I., & Watanabe, M. M. (2014). Diplophrys mutabilis sp. nov., a new member of Labyrinthulomycetes from freshwater habitats. Protist, 165, 50–65.
Takao, Y., Nagasaki, K., Mise, K., Okuno, T., & Honda, D. (2005). Isolation and characterization of a novel single-stranded RNA virus infectious to a marine fungoid protist, Schizochytrium sp.(Thraustochytriaceae, Labyrinthulea). Applied and Environmental Microbiology, 71, 4516–4522.
Thines, M. (2014). Phylogeny and evolution of plant pathogenic oomycetes – A global overview. European Journal of Plant Pathology, 138, 431–447.
Tice, A. K., Silberman, J. D., Walthall, A. C., Le, K. N. D., Spiegel, F. W., & Brown, M. W. (2016). Sorodiplophrys stercorea, another novel lineage of sorocarpic multicellularity. Journal of Eurkaryotic Microbiology, 63, 623–628.
Tsui, C. K., & Vrijmoed, L. L. (2012). A re-visit to the evolution and ecophysiology of the labyrinthulomycetes. Rijeka/Shanghai: INTECH Open Access Publisher.
Tsui, C. K. M., Marshall, W., Yokoyama, R., Honda, D., Lippmeier, J. C., Craven, K. D., & Berbee, M. L. (2009). Labryinthulomycetes phylogeny and its implications for the evolutionary loss of chloroplasts and gain of ectoplasmic gliding. Molecular Phylogenetics and Evolution, 50, 129–140.
Ueda, M., Nomura, Y., Doi, K., & Nakajima, M. (2015). Seasonal dynamics of culturable thraustochytrids (Labyrinthulomycetes, stramenopiles) in estuarine and coastal waters. Aquatic Microbial Ecology, 74, 187–204.
Vergeer, L. H. T., & den Hartog, C. (1991). Occurrence of wasting disease in Zostera noltii. Aquatic Botany, 40, 155–163.
Vergeer, L. H. T., & den Hartog, C. (1994). Omnipresence of Labyrinthulaceae in seagrasses. Aquatic Botany, 48, 1–20.
Wagner-Merner, B. T., Duncan, W. R., & Lawrence, J. M. (1980). Preliminary comparison of Thraustochytriaceae in the guts of a regular and irregular echinoid. Botanica Marina, 23, 95–97.
Winwood, R. J. (2013). Recent developments in the commercial production of DHA and EPA rich oils from micro-algae. OCL, 20(6), D604.
Worden, A. Z., & Not, F. (2008). Ecology and diversity of piceukaryotes. In D. L. Kirchman (Ed.), Microbial ecology of the oceans (2nd ed., pp. 159–205). New York: Wiley.
Yokochi, T., Nakahara, T., Higashihara, T., Yamaoka, M., & Kurane, R. (2001). A new isolation method for labyrinthulids using a bacterium, Psychrobacter phenylpyruvicus. Marine Biotechnology, 3, 68–73.
Yokoyama, R., & Honda, D. (2007). Taxonomic rearrangement of the genus Schizochytrium sensu lato based on morphology, chemotaxonomical characteristics, and 18S rRNA gene phylogeny (Thraustochytriaceae, Labyrinthulomycetes), emendation for Schizochytrium and erection of Aurantiochytrium and Oblongichytrium gen. nov. Mycoscience, 48, 199–211.
Yokoyama, R., Salleh, B., & Honda, D. (2007). Taxonomic rearrangement of the genus Ulkenia sensu lato based on morphology, chemotaxonomical characteristics, and 18S rRNA gene phylogeny (Thraustochytriaceae, Labyrinthulomycetes), emmedation for Ulkenia and erection of Botryochytrium, Parietichytrium, and Sicyoidochytrium gen. nov. Mycoscience, 48, 329–341.
Yubuki, N., Leander, B. S., & Silberan, J. D. (2010). Ultrastructure and molecular phylogenetic position of a novel phagotrophic position of a novel phagotrophic stramenopile from low oxygen environments, Rictus lutensis gen. et sp. nov. (Biocoecida, incertae sedis). Protist, 161, 264–278.
Zopf, F. W. (1892). Zur Kenntniss der Labyrinthuleen, einer Familie der Mycetozoen. Beiträge zur Physiologie und Morphologie niederer Organismen, 2, 36–48.
Acknowledgments
We thank various publishers for allowing the inclusion of their illustrative material. M. Thines has been supported by the excellent initiative of the federal state of Hessen (LOEWE), in the framework of the research cluster for Integrative Fungal Research (IPF). R. M. Bennett has been supported by a fellowship from KAAD and the Studienstiftung Mykologie.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this entry
Cite this entry
Bennett, R.M., Honda, D., Beakes, G.W., Thines, M. (2017). Labyrinthulomycota. In: Archibald, J., Simpson, A., Slamovits, C. (eds) Handbook of the Protists. Springer, Cham. https://doi.org/10.1007/978-3-319-28149-0_25
Download citation
DOI: https://doi.org/10.1007/978-3-319-28149-0_25
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28147-6
Online ISBN: 978-3-319-28149-0
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences