Abstract
Valve degeneration and dysfunction affect an increasing number of aging patients in the developed countries, while congenital and rheumatic diseases affect younger patients worldwide. When possible, valvular disease is treated by valve repair; however, severe pathologies require a surgical or transcatheter valve replacement procedure. Nowadays, clinical-grade valvular prostheses consist of mechanical or bioprosthetic valves, which still present serious disadvantages, such as thrombogenicity and limited durability.
In this chapter, we describe how tissue-engineered heart valves (TEHVs) can solve the shortcomings of current clinically adopted valvular replacements by providing a prosthesis with potential regenerative and remodeling capabilities and, hence, a lifelong durability. Different tissue engineering (TE) approaches, such as in vitro, in vivo, and in situ are described, with particular focus on preclinical and clinical validation of TEHVs. In this context, we will also discuss in silico, in vitro, and in vivo models that can be used to manufacture TEHVs, test their functionality, and predict their remodeling before moving to preclinical and clinical settings. Finally, we address the scientific, regulatory, and clinical challenges that pace the safe clinical translation of TEHVs.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
1 Introduction
The human heart functions as a pump, providing continuous blood flow through the body, and has evolved from an initial tube-like structure to an organ consisting of four chambers (right atrium, right ventricle (RV), left atrium, left ventricle (LV) (Fig. 1a)). The right and left atriums act as blood reservoirs, while the RV and LV act as pumps, responsible for the circulation of the blood in the pulmonary and systemic circulation, respectively (Rehman and Rehman 2018) (Fig. 1b). Each chamber is delimited by specific cardiac valves: the atrioventricular valves (tricuspid and mitral) located between atria and ventricles and the semilunar valves (pulmonary and aortic) (Fig. 1b). The four heart valves act as important inlet and outlet checkpoints during every cardiac cycle, opening and closing in response to different pressure gradients, therefore allowing the unidirectional flow of blood.
Schematic representation of the heart anatomy and physiology. (a) The right atrium (RA) and right ventricle (RV) are connected via the tricuspid valve. The RV is connected to the pulmonary circulation via the pulmonary valve. The right heart works at low-pressure conditions (0–25 mmHg). The left atrium (LA) and left ventricle (LV) are connected via the mitral valve. The LV is connected to the systemic circulation via the aortic valve. The left heart operates at high-pressure conditions (80–120 mmHg). (b) The blood cycle through the heart starts in the RA (i) where deoxygenated blood from the systemic circulation is collected. (ii) The deoxygenated blood flows into the RV, where the blood is then pumped into the pulmonary artery and the lung circulation to get oxygenated. Then, the blood flows back to the heart in the LA in order to be transferred into the LV. (iii) In the LV, the oxygenated blood is pumped in the systemic circulation passing through the aortic valve and into the aorta to supply the organs with oxygen-rich blood. (Images adapted from Servier Medical Art under a creative common attribution 3.0 unported license)
An average adult human heart beats between 60 and 70 times per minute, which leads the valves to open and close up to three billion times during a lifetime (Tao et al. 2012). These high performances are enabled by the continuous remodeling and turnover of heart valve cells and the extracellular matrix (ECM) surrounding them. Both components allow the proper function and maintenance of the organ homeostatic state in a variation of biomechanical and hemodynamic stimuli. However, it should not be surprising that degenerative diseases may affect these organs in the elderly, causing the valve leaflets to become thicker and stiffer due to fibrosis and calcifications (Fishbein and Fishbein 2019). In addition, inflammatory and congenital disease may impact on heart valve functionality in the young.
2 Cardiac Valve Anatomy and Functionality
Heart valves have a fundamental role in the cardiovascular system, by forcing the blood flow in one direction through the heart and into the pulmonary or systemic circulation. The mammalian heart comprises four different valves, which connect either the atrium with the ventricle (i.e., atrioventricular valves) or the ventricle with the artery that leaves the heart (i.e., semilunar valves). Function and morphology of these valves depend on their location in the heart. The right heart (pulmonary circulation) is a low-pressure system (0–25 mmHg) that comprises the atrioventricular tricuspid valve, which controls the direction of the blood flow from the right atrium to the RV, and the pulmonary valve, which leads the blood flow from the RV into the pulmonary artery. The left heart (systemic circulation), on the other hand, is a high-pressure system (80–120 mmHg) that is characterized by the atrioventricular mitral valve, connecting the left atrium to the LV, and the semilunar aortic valve, which controls blood flow direction from the LV to the aorta and the systemic circulation. During the cardiac cycle, the pulmonary circulation delivers blood to the lungs for an oxygen recharge and carbon dioxide discharge. The systemic circulation delivers oxygenated nutrient-rich blood from the heart to the brain and peripheral tissues.
Heart valve leaflets present a unique tri-layered structure that allows them to withstand the specific mechanical environment caused by the differential blood pressure and flow on each side such as pressure, shear stress, and stretch (Merryman et al. 2006).
Valvular heart dysfunction is a disease status for which heart valves are insufficient or stenotic. Nowadays, these pathologies are considered a major societal burden, with a higher incidence of structural diseases in western countries. To understand such pathologies and find a competitive treatment strategy, it is therefore important to comprehend valve anatomy, structure, and function.
2.1 Valve Anatomy and Functionality
2.1.1 Atrioventricular Valves
Both the mitral and the tricuspid valves enable the unidirectional blood flow from the atria to the ventricles and prevent the retrograde blood flow from the ventricles back to the atria. The atrioventricular valves are characterized and supported by a complex network of collagen and elastic fibers attached to the leaflets, known as chordae tendineae. These structures connect the valve leaflets with the papillary muscles, a series of muscles located within the ventricle that, when contract, prevent leaflet prolapse during systole (Anderson et al. 2000; Schoen 2008).
The combination of papillary muscles, chordae tendineae, and leaflets forms a functional unit that controls and ensures the opening and closure of the atrioventricular valves during the cardiac cycle (Millington-Sanders et al. 1998; McCarthy et al. 2010). Despite the similar function, the anatomy of these valves is slightly different.
The mitral valve is composed of four complex structures known as annulus, leaflets, chordae tendineae, and papillary muscles. The annulus designates the opening part of the mitral valve and enables horizontal and vertical geometry changes in the orifice area during cardiac cycle. The mitral valve possesses only two leaflets, the anterior and the posterior, which come together at the two commissures known as the anterolateral and posteromedial commissure (Misfeld and Sievers 2007; Dal-Bianco and Levine 2013).
The tricuspid valve has three thin and translucent leaflets, known as anterior, ventral, and posterior. The leaflets form a complex unit with the annulus, whose structure is subject to dynamic changes during the cardiac cycle (Yacoub and Cohn 2004). Leaflet morphology varies among the three leaflets, with the anterior leaflet being the largest, followed by the medial leaflet, and, finally, posterior leaflet which is the smallest (Buzzatti et al. 2018).
2.1.2 Semilunar Valves
The semilunar valves (i.e., aortic and pulmonary ) have the function of dividing the ventricles from the main arteries and preventing retrograde flow into the ventricles during diastole. Semilunar valves comprise three leaflets, which are attached in a semilunar pattern to the annulus, with no external support from papillary muscles and/or chordae tendineae. The basal point of attachment of every leaflet is characterized by a fundamental anatomical feature, the sinuses of Valsalva, which represents the main load-bearing component of the cusps during diastole. The sinuses collect the regurgitant blood during early diastolic phase, influencing the leaflet closure dynamics, ensuring the blood washout, and, for the aortic valve, allowing blood supply to the coronary arteries (Toninato et al. 2016). This specific geometry allows the valves to be functional and to withstand hemodynamic forces throughout a lifetime.
The aortic valve connects the LV with the aorta and the systemic circulation. Its leaflets are anchored to the aortic root by a fibrous annulus, which supports the aortic valve and has a key role in the proper functioning of the heart, by ensuring the appropriate coronary perfusion and by maintaining the laminar flow in the vascular system (Anderson 2000). The pulmonary valve connects the heart to the pulmonary root thus to the pulmonary circulation. Similar to the aortic valve, the pulmonary valve is composed of the annulus, the sinuses, and the leaflets (Bateman et al. 2013).
The commissures are the highest connection points of the leaflets to the arterial wall and are located above the interleaflet triangles, where they define the sinotubular junctions. The interleaflet triangles are of high importance for the opening and closing motions of the valves.
All the structures composing the aortic valve such as the annulus, the sinuses of Valsalva, commissures, and the leaflets operate together to allow proper valve function.
The three aortic valve leaflets are characterized by three fundamental anatomical features: the sinuses of Valsalva, the lannula, and the noduli of Arantii. As previously mentioned, the Valsalva sinuses correspond to the attachment point of the leaflets to the annulus and transmit the stresses endured by the leaflets to the aortic wall. Importantly, when the leaflets are in closed configuration, they reveal the coronary artery ostia, the access point for perfusing the coronary arteries, which give the name to the three aortic leaflets as left coronary, right coronary, and non-coronary (Underwood et al. 2000). The noduli of Arantii is located in the middle of the free edge of the coapting surface. Next to each side of the nodulus, there is a thin semilunar-shaped structure known as the lannula, which is linked to the aortic root wall close to the commissures and which ensures valve coaptation.
2.2 Valve Cell and Tissue Composition
Heart valve leaflets are subject to three different stress states, bending, shear stress, and stretch caused by blood pressure (Merryman et al. 2006). In order to withstand those mechanical stresses and enable high flow rate with low resistance, the valve leaflets evolved to form a complex microscopic tissue structure. The semilunar valve leaflets have a highly organized three-layered ECM architecture (i.e., fibrosa, spongiosa, and ventricularis/atrialis) populated by valve interstitial cells (VICs) and valve endothelial cells (VECs). This specific tissue composition and structure allows the proper function of the valves, thus enabling unidirectional flow of the blood (Balachandran et al. 2011; Hinton and Yutzey 2011).
2.2.1 Tissue Structure
The three layers that compose the leaflets allow them to withstand perpetual changes of pressure during the cardiac cycle (Lincoln et al. 2006; Balachandran et al. 2011) (Fig. 2, Table 1). The fibrosa layer is mainly composed of collagen fibers (types I and III) and is restricted to the outflow surface (Schoen 2012; Schoen and Gotlieb 2016). In order for the leaflet to have the necessary tensile strength during the opening phase of the valve and to transmit the forces during the closing phase, the collagen fibers position themselves circumferentially (Lincoln et al. 2006; Balachandran et al. 2011). The spongiosa is the intermediate layer characterized by sparse collagen fibers and abundance of proteoglycans and glycosaminoglycans (GAGs). This layer composition acts as a shock absorbent when the valve leaflets are closed, and it confers the ability to compress when the valve is in closed configuration (Sacks et al. 2009). The third and final layer, the ventricularis in the case of the semilunar valves or atrialis in the case of the atrioventricular valves, is located at the inflow surface. The ECM of the ventricularis/atrialis is mainly composed of elastin fibers (e.g., fibrillin and elastin) with radial orientation. The elastin fibers allow better movement of the leaflet by extending and recoiling during the opening and closing motions (Hinton and Yutzey 2011).
Schematic representation of a semilunar heart valve leaflet structure and composition. A cross-section of the heart valve leaflet, with the proximal portion of the valve connecting to the myocardium and the distal portion connecting to the arterial wall. The leaflet connects at the hinge region with the annulus and forms a sinus of Valsalva. A semilunar heart valve leaflet is characterized by three layers: the fibrosa, mainly consisting in circumferentially aligned collagen; the spongiosa, rich in glycosaminoglycans (GAGs); and the ventricularis, with radially oriented elastin. Valve interstitial cells (VICs) are distributed throughout the leaflet, while valve endothelial cells (VECs) are covering the leaflet surfaces in contact with blood. (The image was inspired by the work of Rutkovskiy et al. 2017, and re-created using images adapted from Servier Medical Art under a creative common attribution 3.0 unported license)
2.2.2 Cell Composition
Being a living tissue, native valves are characterized by highly versatile cellular components, such as the VICs and VECs (Fig. 2, Table 1). VICs and VECs ensure tissue homeostasis by maintaining the leaflet layered structure. Importantly, both VICs and VECs are sensitive to the mechanical environment and show great ability to adapt to changes of hemodynamics and to remodel the layered connective tissue of the valve leaflet. The VECs cover the leaflet surfaces in contact with blood and are involved in the regulation process of thromboresistance, inflammation, and maintenance of the valvular structure.
VICs are the most abundant resident cells of the leaflet ECM and are responsible for the remodeling and repair of the leaflet by synthesizing ECM proteins and metalloproteinases to regulate tissue formation and degradation (Liu et al. 2007). In an adult heart, the majority of the VICs have a fibroblast-like phenotype and are in a quiescent state (quiescent VICs). However, a small amount of cells (between 2% and 5%) becomes activated in response to homeostatic changes in the environment (Schoen 2008), and four more phenotypes have been described: (1) embryonic progenitor VICs that act as an internal source, (2) activated VICs that are mostly present during active ECM remodeling processes and tissue repair, (3) osteoblastic VICs that are responsible for valve calcification (Liu et al. 2007), and (4) mesenchymal VICs.
It is this balanced interaction between VECs and VICs that allows to maintain valve tissue integrity throughout life (Butcher and Nerem 2006).
2.3 Cellular Mechanisms of Valvular Disease
Valvular heart diseases are usually progressive diseases, actively regulated by VECs and VICs cellular interactions, that cause damages to the leaflet, disruption of the hemodynamics, and/or inflammation (Gould et al. 2013; Mathieu et al. 2015). The typical resulting inflammation process triggers the production of inflammatory cytokines and chemokines, attracting cells from the immune system into the leaflet. These events determine the continuous activation of VECs and VICs and can result in chronic inflammation which leads to fibrotic thickening, followed by an extensive calcification of the valve (Rutkovskiy et al. 2017; Schoen and Gotlieb 2016).
Leaflet calcification can occur in normal and in congenitally malformed valves, such as bicuspid aortic valves (Roberts and Ko 2005; Rajamannan et al. 2011). Briefly, due to progressive degeneration and/or fibrosis, changes in leaflet stiffness progressively increase the risk of developing calcification. Calcium deposits onto the leaflets reduce the effective orifice area of the valve (i.e., stenosis) and cause an outflow obstruction by limiting leaflet mobility. The molecular and cellular processes regulating calcific valve stenosis are not yet fully characterized. However, VICs and VECs are responsible for the maintenance of valve homeostasis and leaflet integrity by constantly renewing the leaflet ECM and by preserving the characteristic layered composition. Calcific deposit on the valve leaflets are mediated by the resident VICs that, upon pathological cues, can differentiate toward an activated myofibroblast -like and osteoblast -like phenotype (Demer and Tintut 2008; Yip et al. 2009). In addition, as a result of endothelial injury, the immune system has been shown to be involved in VIC activation, causing ECM remodeling, and osteoblastic differentiation, inducing progressive valve mineralization (Coté et al. 2013). Moreover, increased endothelium permeability allows the diffusion of different molecules (i.e., cytokines, growth hormones, low-density lipoproteins) into the valve leaflets that cause the recruitment of immune cells, generating a chronic inflammation and subsequently initiating a phenotype switch of quiescent VICs toward an osteogenic phenotype (Mohty et al. 2008; Mathieu et al. 2015; Schoen and Gotlieb 2016).
On the other hand, myxomatous degeneration is a noninflammatory progressive disease, which is characterized by a disrupted leaflet structure caused by a defect in the synthesis and/or remodeling of collagen. This results in heart valves showing collagen fiber disorganization in the fibrosa layer, resulting in thickened leaflets, and demonstrating thickening of the spongiosa layer due to excessive proteoglycan deposition. These events, driven by improper ECM turnover from the VICs, significantly alter the structural integrity of the leaflet, leading to the mechanical weakening of the valve (Grande-Allen et al. 2003; Schoen and Gotlieb 2016) and causing stretching and elongation (Vesely et al. 1988).
The activation of VICs and VECs on a cellular level results, therefore, in morphological changes of the valve (e.g., leaflet thickening and stiffening) that have a strong impact on valve functionality, causing pathologies such as valve stenosis and valve insufficiency, as detailed in Sect. 3.
3 Heart Valve Pathology
Valvular heart disease constitutes an important clinical problem, causing approximately 25,000 deaths annually in the United States only (Schoen 2018) and contributing to an increased mortality rate (Benjamin et al. 2017), with aortic valve disease accounting for more than half of the deaths (Coffey et al. 2016).
In industrialized countries, aging is the principal cause of the continuous rising of valve disease incidence in patients over 65 years old (Schoen 2018). During an average person lifetime, the valves will open and close up to 100,000 times each day. Hence, it should not be surprising that degenerative diseases may affect these organs in the elderly, causing the valve leaflets to become thicker and stiffer due to fibrosis and calcifications (Fishbein and Fishbein 2019). In developing countries, on the other hand, rheumatic fever (RF) is a leading cause of valvular pathologies and remains a major cause of death and disability in children and young adults (Leal et al. 2019). Congenital heart disease (CHD), affecting up to 2% of the newborn, may also lead to poor valve functionality either early in life (i.e., as in the case of unicuspid aortic valve) or once adulthood is achieved (i.e., bicuspid aortic valve) (Fishbein and Fishbein 2019). Current advancements in pediatric cardiac surgery have resulted in an increasing number of adults with CHD that are followed up at later stages (Marelli et al. 2007). Heart valve disease can affect all the heart valves and can be classified into two categories by their impact on valve functionality: valve stenosis and valve insufficiency (Fig. 3). It is important to consider that most of the time, valve diseases are a consequence of changes in the hemodynamics that impact valvular cell phenotype and functionality.
Schematic representations of the most common valvular diseases. (a) Valve stenosis, (b) inflammatory disease such as endocarditis, (c) acute, and (d) chronic valve insufficiency (or regurgitation). For representative purpose, the affected valve in the schematics is the mitral valve; however, all the heart valves may be subjected to these diseases. (Images adapted from Servier Medical Art under a creative common attribution 3.0 unported license)
3.1 Valve Stenosis
Valve stenosis is a condition characterized by obstruction of the blood flow due to limited opening of the valve (Fig. 3a). The etiologies of valve stenosis can be subdivided into age-related calcific and degenerative disease, CHD, and inflammatory disease.
Age-related valve stenosis mostly affects the aortic valve, and it is caused by stiffening and thickening of the valve cusps due to calcification and fibrosis. Aortic valve stenosis is, therefore, becoming a prevalent pathology in developed countries, due to aging of the population (Chong et al. 2019). Repeated clinical follow-ups are fundamental to assess the progress of aortic stenosis in elderly patients and timely plan a suitable valve replacement.
Congenital malformations are the most common cause of valvular stenosis in pediatric and young adult patients. Aortic bicuspid or unicuspid valves (i.e., the aortic valve is composed of only two cusps with similar size or of one single leaflet, respectively) are prone to progressive degeneration and stenosis about 10–15 years earlier than normal aortic valves. Hence, aortic bicuspid or unicuspid valves are becoming the most common cause of aortic stenosis in patients age 50–70 years old (Fishbein and Fishbein 2019) with an average rate reduction of the valve orifice area that has been estimated to be ∼0.12 cm2/year. Pulmonary stenosis, on the other hand, is less prevalent but can be caused by rare malformations that induce fusion of adjacent leaflets due to CHD.
Inflammatory disease, such as RF and endocarditis (Fig. 2b), can affect multiple valves and is the most prevalent cause of stenosis to affect the atrioventricular mitral and tricuspid valves. Similar to degenerative stenosis, post-inflammatory stenosis is also characterized by cusp thickening, calcification, and fusion of adjacent leaflet at the commissures (Fishbein and Fishbein 2019) that determine limited blood flow through the valve orifice.
Since the progression of valve stenosis can vary significantly between patients, clinical follow-ups are fundamental to assess the hemodynamic parameters and grade the severity of the stenosis (Table 2) via spectral Doppler echocardiography. The recommended primary values for grading aortic stenosis are reported in Table 2.
3.2 Valve Insufficiency
Valve insufficiency (also known as regurgitation or incompetence) refers to the incomplete closure of the valve leaflets, thereby inducing reverse flow in the atrium (when atrioventricular valves are affected) or in the ventricle (when semilunar valves are regurgitant) (Fig. 3).
Compared to valvular stenosis, valve regurgitation is caused by a more extended list of diseases (Fishbein and Fishbein 2019) that differs between acute and chronic regurgitations (Table 3 and Fig. 3). Chronic regurgitation refers to valve damage that determines a progressive increase in regurgitating blood volume over time, causing dilatation of the atrium (when atrioventricular valves are affected) or of the ventricle (when semilunar valves are affected). In contrast, acute aortic regurgitation may lead to sudden elevation of left ventricular filling pressure, reduction in cardiac output, and/or sudden death.
Aortic valve regurgitation may be a consequence of valve leaflet or valve root abnormalities due to congenital and/or degenerative disease, such as dilation of the annulus, cusp prolapse, leaflet retraction, cusp perforation, or rupture. In addition, it may be caused by a variety of other diseases, either inflammatory (i.e., syphilis, endocarditis) or noninflammatory (connective tissue disease, i.e., Marfan syndrome), that usually determine a dilation of the valve root that prevents complete leaflet coaptation. The principal tool to grade aortic valve insufficiency is color Doppler echocardiography, a highly sensitive method to visualize the regurgitant jet (Maurer 2006).
Pulmonary insufficiency is often a consequence of pulmonary hypertension, rheumatic disease, and infective endocarditis. On the other hand, mitral regurgitation is one of the most common forms of valvular disease, and it is often caused by dysfunction of any of the mitral valve structures (i.e., annulus, papillary muscle, chordae tendinae). Specifically, mitral valve prolapse (i.e., the displacement of a mitral valve thickened leaflet into the left atrium during systole) is the most prevalent cause of severe mitral regurgitation in both developed and developing countries (Althunayyan et al. 2019).
4 Current Treatment Options for Valvular Disease
Currently, there is no lifelong medication for valvular disease, and the treatment options and clinical outcome significantly depend on the valve involved, the development of the disease, the overall heart functionality and compensatory mechanisms, as well as the age of the patient.
Whenever possible, valvular repair is the first choice of treatment for a diseased valve, because it comes with several conceptual advantages, such as reduced risk of infection, reduced need for anticoagulants, preservation of valve anatomy, and restoration of patient’s valve functionality, without the need for a prosthesis (Girdauskas et al. 2018). However, the decision between valve repair and valve replacement is evaluated by taking into consideration the severity of the disease, the age of the patient, and whether there are other heart pathologies to correct or comorbidities to consider.
4.1 Valve Repair
In the beginning of the nineteenth century and till the Second World War, approximate techniques of valve repair, such as inserting a finger to free the fused leaflets of a stenotic valve (Dr. Theodore Tuffier, 1912) (Ellis et al. 1996), were used to treat valvular disease. In addition, during and after the Second World War and thanks to the establishment of hypothermia procedures, novel techniques for valve repair were developed as wounded soldiers were injured in the myocardium by explosions. With the introduction of cardiopulmonary bypass in the 1950s and the increased knowledge of cardiac surgeons, heart valve-sparing procedures for semilunar and atrioventricular valves had been constantly developing.
Pulmonary valve stenosis, caused by congenital anomalies that lead to thickening and fusion of the valve leaflets and/or to a bicuspid pulmonary valve, can be efficiently treated via valve-sparing procedures (Parikh et al. 2017). Many patients with pulmonary stenosis are operated with a transannular patch to relieve the stenotic outflow tract of the RV. Briefly, the arterial wall is incised, and a polytetrafluorethylene (PTFE) patch is sutured to enlarge the outflow track (Parikh et al. 2017). While this is an effective procedure to solve the stenosis, pulmonary insufficiency post-intervention, caused by fibrotic tissue formation along the suture, is still a common complication that causes regurgitation and leaflet prolapse (Said et al. 2016). To correct valve insufficiency, an artificial leaflet, made of PTFE or glutaraldehyde-fixed bovine pericardium, is transplanted to recreate a functional three-leaflet pulmonary valve and to preserve the annulus size and the pressure gradient of the outflow tract (Said et al. 2016).
To correct aortic insufficiency, valve repair focusing on ensuring full leaflet coaptation in diastole is used in about 25% of low-risk patients, and it showed good outcomes with low mortality rates even in elderly subjects (Bisleri 2016). As described in, several are the causes of aortic insufficiency (e.g., leaflet retraction, prolapse, calcifications, thickening, and valve annulus dilation). Leaflet retraction and bicuspid aortic valve regurgitation can be corrected by suturing the commissure points or by shortening the belly of the cusps with plicating sutures, also used to solve valve leaflet enlargements, cuspid elevations, and Valsalva sinus remodeling (Girdauskas et al. 2018). Finally, aortic root dilation, commonly observed in bicuspid aortic valves, was initially treated by reducing the perimeter of the aortic root. However, this procedure caused re-dilation of the aortic root over time. To solve this issue, current techniques focus on the placement of an external ring on the aortic root to prevent re-dilation events (Bisleri 2016).
Valve repair is the optimal solution to treat degenerative mitral regurgitation. Together with various surgical techniques, annuloplasty using PTFE rings are typically used to minimize annulus size and, thereby, limit insufficiency (Grasso et al. 2015). In cases of posterior mitral leaflet prolapse, instead, the mitral chordae tendinae are replaced by PTFE- or glutaraldehyde-fixed bovine pericardium-based implants (Shah and Jorde 2019). In elderly and otherwise inoperable patients, valve insufficiency can nowadays also be solved by an edge-to-edge repair of the mitral leaflets using the MitraClip or PASCAL transcatheter system (Mendirichaga et al. 2017). Recently, similar transcatheter strategies have been also developed for the treatment of tricuspid regurgitation. However, current transcatheter strategies for tricuspid regurgitation are still in their early stages (Tabata et al. 2019).
Overall, valve repair is commonly preferred for young patients, because it allows for somatic growth of developing hearts and do not require lifelong anticoagulation treatment. However, valve repair techniques are not always sufficient for the treatment of severe valvular dysfunction associated with degenerative or congenital diseases.
4.2 Valve Replacement
Heart valve repair options provide little improvement for severe conditions, and patients often need a replacement procedure shortly after repair intervention (Gasser et al. 2019). On the other hand, heart valve replacement methods, either surgical or transcatheter, can provide long-term results with low risk of reoperation, in particular for elderly patients (Baumgartner et al. 2017).
Currently, both surgical and transcatheter approaches are in routine clinical use in developed countries, and the selection of one method over the other depends on the pathology, patient age and specific anatomy, gender, and presence of comorbidities.
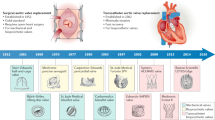
Surgical valve replacement (SVR) is the first treatment option to treat patients affected by severe valvular disease, as it provides good perioperative and long-term results (Baumgartner et al. 2017). SVR is based on an invasive open-heart surgery that involves the use of temporary cardiac arrest and of cardiopulmonary bypass to establish extracorporeal circulation and ventilation during the time required by the surgeon to replace the valve. Considering the consequences of these techniques on the recovery time, patients may be considered inoperable or at a high risk for surgery due to comorbidities. Minimally invasive transcatheter valve replacement (TVR) was developed in the early 2000s (Bonhoeffer et al. 2000; Cribier et al. 2002) (Fig. 4), and, since then, it has revolutionized the treatment options for patients affected by valvular disease. TVR was initially developed to treat patients considered inoperable or at high risk for SVR (Rodés-Cabau et al. 2012), because the minimally invasive implantation procedure significantly reduces the hospital stay and patient recovery and rehabilitation time (Chatterjee et al. 2019). Over the course of two decades, great progresses have been made to deliver valves in a minimally invasive fashion, with specific focus on the replacement of the aortic valve (Rodés-Cabau et al. 2012). Transfemoral aortic valve replacement allows for a fully percutaneous implantation via the retrograde insertion of a long catheter through the femoral artery. However, transfemoral TVR is not suitable to all patients because of poor femoral access and presence of vascular complications (Fioretta et al. 2017). Hence, other access routes have been investigated, such as the transapical (i.e., access to the valve via ventricular apex (Ye et al. 2006)) and the transaortic (i.e., access to the aortic valve from a mini-sternotomy and a puncturing of the aortic wall (Bauernschmitt et al. 2009)) approaches. As of today, TVR has been approved to intermediate- (Leon et al. 2016; Reardon et al. 2017) and low-risk (Mack et al. 2019; Popma et al. 2019) patient cohorts, becoming therefore an available treatment option for all patients affected by aortic stenosis.
Evolution of clinical heart valve prostheses and replacement technique options. Mechanical valves, only eligible for surgical valve replacement (SVR), are in a blue font color. Bioprostheses, on the other hand, were firstly developed as SVR option to overcome the limitations of mechanical valves and are here represented by the red color font. Starting in the 2000s, stented bioprostheses compatible with minimally invasive transcatheter valve replacement (TVR) techniques were developed and are here represented by the green color font
4.3 Heart Valve Prostheses Options
4.3.1 Mechanical Valve Prostheses: A Durable Solution
After the first ball-in-cage mechanical valve replacement (Russo et al. 2017), major progresses in heart valve prostheses design and material selection have been made, until the development of the first bi-leaflet tilting disc mechanical valve (Head et al. 2017) (Fig. 4). Mechanical valves, based on long-lasting metals such as titanium, are characterized by a mechanical durability that lasts more than 20 years, deeming them as the gold standard for the majority of the SVR procedures in younger patients. Despite the remarkable long-term performances, mechanical valves are responsible for altered hemodynamic conditions, turbulent and accelerated blood flow that determine damages to the red blood cells, which consequently cause platelet activation, thereby initiating the blood coagulation cascade (Head et al. 2017). Hence, to prevent thrombus formation, lifelong anticoagulation therapy is recommended. However, blood-thinning medication is not a viable option for a variety of patients (e.g., pregnant women, athletes, patients affected by other comorbidities), because of the increased risk of uncontrolled bleeding (Nishimura and Warnes 2015).
4.3.2 Homografts: A Promising Native-Like Alternative
Valvular homografts are defined as a section of a human donor aorta or pulmonary artery which comprises the intact semilunar valve. Since their introduction (Ross 1962), homografts have been considered the most promising valvular replacements because of their significant advantages when compared to mechanical prostheses (Delmo Walter et al. 2012). In particular, by retaining the native valve anatomy, homografts succeeded to achieve a physiological-like hemodynamics with low thrombogenicity over the course of the patient’s lifespan (Lisy et al. 2017). For these reasons, homografts were mostly used for severe CHD, such as the tetralogy of Fallot (Meijer et al. 2019), where complex surgical reconstruction where required. Long-term follow-ups have, however, highlighted the disadvantages of using this particular valvular prosthesis for SVR. Firstly, homografts can only be implanted via open-heart surgical technique, which complexity may influence the clinical outcome (Delmo Walter et al. 2012). Secondly, homograft tissue quality was reported to differ due to not donor-to-donor variability and to tissue banking methodologies. In particular, differences in cryopreservation and thawing protocols may impact the ECM structure of the homografts, thereby limiting its durability (Delmo Walter et al. 2012). Thirdly, long-term performance is compromised by chronic immunogenicity, onset of calcifications, and valve degeneration over time (Delmo Walter et al. 2012; Bonetti et al. 2019), causing only a third of the implanted graft to reach the 20-year follow-up (Delmo Walter et al. 2012). Last, the limited availability of donor tissues hinders the broader clinical application of these replacement options. Taken together, these limitations significantly impact on the use of homografts for the treatment of congenital and degenerative diseases in pediatric and young patients, where stenosis, degeneration upon implantation, leaflet shortening, and leaflet thickening due to calcifications and fibrosis were observed (Blum et al. 2018).
4.3.3 Xenogeneic Bioprosthetic Valves: An Alternative to Homografts and Mechanical Valves
To overcome the limitations of mechanical and homograft prostheses, animal-derived materials (i.e., tissues derived from bovine or porcine sources treated with glutaraldehyde to limit their immunogenicity) were introduced to manufacture valves with improved hemodynamics and physiological-like tissue composition, based on collagenous ECM (Fig. 4, Table 4). Due to their native-like geometry, bioprostheses are more hemocompatible, and they allow for a reduced need of anticoagulants (Manji et al. 2015). Xenogenic tissues have been also used to manufacture stented bioprostheses compatible with minimally invasive TVR techniques, demonstrating successful preoperational crimping and loading into the delivery catheter (Wiegerinck et al. 2016; Arsalan and Walther 2016). After the promising outcome of recent clinical trials, TVR indication has been recently extended to low-risk patient cohorts (Mack et al. 2019; Popma et al. 2019). Hence, while previously designed to treat the elderly, TVR compatible bioprostheses will be now used also in younger patients (Manji et al. 2015). Nevertheless, bioprostheses are prone to progressive degeneration and, thus, require reoperation after 10–20 years (David 2010; Arsalan and Walther 2016). The major causes of bioprosthesis failure are leaflet degeneration, stiffening, calcification, and immunogenic reactions (Human and Zilla 2017; Fishbein and Fishbein 2019). Importantly, these degenerative phenomena are more prone to occur in pediatric patients, where the immune reaction to the xenogenic material causes severe inflammation (Rabkin-Aikawa et al. 2005).
4.3.4 Non-resorbable Polymeric Valves: A Cost-Effective Solution
Non-resorbable polymers, such as silicon, polytetrafluoroethylene, and polyurethanes, were introduced from the late 1960s as an alternative material to achieve valve prostheses with a physiological design and improved durability (Roe 1969; Mackay et al. 1996). While some polymeric prostheses were successfully used as pulmonary replacement for the treatment of congenital disease (Ando and Takahashi 2009), non-resorbable polymeric valves have shown major flaws, including limited durability, impaired functionality, leaflet stiffening, and thrombogenicity (Hilbert et al. 1987; Nistal et al. 1990; Daebritz et al. 2004). In addition, discrepancies in the manufacturing methods and variability in the polymer batches have impeded their broad clinical translation (Kheradvar et al. 2015). More recently, a siloxane poly-urethane urea polymeric valve has been shown to fully comply with the International Organization for Standardization (ISO)-norm requirements for in vitro testing and demonstrated good functionality with no evidence of calcification or other degenerative phenomena in a preclinical animal model (Bezuidenhout et al. 2015). Next, another novel polymeric aortic valve has been granted permission for clinical trials (ClinicalTrials.gov Identifier: NCT03851068). Ultimately, polymeric valves could be the grounding foundations of cost-effective off-the-self solutions for heart valve replacements using TVR approaches (Bezuidenhout et al. 2015; Scherman et al. 2018). However, it remains a challenge to couple durability of these valves with biocompatibility and proceed further to the clinical evaluation.
4.4 Clinical Impact and Burden on Society
In developed countries, the prevalence of heart valve disease is highest in patients older than 75 years, reaching up to 13.3% (Huygens et al. 2018), and the number of valve replacement procedure is expected to increase to 800,000 annually worldwide by 2050 (Yacoub and Takkenberg 2005). The growing aging population affected by degenerative aortic stenosis that requires a valve replacement procedure will considerably impact on society, with healthcare costs reaching above 1 billion euro just in European countries (Huygens et al. 2018).
TAVR has drastically changed the field of valve replacement over the past 20 years, with the introduction of minimally invasive techniques. Every year, approximately 180,000 patients undergo TAVR in the European Union and Northern America alone. This number is expected to rise up to 270,000 annually (Durko et al. 2018) as recent clinical trials proved non-inferiority of TAVR over SAVR also in intermediate- (Leon et al. 2016; Reardon et al. 2017) and low-risk patients (Mack et al. 2019; Popma et al. 2019). Following these promising results, TAVR valves Sapien 3 and CoreValve Evolut have been approved also for low-risk patients by the FDA (August 2019). This approval makes TAVR available for all patients with severe, symptomatic aortic stenosis, significantly increasing the estimated number of TAVR procedure in European and North American countries of almost 54% (Table 5) (Durko et al. 2018).
Despite this tremendous technical evolution in the field of transcatheter techniques, only little progress has been made on the bioprosthetic materials used for TAVR. Currently available heart valve prostheses are still based on non-regenerative materials (namely, glutaraldehyde-fixed xenogenic or allogenic tissues, titanium, or carbon) that can cause several prostheses-associated problems upon implantation: thrombogenicity, progressive degeneration, limited durability, and, most importantly, the inability to remodel and grow with the patient (Henaine et al. 2012; Head et al. 2017). It should not be of surprise that the quality of life and life expectancy of patients with a valvular replacement is significantly impacted and healthcare costs are considerably higher in the first 3 years post-surgery compared to age-matched healthy individuals (Table 6) (Huygens et al. 2018). To these early costs, further expenses should be taken into account in particular for pediatric and young adult patients, that they will be more likely to undergo multiple reoperation to substitute an outgrown or degenerated valve prosthesis.
Therefore, there is a clear need for new durable valve replacements compatible with transcatheter procedures that are based on novel materials capable to regenerate, remodel, repair, and adjust to the functional and somatic growth of the patient. This led to the development of multidisciplinary approaches, combining cell biology, engineering, and medicine to create tissue-engineered heart valves (TEHVs), a novel concept of valvular prostheses that could remodel and even grow with the patient.
5 Heart Valve Tissue Engineering
“The loss or failure of an organ or tissue is one of the most frequent, devastating, and costly problems in human health care. A new field, tissue engineering, applies the principles of biology and engineering to the development of functional substitutes for damaged tissue,” with those exact words in 1993, Robert Langer and Joseph P. Vacanti opened up a new era for the fields of medicine, biology, and engineering (Langer and Vacanti 1993). The concept was easy and straightforward: generate a new living tissue starting from cells directly isolated from the patient (autologous) and placed on or within biodegradable scaffold matrices. The original in vitro tissue engineering (TE) paradigm embraces the use of three components: (1) a 3D scaffolds; (2) autologous cells, seeded onto the scaffold ; and (3) an in vitro bioreactor system to induce ECM formation by simulating the physiological tissue conditions (Langer and Vacanti 1993; Mayer et al. 1997). Once the new extracellular tissue is formed, the living patient-specific organ substitute can be implanted in the patient enabling the direct function of the prosthesis with further in vivo tissue growth and remodeling. Few years before, an American surgeon pioneer in heart valve surgery highlighted the essential features of the ideal heart valve substitute which included the capacity to grow, self-repair, remodel, adjust to functional changes, be durable over time, prevent thrombus formation, and resist to infections (Harken 1989). Nowadays, the ideal heart valve prostheses which comprise these unique and fundamental properties of native heart valves still do not exist. In this sense, TE offers the possibility to create biodegradable and biocompatible living valve replacements with regenerative capabilities which could potentially overcome the shortcomings of the current clinically used heart valve prostheses. Since the first application of the in vitro TE traditional dogma, various alternative approaches have been developed to produce off-the-shelf available TEHV prostheses by reducing the production costs and time. This chapter deals with the different approaches, requirements, and potential benefits that characterize TEHVs and the various experimental and technological challenges that are being faced to achieve a broad use of TE in clinics. Section 5 deals with the different technological concepts characterizing TEHVs nowadays. Section 6 describes the multitude of cell sources and scaffold types that are used for engineering heart valves in vitro, in vivo, and in situ. To conclude, Sects. 7 and 8 provide an outlook on the current testing platforms for TEHVs and on the next-in-line steps towards clinical translation of such prostheses.
Given the extensive body of literature on the multiple approaches existing to generate TEHVs, this chapter gives the reader a general overview of the main concepts involved in heart valve TE field. Conceptually, we can distinguish three main categories: (1) in vitro TE (the classical paradigm), (2) in vivo TE, and (3) in situ TE.
5.1 In Vitro Heart Valve Tissue Engineering
The classic in vitro heart valve TE concept (Fig. 5) starts with the isolation and expansion of autologous cells from the patient using in vitro cell culture techniques. When in sufficient amount, cells are seeded onto a biodegradable, biocompatible porous scaffold, either of synthetic or biological origin, which provides a temporary structural support for the cells to produce their own ECM. This cell-scaffold construct is then transferred and cultured into a bioreactor system over a predetermined period of time. Here, cells are exposed to different biomechanical and/or biochemical cues to induce cell proliferation first and in a second time tissue formation. The purpose of the bioreactor is to mimic the physiological environment of native heart valves, which tissue organization is heavily influenced by the hemodynamic loading of the cardiac cycle (Barron et al. 2003). Hence, mechanical properties, pressure, and shear stresses are culturing conditions which have been optimized in the years to enable the engineering of lab-grown valves that mimic their native counterparts as best as possible.
Schematic representation of in vitro heart valve tissue engineering. This approach aims at the development of an autologous TEHV by isolating cells from the patient. After expansion, the cells are seeded onto a bioresorbable scaffold, cultured in a bioreactor system to ensure nutrient exchange and chemical and mechanical stimulation to favor ECM deposition. After a predetermined culture time, the autologous TEHV is ready for implantation into the patient. (Images adapted from Servier Medical Art under a creative common attribution 3.0 unported license)
Cells of various origin, such as adipose-derived stem cells, endothelial cells, myofibroblasts, dermal fibroblasts, valve interstitial cells, bone marrow-derived stem cells, blood progenitor cells, umbilical cord vascular cells, and/or amniotic fluid cells, have been used for seeding TEHVs over the course of the years (Jana et al. 2016). Vascular-derived myofibroblasts isolated from saphenous veins or forearm vein have been extensively investigated as a relevant cell source for autologous cardiovascular TE applications (Jana et al. 2016). These cells have, indeed, good proliferation potential and can produce collagenous ECM in vitro.
First attempt to generate TEHVs were first introduced in 1995 by Shinoka et al. (1995), where polyglycolic acid (PGA) sheets were seeded with autologous myofibroblasts and endothelial cells and used to reconstruct and replace the right posterior pulmonary heart valve leaflet in lambs. These first reports were then followed by proof-of-concept studies showing the complete valve replacement via in vitro engineered pulmonary heart valves in lambs (Dijkman et al. 2012a). Since then, many approaches for the production of TEHVs were reported in literature and reviewed elsewhere (Mol et al. 2009; Fioretta et al. 2017), and significant progresses have been made in the development of different scaffold materials and cell sources for the development of in vitro TE. However, the long-term proof of such concepts when applied to TEHVs is still pending. Indeed, latest studies investigating TEHV performance and tissue remodeling into preclinical animal models have been characterized by gradual loss of functionality within few months due to adverse remodeling, which was caused by the development of leaflet retraction and valvular incompetence (Flanagan et al. 2009; Gottlieb et al. 2010; Schmidt et al. 2010; Weber et al. 2013; Driessen-Mol et al. 2014; Syedain et al. 2015; Reimer et al. 2017; Motta et al. 2018). Finally, from a translational perspective, in vitro TE is recognized to be logistically complex, costly, and time-consuming, all aspects that hinder the scalability and translatability into clinical trials and commercialization.
5.2 In-Body Heart Valve Tissue Engineering
In-body TE, also known as in vivo TE (Fioretta et al. 2017), is an additional research line developed to exploit the foreign body reaction of the host upon subcutaneous implantation of a non-degradable mold (Hayashida et al. 2007, 2008). The aim is to generate a fibrotic collagen-rich tissue which is able to encapsulate and take the shape of the implanted mold. The final TEHV product, which researcher in the field has named biovalve, can be harvested and implanted as autologous non-immunogenic replacement in the same host (Fig. 6). Several prototypes and different generations of biovalves have been already tested in vitro and in vivo under pulmonary and aortic conditions (Takewa et al. 2013; Funayama et al. 2015a, b; Sumikura et al. 2015), even in combination to minimally invasive implantation techniques (Nakayama et al. 2015; Funayama et al. 2015b; Sumikura et al. 2015). In addition, tools to noninvasively monitor tissue formation have been developed to ensure complete tissue formation around the mold over time (Funayama et al. 2015c).
Schematic representation of in-body heart valve tissue engineering. This approach creates an autologous TEHV by implanting subcutaneously a valvular mold into the patient. Due to the host response, the mold is covered by fibrotic collagenous tissue and, after a predetermined amount of time, is removed to obtain a TEHV. The valve is then implanted back into the patient heart as valvular substitute. (Images adapted from Servier Medical Art under a creative common attribution 3.0 unported license)
Despite the early positive results demonstrated in both in vitro and in vivo studies, even in vascular graft applications (Terazawa et al. 2019), the in-body manufacturing of such matrices in humans is questionable: (1) the approach is highly invasive; (2) the technique is expected to require at least 4 months to obtain a biovalve in human; (3) foreign body response and fibrotic capsule formation are uncontrolled phenomena that may lead to unpredictable tissue thickness over time; (4) tissue durability may be limited by the lack of elastin in the construct (Hayashida et al. 2007, 2008); and (5) the regenerative potential of fibrotic tissues in human is limited.
5.3 In Situ Heart Valve Tissue Engineering
Also known as “the remodeling within the host,” in situ TE attempts to regenerate implanted tissues by harnessing the natural regenerative potential of the human body. This approach exploits the active or passive recruitment of endogenous cells to the acellular implant to induce remodeling and regeneration of the new prosthesis into a native-like tissue (Fioretta et al. 2017; Wissing et al. 2017) (Fig. 7). In this regard, biomaterials might be designed to enhance cell infiltration and adhesion into the scaffold, favor proliferation and overtime formation of new ECM, and reabsorb and degrade gradually over time. After its initial role as mechanical support for endogenous cells, the scaffold is slowly degraded by hydrolysis and replaced by newly deposited ECM. These implants are designed to favor, guide, and control cell recruitment and tissue remodeling to achieve a native-like functional substitute that can last a lifetime. When compared to in vitro and in-body TE methods, in situ TE approach represents a straightforward and less complex alternative to produce off-the-shelf available prostheses that can be implanted in response to the specific patient’s needs. Within the field of in situ heart valve TE, a multitude of scaffolds have been tested, as described in Sect. 6.
Schematic representation of in situ heart valve tissue engineering. This approach simplifies the logistical complexity and reduces the costs of the in vitro TE method. In situ TE aims at the implantation of an off-the-shelf available, cell-free valve replacement directly into the patient. Following the natural inflammatory response to the implanted material, the remodeling cascade will be initiated, with host cell adhesion and differentiation and de novo ECM deposition, while scaffold degradation will occur. TEHVs used for this approach can be based on different scaffold materials: (a) a biodegradable polymers, (b) decellularized xenogenic tissue, (c) decellularized allogenic tissue, or (d) in vitro manufactured tissue-engineered matrix. (Images adapted from Servier Medical Art under a creative common attribution 3.0 unported license)
6 Scaffolds for Heart Valve Tissue Engineering
The field of heart valve TE can count on a multitude of scaffold materials that are suitable for either in vitro and in situ applications: (1) native tissue-derived scaffolds, which are based on existing native tissues, either from human (allogenic) or animal (xenogenic) origin; (2) tissue-engineered matrices (TEM), obtained by in vitro culture of cells onto a scaffold and subsequent off-the-shelf treatments, such as decellularization; and (3) natural, synthetic, or hybrid polymers. In the following paragraphs, we will focus on the scaffold materials used for in situ TEHV concepts, referring the reader to other publications where more broad information about scaffolds for cardiovascular TE can be found (Fioretta et al. 2012; Generali et al. 2014; Jana et al. 2014; Dijkman et al. 2016).
For in situ TEHV applications, the scaffold of choice plays an important role as it needs to provide sufficient strength to sustain in vivo valve functionality immediately upon implantation, by providing mechanical stability and durability over time. At the same time, the scaffold should favor host cell infiltration and promote new tissue formation and ECM remodeling.
6.1 Native Tissue-Derived Scaffolds
Native tissue-derived TEHVs, based on human or animal matrix depleted of cells, aim to mimic the characteristics and function of native tissues by retaining the physiological ECM composition and eliminating the immunogenic cellular component via decellularization. Decellularization is a technique used to preserve the complex structure and protein composition of the ECM by physical, chemical, and/or enzymatic removal of the cellular and nuclear components (Crapo et al. 2011). When compared to cryopreservation or glutaraldehyde fixation techniques, decellularization of allogenic and xenogenic matrices results in a more promising concept where off-the-shelf tissues with remodeling potential are achieved and immunogenicity and disease transmission risks are reduced.
6.1.1 Decellularized Homografts
Pioneering studies investigating the employment of decellularized homografts (or allografts) date back to nearly two decades ago. Human heart valve homografts still represent the most valid alternative as heart valve substitute due to characteristics such as maintained anatomy and physiological-like hemodynamics, limited immunological reactions, low infection risk, and low thromboembolic risks compared to clinically available prostheses. For preclinical and clinical evaluation (Table 7), homografts are firstly decellularized to reduce potential immunological responses and then cryopreserved until further use. In this context, results have showed reduced immune response, repopulation by endogenous cells, positive remodeling, promising midterm functionality, and no signs of degeneration (Miller et al. 2006; Dohmen et al. 2007). Additionally, midterm results of decellularized homografts in young patients as pulmonary and aortic valve replacement demonstrated good functionality and, in comparison to standard xenogeneic prostheses, reduced reoperation rates (Cebotari et al. 2011; Sarikouch et al. 2016). However, a recent 10-year study involving decellularized aortic homografts reported extensive fibrosis, calcification, minimal recellularization, and degeneration, questioning the safety of such prostheses on the longer term (Helder et al. 2016). Notwithstanding, their potential, decellularized homografts present several disadvantages. First, there is limited availability of human tissue donors that, combined with the increasing number of valve replacement procedures, makes this approach a nonviable solution for broad clinical adoption. Second, growth potential of this prosthesis is still debated, with only one published study where signs of growth were reported (Cebotari et al. 2011). Third, decellularized homografts can be only implanted via high-risk open-heart SVR, and, because of the limited remodeling and growth in human, multiple reoperations may be required during the course of the life, in particular for younger patients, thereby reducing their life quality and increasing risk of comorbidities.
6.1.2 Decellularized Xenografts
Decellularized heart valves based on xenogeneic-derived materials (e.g., bovine pericardium, pig heart valves) have been investigated as a potential alternative to glutaraldehyde-fixed bioprostheses in both preclinical and clinical studies (Table 8). Similar to human homografts, decellularized xenografts preserve the native valve ECM structure, providing a physiological-like template for the host cells and good hemodynamic performance. Despite the first promising preclinical results, which demonstrated complete cell infiltration, good hemodynamics, and absence of calcifications (Table 8B), clinical experiences resulted in stenosis, inflammation, pseudo-aneurysm, and dilation (Table 8A). Most importantly, the implantation of xenograft-based TEHVs in pediatric patients resulted in severe immune reactions, driven by an incomplete decellularization of the implant, which unfortunately led to early fatal failure of the implanted prosthesis in three patients (Simon et al. 2003). These results suggested that the use of cell-free xenografts is not recommended in human applications because of the severe immune and inflammatory response observed in multiple clinical studies, which lead to early valve failure for stenosis and/or insufficiency, and a high incidence of reoperation (Simon et al. 2003; Woo et al. 2016). However, improved decellularization protocols capable of eradicating any residual antigens of the xenogeneic valves may give rise to improved xenogenic TEHVs with remodeling potential (Helder et al. 2017).
6.2 In Vitro-Derived TEM-Based Scaffolds
Tissue-engineered matrices (TEM) obtained via classic in vitro TE concept by using non-autologous cell sources can be successfully decellularized to achieve an off-the-shelf and ready-to-use constructs for in situ applications (Fig. 8).
Schematic representation of in situ heart valve tissue engineering using a tissue-engineered matrix (TEM). This method uses classic TE methodologies to obtain a scaffolds consisting of in vitro grown ECM depleted of cells (i.e., tissue-engineered matrix (TEM)). Compared to the autologous in vitro TE (Fig. 5), this approach uses allogenic cells to create the TEHV. After culture, off-the-shelf availability and immunocompatibility are granted by the decellularization process. The TEHV can be efficiently stored until implantation. (Images adapted from Servier Medical Art under a creative common attribution 3.0 unported license)
Common cell sources for this approach are easily accessible cells, such as umbilical cord vascular cells or dermal fibroblasts. Both these fibroblastic cells have shown potential to generate dense and organized collagenous matrices for vascular (Niklason et al. 1999; Syedain et al. 2014, 2016; Lawson et al. 2016; Kirkton et al. 2019) and valvular applications (Syedain et al. 2015; Reimer et al. 2017; Motta et al. 2018, 2019; Lintas et al. 2018).
Decellularization of TEM-based TEHVs dates back to 2012, when it was firstly introduced to simplify the manufacturing procedures of in vitro engineered valves obtained with autologous cells. In addition, decellularization was able to prevent the leaflet retraction observed immediately upon leaflet separation in in vitro cultured TEHVs, with significant impact on in vivo valve functionality (Dijkman et al. 2012b; Driessen-Mol et al. 2014). Since then, a multitude of studies have shown the potential of using decellularized TEM-based TEHVs in preclinical animal models, showing good functionality, host cell repopulation, and remodeling over time in both pulmonary and aortic positions (Table 9). Remarkably, TEM-based TEHVs proved to be competent and functional as aortic valve replacement in sheep, with good functionality and almost complete cellular repopulation 6 months after implantation (Syedain et al. 2015). Remarkably, TEM-based TEHVs were proved to even be compatible with transcatheter implantation techniques in several preclinical studies as pulmonary (Weber et al. 2013; Driessen-Mol et al. 2014; Emmert et al. 2018; Motta et al. 2018, 2019) and aortic (Lintas et al. 2018) replacements.
Till 2018, the first cause of failure for all TEM-based TEHV was the development of progressive insufficiency in vivo, most probably ascribable to leaflet thickening and/or shortening and retraction (Flanagan et al. 2009; Gottlieb et al. 2010; Schmidt et al. 2010; Weber et al. 2013; Driessen-Mol et al. 2014; Syedain et al. 2015; Reimer et al. 2017; Motta et al. 2018).
To solve this long-standing problem, Emmert et al. introduced the use of computational modeling tools to optimize the design of a TEM-based TEHV (Sanders et al. 2016; Emmert et al. 2018) to control tissue remodeling and to ensure long-term functionality upon implantation in vivo. As more thoroughly explained in Sect. 6.1.2, the simplified TEM-based valve geometry, lacking a belly region, used in these studies lead to radial leaflet compression under physiological hemodynamics, with consequent leaflet shortening (Loerakker et al. 2016). To prevent leaflet retraction, computational modeling suggested a new valve geometry that was achieved by using a constraining bioreactor insert during TEHV culture (Sanders et al. 2016). TEHVs manufactured with the novel geometry were then implanted in the pulmonary position in the ovine model for up to 1 year, reporting excellent performance, host cell repopulation, native-like remodeling, and sustained durability, without leaflet thickening nor shortening (Emmert et al. 2018). In this regard, valve design, hemodynamic loading, and cell contractile forces have been predicted to be crucial in influencing the remodeling outcome under physiological conditions.
6.3 Bioresorbable Polymeric Scaffolds
Bioresorbable, natural, synthetic, or hybrid polymeric scaffolds are widely used in the medical field due to their high versatility and associated low costs. The main advantage of using bioresorbable polymers for in situ TE applications is the possibility to obtain a cell-free TEHV with tunable scaffold architecture, mechanical properties, stability, and degradation rate, hence assuring reproducibility, unlimited supply, and scalability in the production process. The polymeric valve has inherent off-the-shelf availability and can be directly implanted into the patient when needed, without the use of further expensive and complicated in vitro techniques (Fig. 8). Natural polymers are mostly represented in the form of hydrogels and comprise components of the native tissue composition (e.g., collagen, fibrin, chitosan, silk, and keratin) as starting material (Jana et al. 2014). Such protein-based scaffolds are fast degrading and nontoxic materials, with low immunogenicity and tunable architecture, degradation rate, and porosity. However, they are limited by the inherently low mechanical properties of hydrogels, which make them unsuitable as starting matrix for in situ TEHV applications.
6.3.1 Bioresorbable Synthetic Polymeric Scaffolds
Biodegradable synthetic polymers, such as polyglycolic acid, polylactic acid, polycaprolactone, and bisurea-modified polycarbonate, have been extensively used as starting matrices for both in vitro and in situ TE applications because they can be degraded and absorbed by the human body. Such polymers possess tunable mechanical, chemical, and structural properties that can be tailored for the development of TEHVs with long-term durability, gradual degradation rates, flexible and elastic leaflets, and potential for further functionalization (Fioretta et al. 2012; Wissing et al. 2017). Synthetic materials are attractive for their fast production, unlimited availability, and lack of disease transmission. In light of these properties, numerous preclinical large animal studies investigating such synthetic materials in the context of TEHVs have been performed, even in combination to transcatheter implantation techniques (Table 10). In this context, TEHVs based on bioresorbable supramolecular elastomeric polymers (i.e., bisurea-modified poly(carbonate) and 2-ureido-4[1H]-pyrimidinone-modified polycaprolactone) were manufactured and implanted with both surgical and transcatheter approaches, demonstrating reasonable performance for up to 1 and 2 years as pulmonary valve replacement. In these studies, ECM deposition, partial degradation, and endogenous cellularization were observed. Following these results supramolecular polymer-based valve replacements have advanced into clinical trials, with however heterogenous outcomes. Hence, further chronic animal studies are required to further assess the long-term durability and remodeling of such prostheses. Indeed, a recent study showed important differences in tissue remodeling between valves but also in between leaflets of the very same valve (Fioretta et al. 2019a). These latter results suggest that a further investigation of polymer degradation mechanisms and tissue remodeling pathways is needed to ensure safe clinical translation of this technology into patients.
6.3.2 Autologous Cell Pre-seeding onto Bioresorbable Polymeric TEHVs
To favor early remodeling responses, researchers have also investigated the use of autologous cell pre-seeding onto biodegradable scaffold materials. These on-the-fly concepts proved to be attractive especially when combined with bone marrow-derived mononuclear cells (BMMNCs) because they could be performed in a one-step procedure prior implantation (Table 10) and where previously proved to favor vascular graft remodeling in small animal models. In light of these results, BMMNC were used to pre-seed synthetic scaffolds for both pulmonary and aortic TVR procedures (Table 10). While most of these studies are proof of concept with an acute or short-term follow-up (up to 4 weeks) (Weber et al. 2011; Emmert et al. 2011, 2012, 2014), a recent publication showed detrimental effects of BMMNC pre-seeding onto bioresorbable supramolecular TEHVs, with loss of functionality over time due to regurgitation, leaflet thickening, and calcifications (Fioretta et al. 2019a) within 16 weeks from implantation.
6.3.3 Hybrid Polymeric Scaffolds
Natural proteins (e.g., gelatin, alginate, hyaluronic acid) can be efficiently combined to synthetic polymeric scaffolds to form hybrids, mainly manufactured via rotary jet spinning, electrospinning, or 3D printing techniques. The advantages of applying synthetic materials to create hybrid scaffolds are multiple and allow for tunable mechanical properties, controlled biodegradation, off-the-shelf availability, scalability, and reproducibility. Additionally, to further improve polymer biocompatibility and reduce thrombogenicity, scaffolds can be functionalized using natural proteins. In this regard, synthetic polymers are linked to natural proteins via physical absorption, chemical binding, or co-spinning (Rossi and Van Griensven 2014). Hence, hybrid material organization should ideally mimic the physical properties and structure of native tissues, thereby improving the cellular adhesion, distribution, and proliferation. As an example, cell-free biomimetic TEHVs were recently manufactured starting from a blend of synthetic polymer (i.e., poly(4-hydroxybutyrate) and natural proteins (i.e., gelatin) processed via rotary jet spinning (Capulli et al. 2017). These valves proved to be compatible with transcatheter pulmonary valve implantation techniques proving feasibility and functionality in an acute proof-of-concept study in sheep with early cellular adhesion and infiltration (Capulli et al. 2017).
Beyond mechanical stability and scaffold architecture properties, hybrid and synthetic scaffolds offer the possibility to include bioactive molecules and serve as vehicles for the delivery of components such as peptides, growth factors, antibodies, and drugs (Fioretta et al. 2012). This functionalization aims at improving scaffold performance by favoring the recruitment of host cells, promoting tissue formation, ensuring hemocompatibility, and controlling the early steps of the inflammatory cascade (Roh et al. 2010). Additionally, the inflammatory response triggered by those treatments might be modulated toward a positive and native-like tissue remodeling (Muylaert et al. 2016).
7 Testing of Tissue-Engineered Heart Valves
Physiologically, heart valves open once every second, and, for each cardiac cycle, the valve leaflets are exposed to complex deformation and hemodynamic forces. The hemodynamics is a combination of mechanical forces that control blood flow and blood pressure in the body. Non-physiological values of blood flow and/or pressure are associated with changes in valvular structure and, therefore, play a significant role in the etiology of valvular disease (Chandran 2010). However, there is a new general consensus that these forces are also responsible for adaptive or maladaptive remodeling of TEHVs (Emmert et al. 2018). Hence, it is important to validate a novel TEHV by using an array of testing platform consisting of in silico models, in vitro models, and, finally, in vivo preclinical animal models.
7.1 In Silico Models to Optimize Valve Design
In silico models are capable of simulating the complex hemodynamic environment of a heart valve. Computational simulations are, therefore, becoming an important tool to improve our understanding of heart valve physiology and pathology, but also to assess valve prosthesis functionality, and to improve surgical planning using precision medicine tools.
The hemodynamics plays a key role in controlling the physiological or pathological remodeling of the native leaflet. By using computational modeling, the hemodynamic forces on the valve can be simulated to understand structural and biological changes in response to (non-)physiological stresses; as an example, higher values of blood pressure can lead to increased leaflet deformation that, on a microscopic scale, induces changes in the valvular interstitial and endothelial cells (Gould et al. 2013). As explained in Sect. 2, this may result in VIC activation toward a fibrotic or osteoblastic phenotype, setting the basis for fibrosis and calcification. Hence, understanding the distribution of stress and strain in the valve leaflets is fundamental to predict the remodeling potential of TEHVs.
7.1.1 Design Optimization for Artificial Heart Valve Replacements
In silico models have significantly advanced in the past decades, and they are now a valid tool to better understand the complexity of the valve hemodynamics: cusp opening and closure pattern, flow pattern, and stress and strain distribution in the leaflets (Chandran 2010). More importantly, computational simulations have been used to assess the efficacy of novel heart valve prosthesis designs, significantly reducing the number of prototype manufacturing, bench testing, and in vivo testing (Morris et al. 2016).
Finite element analysis (FEA) is a modeling technique that focus on the assessment of stress and strain distribution in a region of interest. FEA is used to optimize valve design by implementing valve geometry and dimension into the computational tool. Initially, FEA was using simplified symmetric geometries and valves in closed configuration (Cataloglu et al. 1977; Ghista and Reul 1977; Sabbah et al. 1985). However, with the increased computational power of modern computers, the dynamic opening and closure configuration of a heart valve can be also modeled and combined to patient-specific geometries based on a detailed 3D reconstruction of the valve via cardiac imaging techniques (Gnyaneshwar et al. 2002; Sripathi et al. 2004).
More recently, a proof-of-concept study developed machine learning techniques to estimate stress and strain forces on transcatheter valves, starting with a set of TAVI-compatible leaflet designs (Liang and Sun 2019). The results were promising, with the model being able to accurately estimate the deformed leaflet geometries and stress distribution within seconds, therefore considerably reducing the modeling and simulation complexity of standard FEA techniques.
Computational fluid dynamics (CFD) is another method to provide a quantitative description of flow characteristics. CFD is commonly used to calculate the values of wall shear stress in a noninvasive manner, providing information on the flow pattern characteristics (i.e., laminar or turbulent flow) (Jin et al. 2004). CFD is also efficiently used to understand the flow pattern through a valve prosthesis, providing crucial information to optimize valve design in order to limit thrombotic events (Kelly 2002; Yoganathan et al. 2005; Simon et al. 2010; Zakaria et al. 2017).
Finally, fluid-structure interaction (FSI) models focus on the interactions between valve structures (i.e., leaflets) and blood flow. Specifically, FSI has been used in combination to platelet activation models to assess the hemodynamics of valvular replacement and to understand the interaction between blood cells and valve structures (Borazjani 2015). FSI models can, for example, provide detailed information on platelet distribution and accumulation and on blood flow patterns within a specific valve design. Hence, FSI models provide important information on the thrombogenic risk profile of the tested valve design and suggest where improvement is needed to prevent thrombotic events (Piatti et al. 2015).
7.1.2 Design Optimization for Tissue-Engineered Heart Valves
Design optimization in silico is, therefore, a common application in artificial valves to predict the consequences of changes in artificial valve design on the overall outcome.
However, computational modeling has hardly been utilized to improve the performance of TEHVs, nor has it been validated in clinically relevant in vivo models (Soares et al. 2014a, b).
Recently, a multidisciplinary team of researchers showed the potential of using computational modeling tools to optimize the design of a TEHV (Sanders et al. 2016; Emmert et al. 2018) to manufacture a TEM-based TEHV capable of controlling tissue remodeling to ensure long-term functionality upon implantation in vivo. Indeed, most TEHVs based on TEM lose their functionality within a few months due to uncontrolled (adverse) tissue remodeling phenomena which translate into leaflet shortening, resulting in valve insufficiency (Flanagan et al. 2009; Schmidt et al. 2010; Weber et al. 2013; Driessen-Mol et al. 2014; Reimer et al. 2015, 2017; Schmitt et al. 2016; Motta et al. 2018).
Computational simulations showed that the simplified valve geometry used in most of these studies led to radial leaflet compression when exposed to physiological pulmonary pressure and in the presence of contractile cells (Loerakker et al. 2016). To counteract cusp tissue compaction, a new valve geometry was firstly computationally derived and then implemented in TEHVs by using a constraining bioreactor insert during culture (Sanders et al. 2016). The novel valve geometry showed limited radial tissue compression compared to previous designs, suggesting that these TEHVs would be less prone to host cell-mediated tissue retraction upon implantation.
To confirm this hypothesis, the researchers implanted such TEHVs minimally invasively as pulmonary valve replacements in sheep. The results of this study were outstanding, with preserved and good long-term in vivo performance for up to 1 year, as predicted by and consistent with our computational modeling. Additionally, tissue remodeling was profound, with collagen and elastin deposition, polymer reabsorption, and even the initial formation of sinuses of Valsalva in some of the explants. This study indicated the high relevance of an integrated in silico, in vitro, and in vivo bioengineering approach for developing functional TEHVs.
7.2 In Vitro Models to Test Valve Functionality
Similar to artificial valve prostheses, TEHVs should be tested in vitro to characterize valve performance by measuring parameters such as pressure difference, regurgitation, and durability using hydrodynamic studies. Two systems are frequently used in the characterization and in vitro validation of a valvular replacement: the pulse duplicator system and the durability system. More recently, cardiac biosimulator platform have been developed to assess valve performance inside a real heart. These testing platforms are also regulated by the ISO 5840 (cardiovascular implants: cardiac valve prostheses) that provides a series of requirements a valve prosthesis should fulfil before clinical translation.
7.2.1 Pulse Duplicator Systems
Pulse duplicator systems (e.g., HDT-500 Heart Valve Pulse Duplicator System by BDC Laboratories or the ViVitro Pulse Duplicator System by ViVitroLabs) (Fig. 9) can be used to simulate left or right heart functionality and to measure forward and backflow flow and pressure gradients along with an accurate visualization of the valve’s opening and closing behavior from optical visualization ports. This system is usually characterized by two chambers connected by a fixture where the valve prosthesis is allocated for the testing. A pulsatile pump provides the desired pressure and flow conditions to mimic the left or right heart, which are monitored real time with pressure and flow sensors in the cardiac chambers. The system is finally connected to a computer where a software provides you with the acquired information on valve performance. The pulse duplicator systems can be used to simulate physiological and non-physiological cardiac conditions by varying the resistance and compliance of the system.
Representative images of in vitro valve testers. (a) A pulse duplicator system (HDT-500 Heart Valve Pulse Duplicator System by BDC Laboratories) and (b) a durability testing platform (VDT-3600i Heart Valve Durability System by BDC Laboratories). (Pictures courtesy of Nikolaos Poulis, Institute for Regenerative Medicine, University of Zurich, Switzerland)
While initially developed to test mechanical and bioprosthetic valves, these systems have been recently extended also for the testing of TEHVs by using customized fixtures to allocate TEHVs with different stent designs (Sanders et al. 2016; Kluin et al. 2017; Emmert et al. 2018; Lintas et al. 2018; Motta et al. 2019) and even biological valved conduits (Buse et al. 2016).
However, it is important to notice that considerable differences were observed in the hydrodynamic performance (i.e., pressure gradient and backflow leakage) of a single valve tested in different ISO 5840 compliant pulse duplicators (Retta et al. 2017). These results suggest the importance of using a known reference valve in hydrodynamic performance testing to assess the individual measurement conditions in the duplicator.
7.2.2 Durability Systems
Valve durability systems (e.g., VDT-3600i Heart Valve Durability System by BDC Laboratories and DuraPulse Heart Valve Test Instrument, TA Instruments) (Fig. 9) perform accelerated durability tests to assess the fatigue behavior of the valve (Black et al. 1994; Sanders et al. 2016; Buse et al. 2016). The platform can consist of multiple independent testing stations where different valves can be allocated and tested independently at accelerated frequencies (up to 40 Hz) to simulate long-term in vivo performance. Despite the wide range of accelerated frequencies that can be used to test the valve, it is important to ensure that even at high frequency, the valve is subjected to a complete opening and closing cycle to prevent incorrect and/or insufficient loading of the valve cusps that would affect valve durability (Sanders et al. 2016).
The physiological environment can be further simulated by replicating blood viscosity via the addition of xanthan gum or polyacrylamide to the water solution (Pohl et al. 1996). This method will mimic the complex viscoelastic behavior of blood and will improve the accuracy of the recorded leakage flow and leakage volume.
ISO5840 provides guidelines to assess artificial valve prosthesis durability and performance, suggesting that bioprostheses should sustain at least 200 million cycles while mechanical valves 400 million cycles (corresponding to 5 and 10 years in vivo, respectively). However, these norms have been specifically designed for artificial non-regenerative valve prostheses and may not be applicable to TEHVs. Indeed, TEHV functionality and durability are closely related to the in vivo remodeling potential, and performing a durability test on TEHVs will most likely result in underestimation of valve durability due to the lack of remodeling potential in the in vitro settings. As an example, TEM-based TEHV durability in vitro was reported to be the equivalent of 16 weeks (Sanders et al. 2016). However, upon implantation in a preclinical sheep model, these valves showed good functionality for up to 1 year (Emmert et al. 2018), thereby proving that upon remodeling, durability was improved. Hence, there is a clear need to improve guidelines for the testing of regenerative heart valves, in order to take into account their regenerative potential.
7.2.3 Cardiac Biosimulator and Beating Heart Platforms
Pulse duplicator and durability systems have the advantage of providing automatic feedback control and independent test chambers to ensure that test conditions are maintained throughout the test. However, assessing valve functionality inside a real heart will provide even more insights on the efficacy and efficiency of the developed TEHV. A cardiac biosimulator platform (e.g., Cardiac Biosimulator Platform by LifeTec Group) consists of a mock apparatus where a full (animal) heart is connected to a pump that actuates the heart to simulate the hemodynamic environment in an anatomical setting, thereby providing in vitro the most physiological-like environment to assess valve performance (Leopaldi et al. 2015). Modern imaging techniques can be connected to the platform to obtain precise information on valve hemodynamics and performance in the heart (Leopaldi et al. 2012). Compared to pulse duplicator and durability systems, the cardiac biosimulator platform allows to test the valve in a pressurized heart with physiological-like hydrodynamics and heart wall movements, which will efficiently replicate the in vivo animal model in assessing functionality and implantability of the valve, but with limited ethical concerns.
Another interesting setup aimed at testing new cardiovascular products in a relevant physiological environment is the beating heart platform (e.g., PhysioHeart by LifeTec Group). Compared to the cardiac biosimulator, the heart used in this platform is the actual pump that, by retained physiological contraction, provides realistic physiological cardiac dynamic conditions analogous to an animal model. However, it has the advantage of allowing endoscopy to study valve implantation, efficacy, and performance over time (de Weger et al. 2010).
Both the cardiac biosimulator and the beating heart apparatus have been validated with different mammalian hearts and have been successfully used to train cardiac surgeon in novel surgical and transcatheter interventions (Leopaldi et al. 2018).
7.3 In Vivo Animal Models to Assess Remodeling
Heart valve functionality depends on a complex combination of mechanical, biological, and physical processes that are only partly replicated by in vitro models. In vitro model systems are, therefore, not yet able of fully replicating the complexity of a living being, in particular regarding remodeling and growth potential, fundamental aspect of a tissue-engineered prosthesis (Fioretta et al. 2019b). Therefore, clinical translation of valvular prostheses still relays on in vivo experiments. Research aiming at understanding the basic mechanisms involved in heart organogenesis can be performed in non-mammalian models, such as the zebrafish, because of easy reproduction, external embryonic development, and transparency of the embryos (Cesarovic et al. 2020).
Small mammalians, such as mice and rats, are, on the other hand, extremely helpful in assessing the molecular mechanisms of heart physiology and pathophysiology, since they are genetically similar to humans, they have a four-chambered heart that better represent the human anatomy compared to non-mammalian animal models, and they can be genetically modified to express certain disease characteristics (Cesarovic et al. 2020). In addition, they have been also extensively used to assess functionality and host cell-mediated remodeling of tissue-engineered vascular grafts (Roh et al. 2010; Hibino et al. 2011; Lee et al. 2014). However, due to size and hemodynamic differences, they do not provide a suitable animal model for the assessment of heart valve replacements.
The ideal animal model to assess valve implantation and functionality should closely resemble the human heart in size, anatomy, and hemodynamics. In addition, similar coagulation cascade and immune response will be fundamental to better interpret the remodeling potential of a TEHV. While guidelines are provided for the in vivo preclinical validation of artificial valve prostheses (i.e., ISO5840) and to design and execute animal experiments to evaluate medical devices (ISO10993), there is not yet a standard protocol to test TEHV prostheses. TEHVs have been tested in different large mammals, such as sheep, pig, and nonhuman primates, which provide a comparable size, anatomy, functionality, and physiology to the human heart. These similarities allow for the use of clinical-grade imaging and diagnostic devices to determine feasibility of valve implantation procedure and to assess valve functionality in clinical-like settings.
7.3.1 Nonhuman Primate Model
Nonhuman primates, such as baboons, are the animal model that is most similar to human in terms of anatomy, physiology, and growth curve, making them an ideal candidate for long-term chronic studies and growth assessment.
Only a handful of studies have tested TEHVs in baboons. Decellularized human and baboon pulmonary valves have been implanted as pulmonary replacement in baboon and the results compared to clinical-grade porcine valved conduits (Hopkins et al. 2013). All animals showed excellent valve functionality upon implantation. While decellularized valves remained functional over time, a reduction of effective orifice area was detected for the porcine conduits, and it was associated with a more intense inflammatory response. In another study, bioresorbable valves were pre-seeded with autologous bone marrow mononuclear cells prior to implantation and delivered as pulmonary valve replacement in baboon (Weber et al. 2011). Valve functionality was adequate up to 4 weeks upon implantation, with substantial remodeling and cellular infiltration. Finally, TEM-based TEHVs were implanted as pulmonary replacement in baboons to assess functionality and remodeling (Weber et al. 2013) and the results compared to decellularized human valves. The results indicated better cellular infiltration for TEM-based TEHVs but leaflet shortening over time associated with moderate regurgitation.
Nonhuman primates are, however, a strictly regulated and costly animal model, which is also often prohibited for ethical and regulatory reasons (Rashid et al. 2004; Cesarovic et al. 2020).
7.3.2 Swine Model
Swine heart anatomy and physiology closely resemble the human heart, making pigs a good animal model for cardiovascular research (Suzuki et al. 2011), in particular for the investigation of coronary artery disease and myocardial infarction (Patterson and Kirk 1983). In addition, other biological processes, such as the immune systems, the coagulation cascade, and the endothelialization potential, are similar to humans (Gallo et al. 2017).
Despite these advantages, pigs have a fast-growing curve that may determine size mismatches with the implanted prosthesis during long-term follow-ups, thereby affecting the chronic study outcome (Rashid et al. 2004; Kheradvar et al. 2017). In the field of heart valve TE, pigs have been used to investigate feasibility of the implantation procedure of novel fully bioresorbable stented prostheses in acute studies (Coyan et al. 2019).
To limit size-related issues, the Vietnamese mini-pig has been used to investigate functionality and remodeling of TEHVs based on decellularized porcine aortic roots (Bottio et al. 2010; Gallo et al. 2016, 2017). This homologous replacement showed promising results over a 15-month follow-up, with retained functionality, host cell repopulation, tissue remodeling, and no calcification observed. Taken together, these results suggest that pigs can be used for assessing TEHV performance and may become a valid preclinical animal model in the future to evaluate regenerative replacements.
7.3.3 Ovine Model
The sheep is the FDA-approved and ISO-norm recommended animal model for the in vivo preclinical validation of heart valve replacements, with similar heart anatomy, heart rate, pressure values, and, most importantly, heart valve sizes to human’s (Kheradvar et al. 2017). In addition, the high calcium level in the lamb serum (up to 12 months old), makes this animal model “the worst-case scenario” (Taramasso et al. 2015) to investigate durability and susceptibility to calcification of an implanted valve prosthesis (Barnhart et al. 1982). Finally, the growth curve of a sheep is moderate compared to the swine model, making it a valid choice for the assessment of regenerative tissue-engineered replacements with growth potential, as extensively demonstrated in literature (Iop et al. 2014; Zafar et al. 2015; Quinn et al. 2016; Reimer et al. 2017; Hennessy et al. 2017).
The sheep is, therefore, the gold standard animal model for assessing TEHV functionality and remodeling when implanted as pulmonary or aortic valve replacement, as well as for determining growth potential of TEHVs (Tables 6, 7, 8, and 9).
Although the heart anatomy is similar to the human’s, animal models still present several limiting factors. Firstly, the orientation of the great arteries and of the heart differs considerably in quadrupeds compared to humans, making it difficult to test devices (e.g., transcatheter delivery systems) that are designed for the human anatomy. In addition, large animal models do not acquire the same valvular disease than human, and there is no animal model capable of replicating valve stenosis, insufficiency, or calcification. Moreover, due to financial and ethical constraints, long-term studies (>1 year) in large animal models are rare (Kheradvar et al. 2017; Cesarovic et al. 2020).
8 Challenges Toward Clinical Translation
By simplifications of manufacturing methods to grant off-the-shelf availability and scalability, TEHVs with in situ remodeling potential could potentially see a way to commercialization in the near future. However, despite the promising outcomes in both preclinical and clinical trials, routinely application of TEHVs into clinics is to date limited. From a scientific point of view, TEHVs need to provide proof of successful acute feasibility, short- and long-term functionality, over time durability, self-regeneration, and growth capabilities. However, several other aspects need to be equally considered.
8.1 Regulatory Challenges
Following FDA guidelines, cardiac valve replacements are devices classified as class III, according to their risk-based classification scheme. More specifically, such devices belong to the group of “support or sustainment of human life, which are of substantial importance in preventing impairment of human health or present a potential, unreasonable risk of illness or injury.” However, the precise classification of such TEHVs might be difficult due to the variety of manufacturing approaches reported in literature and the heterogeneity in the international guidelines of the different regulatory agencies (Ram-Liebig et al. 2015). For instance, class III devices require a premarket approval (PMA), showing that for this particular device, there is no approved/existing equivalent device and demonstrating via research studies that the device is safe and effective. Preclinical studies for the translation of TEHVs need to withstand additional technical requirements in compliance to the guidelines ISO-5840 of the International Organization for Standardization (ISO). However, ISO-5840 document was developed for mechanical and bioprosthetic valves only, and therefore, current standards and regulations must be adapted to determine preclinical safety and efficacy of TEHVs.
8.2 Logistical Challenges
With the introduction in 1978 of the Good Laboratory Practice (GLP) concepts, standardized premarket evaluation rules were established. Hence, the translation of TEHV into clinics will only be effective when device production processes will also follow good manufacturing practices (GMP). Both regulation systems help the simplification and standardization of the clinical translation of TEHVs. Hence, researchers need to collaborate with GMP-compliant facility and perform GLP-compliant testing in order to further advance their prototypes into clinical trials. However, these facilities are not usually broadly available, and renting of special laboratories, such as clean rooms, is very expensive, thereby limiting the possibilities for academic researchers. In this regard, foundations to help accelerate the translation process are fundamental. These foundations may provide funding, facilities, and/or expertise to ensure that academic research and scientific discoveries are translated into clinical applications, even without the collaboration of industry and investors.
8.3 Clinical Requirements
The complete regeneration and repair of the implanted scaffold material is of utmost importance for any in situ approach. However, the regenerative capabilities of the host recipient play a pivotal role in the acceptance and remodeling of the implanted engineered construct, hence directly influencing the quality and success rate of the TEHVs. An in-depth clinical screening of the patient to determine his/her own regenerative potential is one of the requirements to anticipate when translating TEHVs into clinical practice and to ensure the best chance of remodeling upon implantation. Specifically, some patient-specific risk factors (e.g., diabetes, age, and smoke) are associated with impaired regenerative processes by the cells, hence possibly affecting TEHV remodeling and, therefore, functionality and durability on the long term. In addition, implant monitoring strategies using noninvasive imaging techniques, such as echocardiography, CT scan, and/or MRI, will be required to assess the status of the remodeling over time and ensure correct valve functionality. Finally, emergency treatments and strategies to adopt in case of failure should be considered and planned for safe clinical translation.
9 Conclusions
The prevalence of valvular diseases requiring a replacement is growing in light of our aging population and the ability to correct congenital heart defects. There are various treatment options for patients affected by valvular disease, such as valve repair and surgical or transcatheter valve replacement techniques. However, current clinically adopted valve replacements are a suboptimal solution, requiring the need for multiple reoperations either to accommodate for patient’s growth or to substitute a degenerated valve. In addition, after the recent FDA approval of TAVI for low-risk case of aortic stenosis, the number of (younger) patients now eligible for TAVI has significantly increased. There is, therefore, a clear need for novel valve replacement solutions compatible with TVR techniques that could remodel and even grow upon implantation, ensuring a lifelong durability even for younger patient cohorts. Off-the-shelf available TEHVs with in situ regenerative and growth potential may overcome the limitations of currently used heart valve replacements, providing a novel durable option even compatible with transcatheter techniques. Clinical translation of decellularized homografts showed promising performance and recellularization potential (Table 7). In contrast, decellularized xenografts failed because of severe inflammation and stenosis (Table 8). Based on encouraging results from a first clinical trial using tissue-engineered vascular grafts based on supramolecular polymers (i.e., extracardiac total cavopulmonary connection to treat CHD) (Bockeria et al. 2017), clinical pilot trials using bioresorbable supramolecular polymer-based TEHVs are underway (trial number NCT02700100, NCT03022708, and NCT03405636). Furthermore, it is expected that TEM-based transcatheter TEHVs (Emmert et al. 2018; Lintas et al. 2018), will reach clinical translation in the near future.
By providing native-like remodeling, and long-term durability, TEHVs will be also able to reduce the healthcare costs.
References
Althunayyan A, Petersen SE, Lloyd G, Bhattacharyya S (2019) Mitral valve prolapse. Expert Rev Cardiovasc Ther 17:43–51. https://doi.org/10.1080/14779072.2019.1553619
Anderson RH (2000) Clinical anatomy of the aortic root. Heart 84:670–673
Anderson RH, Ho SY, Becker AE (2000) Anatomy of the human atrioventricular junctions revisited. Anat Rec 260:81–91. https://doi.org/10.1002/1097-0185(20000901)260:1<81::AID-AR90>3.0.CO;2-3
Ando M, Takahashi Y (2009) Ten-year experience with handmade trileaflet polytetrafluoroethylene valved conduit used for pulmonary reconstruction. J Thorac Cardiovasc Surg 137:124–131. https://doi.org/10.1016/j.jtcvs.2008.08.060
Arsalan M, Walther T (2016) Durability of prostheses for transcatheter aortic valve implantation. Nat Rev Cardiol 13:360–367. https://doi.org/10.1038/nrcardio.2016.43
Backhoff D, Steinmetz M, Sigler M, Schneider H (2014) Formation of multiple conduit aneurysms following Matrix P® conduit implantation in a boy with tetralogy of Fallot and pulmonary atresia. Eur J Cardiothorac Surg 46:500–502. https://doi.org/10.1093/ejcts/ezt635
Balachandran K, Sucosky P, Yoganathan AP (2011) Hemodynamics and Mechanobiology of Aortic Valve Inflammation and Calcification. Int J Inflamm 2011:1–15. https://doi.org/10.4061/2011/263870
Barnhart GR, Jones M, Ishihara T et al (1982) Bioprosthetic valvular failure. Clinical and pathological observations in an experimental animal model. J Thorac Cardiovasc Surg 83:618–631
Barron V, Lyons E, Stenson-Cox C et al (2003) Bioreactors for cardiovascular cell and tissue growth: a review. Ann Biomed Eng 31:1017–1030
Bateman MG, Quill JL, Hill AJ, Iaizzo PA (2013) The anatomy and function of the semilunar valves. In: Heart valves: from design to clinical implantation. Springer US, Boston, pp 27–43
Bauernschmitt R, Schreiber C, Bleiziffer S et al (2009) Transcatheter aortic valve implantation through the ascending aorta: an alternative option for no-access patients. Heart Surg Forum 12. https://doi.org/10.1532/HSF98.20081112
Baumgartner H, Falk V, Bax JJ et al (2017) 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 38:2739–2791. https://doi.org/10.1093/eurheartj/ehx391
Benjamin EJ, Blaha MJ, Chiuve SE et al (2017) Heart disease and stroke statistics – 2017 update: a report from the American Heart Association. Circulation 135:e146–e603. https://doi.org/10.1161/CIR.0000000000000485
Bennink G, Torii S, Brugmans M et al (2018) A novel restorative pulmonary valved conduit in a chronic sheep model: mid-term hemodynamic function and histologic assessment. J Thorac Cardiovasc Surg 155:2591–2601.e3. https://doi.org/10.1016/j.jtcvs.2017.12.046
Bezuidenhout D, Williams DF, Zilla P (2015) Polymeric heart valves for surgical implantation, catheter-based technologies and heart assist devices. Biomaterials 36:6–25
Bisleri G (2016) Aortic valve repair. Curr Opin Cardiol 31:581–584. https://doi.org/10.1097/HCO.0000000000000334
Black MM, Hose DR, Lamb CJ et al (1994) In vitro heart valve testing: steady versus pulsatile flow. J Heart Valve Dis 3:212–215
Blum KM, Drews JD, Breuer CK (2018) Tissue-engineered heart valves: a call for mechanistic studies. Tissue Eng Part B Rev 24:240–253. https://doi.org/10.1089/ten.teb.2017.0425
Bobylev D, Sarikouch S, Tudorache I et al (2019) Double semilunar valve replacement in complex congenital heart disease using decellularized homografts. Interact Cardiovasc Thorac Surg 28:151–157. https://doi.org/10.1093/icvts/ivy212
Bockeria L, Svanidze O, Kim A et al (2017) Total cavopulmonary connection with a new bioabsorbable vascular graft: first clinical experience. J Thorac Cardiovasc Surg 153:1542–1550
Boethig D, Horke A, Hazekamp M et al (2019) A European study on decellularized homografts for pulmonary valve replacement: initial results from the prospective ESPOIR Trial and ESPOIR Registry data†. Eur J Cardiothorac Surg 56:503–509. https://doi.org/10.1093/ejcts/ezz054
Bonetti A, Marchini M, Ortolani F (2019) Ectopic mineralization in heart valves: new insights from in vivo and in vitro procalcific models and promising perspectives on noncalcifiable bioengineered valves. J Thorac Dis 11:2126–2143. https://doi.org/10.21037/jtd.2019.04.78
Bonhoeffer P, Boudjemline Y, Saliba Z et al (2000) Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet 356:1403–1405. https://doi.org/10.1016/S0140-6736(00)02844-0
Borazjani I (2015) A review of fluid-structure interaction simulations of prosthetic heart valves. J Long-Term Eff Med Implants 25:75–93
Bottio T, Tarzia V, Dal Lin C et al (2010) The changing hydrodynamic performance of the decellularized intact porcine aortic root: considerations on in-vitro testing. J Heart Valve Dis 19:485–491
Breitenbach I, El-Essawi A, Pahari D et al (2014) Early failure of decellularized xenogenous pulmonary valve conduit (Matrix-P-Plus) for reconstruction of the right ventricular outflow tract in the ross procedure. Thorac Cardiovasc Surg 62. https://doi.org/10.1055/s-0034-1367197
Brown JW, Ruzmetov M, Eltayeb O et al (2011) Performance of synergraft decellularized pulmonary homograft in patients undergoing a ross procedure. Ann Thorac Surg 91:416–423. https://doi.org/10.1016/j.athoracsur.2010.10.069
Burch PT, Kaza AK, Lambert LM et al (2010) Clinical performance of decellularized cryopreserved valved allografts compared with standard allografts in the right ventricular outflow tract. Ann Thorac Surg 90:1301–1306. https://doi.org/10.1016/j.athoracsur.2010.05.024
Buse EE, Hilbert SL, Hopkins RA, Converse GL (2016) Pulse duplicator hydrodynamic testing of bioengineered biological heart valves. Cardiovasc Eng Technol 7:352–362. https://doi.org/10.1007/s13239-016-0275-9
Butcher JT, Nerem RM (2006) Valvular endothelial cells regulate the phenotype of interstitial cells in co-culture: effects of steady shear stress. Tissue Eng 12:905–915. https://doi.org/10.1089/ten.2006.12.905
Buzzatti N, De Bonis M, Moat N (2018) Anatomy of the tricuspid valve, pathophysiology of functional tricuspid regurgitation, and implications for percutaneous therapies. Interv Cardiol Clin 7:1–11
Capulli AK, Emmert MY, Pasqualini FS et al (2017) JetValve: rapid manufacturing of biohybrid scaffolds for biomimetic heart valve replacement. Biomaterials 133:229–241. https://doi.org/10.1016/j.biomaterials.2017.04.033
Cataloglu A, Clark RE, Gould PL (1977) Stress analysis of aortic valve leaflets with smoothed geometrical data. J Biomech 10:153–158
Cebotari S, Tudorache I, Ciubotaru A et al (2011) Use of fresh decellularized allografts for pulmonary valve replacement may reduce the reoperation rate in children and young adults: early report. Circulation 124:115–124. https://doi.org/10.1161/CIRCULATIONAHA.110.012161
Cesarovic N, Lipiski M, Falk V, Emmert MY (2020) Animals in cardiovascular research. Eur Heart J 41:200–203. https://doi.org/10.1093/eurheartj/ehz933
Chandran KB (2010) Role of computational simulations in heart valve dynamics and design of valvular prostheses. Cardiovasc Eng Technol 1:18–38. https://doi.org/10.1007/s13239-010-0002-x
Chatterjee A, Bhatia N, Torres MG et al (2019) Comparison of transcatheter pulmonic valve implantation with surgical pulmonic valve replacement in adults (from the National Inpatient Survey Dataset). Am J Cardiol. https://doi.org/10.1016/j.amjcard.2019.09.031
Chong A, Senior R, Wahi S (2019) Contemporary imaging of aortic stenosis. Heart Lung Circ 28:1310–1319. https://doi.org/10.1016/j.hlc.2019.05.177
Coffey S, Cairns BJ, Iung B (2016) The modern epidemiology of heart valve disease. Heart 102:75–85. https://doi.org/10.1136/heartjnl-2014-307020
Coté N, Mahmut A, Bosse Y et al (2013) Inflammation is associated with the remodeling of calcific aortic valve disease. Inflammation 36:573–581. https://doi.org/10.1007/s10753-012-9579-6
Coyan GN, D’Amore A, Matsumura Y et al (2019) In vivo functional assessment of a novel degradable metal and elastomeric scaffold-based tissue engineered heart valve. J Thorac Cardiovasc Surg 157:1809–1816. https://doi.org/10.1016/j.jtcvs.2018.09.128
Crapo PM, Gilbert TW, Badylak SF (2011) An overview of tissue and whole organ decellularization processes. Biomaterials 32:3233–3243. https://doi.org/10.1016/j.biomaterials.2011.01.057
Cribier A, Eltchaninoff H, Bash A et al (2002) Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 106:3006–3008
Da Costa FDA, Costa ACBA, Prestes R et al (2010) The early and midterm function of decellularized aortic valve allografts. Ann Thorac Surg 90:1854–1860. https://doi.org/10.1016/j.athoracsur.2010.08.022
Daebritz SH, Fausten B, Hermanns B et al (2004) New flexible polymeric heart valve prostheses for the mitral and aortic positions. Heart Surg Forum 7:E525–E532. https://doi.org/10.1532/HSF98.20041083
Dal-Bianco JP, Levine RA (2013) Anatomy of the mitral valve apparatus. Role of 2D and 3D echocardiography. Cardiol Clin 31:151–164
David TE (2010) Reoperations after the ross procedure. Circulation 122:1139–1140. https://doi.org/10.1161/CIRCULATIONAHA.110.977991
de Weger A, van Tuijl S, Stijnen M et al (2010) Direct endoscopic visual assessment of a transcatheter aortic valve implantation and performance in the physioheart, an isolated working heart platform. Circulation 121:e261–e262. https://doi.org/10.1161/CIR.0b013e3181d9b879
Delmo Walter EM, de By TMMH, Meyer R, Hetzer R (2012) The future of heart valve banking and of homografts: perspective from the Deutsches Herzzentrum Berlin. HSR Proc Intensive Care Cardiovasc Anesth 4:97–108
Demer LL, Tintut Y (2008) Vascular calcification: pathobiology of a multifaceted disease. Circulation 117:2938–2948. https://doi.org/10.1161/CIRCULATIONAHA.107.743161
Dijkman PE, Driessen-Mol A, de Heer LM et al (2012a) Trans-apical versus surgical implantation of autologous ovine tissue-engineered heart valves. J Heart Valve Dis 21:670–678
Dijkman PE, Driessen-Mol A, Frese L et al (2012b) Decellularized homologous tissue-engineered heart valves as off-the-shelf alternatives to xeno- and homografts. Biomaterials 33:4545–4554. https://doi.org/10.1016/j.biomaterials.2012.03.015
Dijkman PEPE, Fioretta ESES, Frese L et al (2016) Heart valve replacements with regenerative capacity. Transfus Med Hemother 43:282–290. https://doi.org/10.1159/000448181
Dohmen P, da Costa F (2012) An experimental study of decellularized xenografts implanted into the aortic position with 4 months of follow up. J Clin Exp Cardiol s4. https://doi.org/10.4172/2155-9880.s4-004
Dohmen PM, Ozaki S, Nitsch R et al (2003) A tissue engineered heart valve implanted in a juvenile sheep model. Med Sci Monit 9:BR97–BR104
Dohmen PM, da Costa F, Lopes SV et al (2005) Results of a decellularized porcine heart valve implanted into the juvenile sheep model. Heart Surg Forum 8:100. https://doi.org/10.1532/HSF98.20041140
Dohmen PM, Lembcke A, Holinski S et al (2007) Mid-term clinical results using a tissue-engineered pulmonary valve to reconstruct the right ventricular outflow tract during the ross procedure. Ann Thorac Surg 84:729–736. https://doi.org/10.1016/j.athoracsur.2007.04.072
Driessen-Mol A, Emmert MY, Dijkman PE et al (2014) Transcatheter implantation of homologous “off-the-shelf” tissue-engineered heart valves with self-repair capacity: long-term functionality and rapid in vivo remodeling in sheep. J Am Coll Cardiol 63:1320–1329. https://doi.org/10.1016/j.jacc.2013.09.082
Durko AP, Osnabrugge RL, Van Mieghem NM et al (2018) Annual number of candidates for transcatheter aortic valve implantation per country: current estimates and future projections. Eur Heart J 39:2635–2642. https://doi.org/10.1093/eurheartj/ehy107
Ellis JT, Healy TM, Fontaine AA et al (1996) Velocity measurements and flow patterns within the hinge region of a Medtronic Parallel bileaflet mechanical valve with clear housing. J Heart Valve Dis 5:591–599
Emmert MY, Weber B, Behr L et al (2011) Transapical aortic implantation of autologous marrow stromal cell-based tissue-engineered heart valves. JACC Cardiovasc Interv 4:822–823. https://doi.org/10.1016/j.jcin.2011.02.020
Emmert MY, Weber B, Wolint P et al (2012) Stem cell-based transcatheter aortic valve implantation: first experiences in a pre-clinical model. JACC Cardiovasc Interv 5:874–883. https://doi.org/10.1016/j.jcin.2012.04.010
Emmert MY, Weber B, Behr L et al (2014) Transcatheter aortic valve implantation using anatomically oriented, marrow stromal cell-based, stented, tissue-engineered heart valves: technical considerations and implications for translational cell-based heart valve concepts. Eur J Cardiothorac Surg 45:61–68. https://doi.org/10.1093/ejcts/ezt243
Emmert MY, Schmitt BA, Loerakker S, et al (2018) Computational modeling guides tissue-engineered heart valve design for long-term in vivo performance in a translational sheep modelSci Transl Med 10. https://doi.org/10.1126/scitranslmed.aan4587
Fioretta ESES, Fledderus JO, Burakowska-Meise EAEA et al (2012) Polymer-based scaffold designs for in situ vascular tissue engineering: controlling recruitment and differentiation behavior of endothelial colony forming cells. Macromol Biosci 12. https://doi.org/10.1002/mabi.201100315
Fioretta ESES, Dijkman PEPE, Emmert MYMY, Hoerstrup SPSP (2017) The future of heart valve replacement: recent developments and translational challenges for heart valve tissue engineering. J Tissue Eng Regen Med. https://doi.org/10.1002/term.2326
Fioretta ES, Lintas V, Mallone A et al (2019a) Differential leaflet remodeling of bone marrow cell pre-seeded versus nonseeded bioresorbable transcatheter pulmonary valve replacements. JACC Basic Transl Sci. https://doi.org/10.1016/j.jacbts.2019.09.008
Fioretta ES, von Boehmer L, Motta SE et al (2019b) Cardiovascular tissue engineering: from basic science to clinical application. Exp Gerontol 117:1–12. https://doi.org/10.1016/j.exger.2018.03.022
Fishbein GA, Fishbein MC (2019) Pathology of the aortic valve: aortic valve stenosis/aortic regurgitation. Curr Cardiol Rep 21:81. https://doi.org/10.1007/s11886-019-1162-4
Flanagan TC, Sachweh JS, Frese J et al (2009) In vivo remodeling and structural characterization of fibrin-based tissue-engineered heart valves in the adult sheep model. Tissue Eng Part A 15:2965–2976. https://doi.org/10.1089/ten.tea.2009.0018
Funayama M, Furukoshi M, Moriwaki T, Nakayama Y (2015a) Development of an in vivo tissue-engineered valved conduit (type S biovalve) using a slitted mold. J Artif Organs 18:382–386. https://doi.org/10.1007/s10047-015-0856-7
Funayama M, Matsui Y, Tajikawa T et al (2015b) Successful implantation of autologous valved conduits with self-expanding stent (stent-biovalve) within the pulmonary artery in beagle dogs. J Vet Cardiol 17:54–61. https://doi.org/10.1016/j.jvc.2014.12.003
Funayama M, Takewa Y, Oie T et al (2015c) In situ observation and enhancement of leaflet tissue formation in bioprosthetic “biovalve”. J Artif Organs 18:40–47. https://doi.org/10.1007/s10047-014-0793-x
Gallo M, Bonetti A, Poser H et al (2016) Decellularized aortic conduits: could their cryopreservation affect post-implantation outcomes? A morpho-functional study on porcine homografts. Heart Vessel 31:1862–1873. https://doi.org/10.1007/s00380-016-0839-5
Gallo M, Poser H, Bottio T et al (2017) The Vietnamese pig as a translational animal model to evaluate tissue engineered heart valves: promising early experience. Int J Artif Organs 40:142–149. https://doi.org/10.5301/ijao.5000568
Gasser S, von Stumm M, Sinning C et al (2019) Can we predict failure of mitral valve repair? J Clin Med 8:526. https://doi.org/10.3390/jcm8040526
Generali M, Dijkman PE, Hoerstrup SP (2014) Bioresorbable scaffolds for cardiovascular tissue engineering. EMJ Interv Cardiol 1:91–99
Ghista DN, Reul H (1977) Optimal prosthetic aortic leaflet valve: design parametric and longevity analyses: development of the Avcothane-51 leaflet valve based on the optimum design analysis. J Biomech 10:313–324
Girdauskas E, Petersen J, Sachweh J et al (2018) Aortic valve repair in adult congenital heart disease. Cardiovasc Diagn The 8:811–813
Gnyaneshwar R, Kumar RK, Balakrishnan KR (2002) Dynamic analysis of the aortic valve using a finite element model. Ann Thorac Surg 73:1122–1129
Goecke T, Theodoridis K, Tudorache I et al (2018) In vivo performance of freeze-dried decellularized pulmonary heart valve allo- and xenografts orthotopically implanted into juvenile sheep. Acta Biomater 68:41–52. https://doi.org/10.1016/j.actbio.2017.11.041
Gottlieb D, Kunal T, Emani S et al (2010) In vivo monitoring of function of autologous engineered pulmonary valve. J Thorac Cardiovasc Surg 139:723–731. https://doi.org/10.1016/j.jtcvs.2009.11.006
Gould ST, Srigunapalan S, Simmons CA, Anseth KS (2013) Hemodynamic and cellular response feedback in calcific aortic valve disease. Circ Res 113:186–197. https://doi.org/10.1161/CIRCRESAHA.112.300154
Grande-Allen KJ, Griffin BP, Ratliff NB et al (2003) Glycosaminoglycan profiles of myxomatous mitral leaflets and chordae parallel the severity of mechanical alterations. J Am Coll Cardiol 42:271–277. https://doi.org/10.1016/S0735-1097(03)00626-0
Grasso C, Capodanno D, Tamburino C, Ohno Y (2015) Current status and clinical development of transcatheter approaches for severe mitral regurgitation. Circ J 79:1164–1171
Harken DE (1989) Heart valves: ten commandments and still counting. Ann Thorac Surg 48:S18–S19. https://doi.org/10.1016/0003-4975(89)90623-1
Hawkins JA, Hillman ND, Lambert LM et al (2003) Immunogenicity of decellularized cryopreserved allografts in pediatric cardiac surgery: comparison with standard cryopreserved allografts. J Thorac Cardiovasc Surg 126:247–253. https://doi.org/10.1016/S0022-5223(03)00116-8
Hayashida K, Kanda K, Yaku H et al (2007) Development of an in vivo tissue-engineered, autologous heart valve (the biovalve): preparation of a prototype model. J Thorac Cardiovasc Surg 134:152–159. https://doi.org/10.1016/j.jtcvs.2007.01.087
Hayashida K, Kanda K, Oie T et al (2008) “In vivo tissue-engineered” valved conduit with designed molds and laser processed scaffold. J Cardiovasc Nurs 23:61–64. https://doi.org/10.1097/01.JCN.0000305053.50506.97
Head SJ, Çelik M, Kappetein AP (2017) Mechanical versus bioprosthetic aortic valve replacement. Eur Heart J 38:2183–2191. https://doi.org/10.1093/eurheartj/ehx141
Helder MRKK, Kouchoukos NT, Zehr K et al (2016) Late durability of decellularized allografts for aortic valve replacement: a word of caution. J Thorac Cardiovasc Surg 152:1197–1199. https://doi.org/10.1016/j.jtcvs.2016.03.050
Helder MRK, Stoyles NJ, Tefft BJ et al (2017) Xenoantigenicity of porcine decellularized valves. J Cardiothorac Surg:12. https://doi.org/10.1186/s13019-017-0621-5
Henaine R, Roubertie F, Vergnat M, Ninet J (2012) Valve replacement in children: a challenge for a whole life. Arch Cardiovasc Dis 105:517–528. https://doi.org/10.1016/j.acvd.2012.02.013
Hennessy RSRR, Go JL, Hennessy RSRR et al (2017) Recellularization of a novel off-the-shelf valve following xenogenic implantation into the right ventricular outflow tract. PLoS One 12:e0181614. https://doi.org/10.1371/journal.pone.0181614
Hibino N, Yi T, Duncan DR et al (2011) A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. FASEB J 25:4253–4263. https://doi.org/10.1096/fj.11-186585
Hilbert SL, Ferrans VJ, Tomita Y et al (1987) Evaluation of explanted polyurethane trileaflet cardiac valve prostheses. J Thorac Cardiovasc Surg 94:419–429
Hinton RB, Yutzey KE (2011) Heart valve structure and function in development and disease. Annu Rev Physiol 73:29–46. https://doi.org/10.1146/annurev-physiol-012110-142145
Hopkins RA, Bert AA, Hilbert SL et al (2013) Bioengineered human and allogeneic pulmonary valve conduits chronically implanted orthotopically in baboons: hemodynamic performance and immunologic consequences. J Thorac Cardiovasc Surg 145:1098–1107.e3. https://doi.org/10.1016/j.jtcvs.2012.06.024
Human P, Zilla P (2017) The neglected villain of bioprosthetic degeneration: inflammatory and immune processes. J Long-Term Eff Med Implants 27:159–180. https://doi.org/10.1615/JLongTermEffMedImplants.v27.i2-4.50
Huygens SA, Goossens LMA, Van Erkelens JA et al (2018) How much does a heart valve implantation cost and what are the health care costs afterwards? Open Heart 5:e000672. https://doi.org/10.1136/openhrt-2017-000672
Iop L, Bonetti A, Naso F et al (2014) Decellularized allogeneic heart valves demonstrate self-regeneration potential after a long-term preclinical evaluation. PLoS One:9. https://doi.org/10.1371/journal.pone.0099593
Iwai S, Torikai K, Coppin CM, Sawa Y (2007) Minimally immunogenic decellularized porcine valve provides in situ recellularization as a stentless bioprosthetic valve. J Artif Organs 10:29–35. https://doi.org/10.1007/s10047-006-0360-1
Jana S, Tefft BJ, Spoon DB, Simari RD (2014) Scaffolds for tissue engineering of cardiac valves. Acta Biomater 10:2877–2893. https://doi.org/10.1016/j.actbio.2014.03.014
Jana S, Tranquillo RT, Lerman A (2016) Cells for tissue engineering of cardiac valves. J Tissue Eng Regen Med 10:804–824. https://doi.org/10.1002/term.2010
Jin S, Yang Y, Oshinski J et al (2004) Flow patterns and wall shear stress distributions at atherosclerotic-prone sites in a human left coronary artery – an exploration using combined methods of CT and computational fluid dynamics. Conf Proc IEEE Eng Med Biol Soc 5:3789–3791. https://doi.org/10.1109/IEMBS.2004.1404062
Kelly SGD (2002) Computational fluid dynamics insights in the design of mechanical heart valves. Artif Organs 26:608–613. https://doi.org/10.1046/j.1525-1594.2002.07083.x
Kheradvar A, Groves EM, Dasi LP et al (2015) Emerging trends in heart valve engineering: part I. Solutions for future. Ann Biomed Eng 43:833–843. https://doi.org/10.1007/s10439-014-1209-z
Kheradvar A, Zareian R, Kawauchi S et al (2017) Animal models for heart valve research and development. Drug Discov Today Dis Model 24:55–62. https://doi.org/10.1016/j.ddmod.2018.04.001
Kirkton RD, Santiago-Maysonet M, Lawson JH et al (2019) Bioengineered human acellular vessels recellularize and evolve into living blood vessels after human implantation. Sci Transl Med 11:eaau6934. https://doi.org/10.1126/scitranslmed.aau6934
Kluin J, Talacua H, Smits AIPMAIPM et al (2017) In situ heart valve tissue engineering using a bioresorbable elastomeric implant – from material design to 12 months follow-up in sheep. Biomaterials 125:101–117. https://doi.org/10.1016/j.biomaterials.2017.02.007
Konertz W, Dohmen PM, Liu J et al (2005) Hemodynamic characteristics of the Matrix P decellularized xenograft for pulmonary valve replacement during the Ross operation. J Heart Valve Dis 14:78–81
Konertz W, Angeli E, Tarusinov G et al (2011) Right ventricular outflow tract reconstruction with decellularized porcine xenografts in patients with congenital heart disease. J Heart Valve Dis 20:341–347
Langer R, Vacanti JP (1993) Tissue engineering. Science 260:920–926. https://doi.org/10.1126/science.8493529
Lawson JH, Glickman MH, Ilzecki M et al (2016) Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials. Lancet 387:2026–2034. https://doi.org/10.1016/S0140-6736(16)00557-2
Leal MTBC, Passos LSA, Guarçoni FV et al (2019) Rheumatic heart disease in the modern era: recent developments and current challenges. Rev Soc Bras Med Trop 52:e20180041. https://doi.org/10.1590/0037-8682-0041-2019
Lee Y-U, Yi T, Tara S et al (2014) Implantation of inferior vena cava interposition graft in mouse model. J Vis Exp. https://doi.org/10.3791/51632
Leon MB, Smith CR, Mack MJ et al (2016) Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 374:1609–1620. https://doi.org/10.1056/NEJMoa1514616
Leopaldi AM, Vismara R, Lemma M et al (2012) In vitro hemodynamics and valve imaging in passive beating hearts. J Biomech 45:1133–1139. https://doi.org/10.1016/j.jbiomech.2012.02.007
Leopaldi AM, Vismara R, van Tuijl S et al (2015) A novel passive left heart platform for device testing and research. Med Eng Phys 37:361–366. https://doi.org/10.1016/j.medengphy.2015.01.013
Leopaldi AM, Wrobel K, Speziali G et al (2018) The dynamic cardiac biosimulator: a method for training physicians in beating-heart mitral valve repair procedures. J Thorac Cardiovasc Surg 155:147–155. https://doi.org/10.1016/j.jtcvs.2017.09.011
Leyh RG, Wilhelmi M, Walles T et al (2003) Acellularized porcine heart valve scaffolds for heart valve tissue engineering and the risk of cross-species transmission of porcine endogenous retrovirus. J Thorac Cardiovasc Surg 126:1000–1004. https://doi.org/10.1016/s0022-5223(03)00353-2
Liang L, Sun B (2019) A proof of concept study of using machine-learning in artificial aortic valve design: from leaflet design to stress analysis. Bioengineering 6:104. https://doi.org/10.3390/bioengineering6040104
Lincoln J, Lange AW, Yutzey KE (2006) Hearts and bones: shared regulatory mechanisms in heart valve, cartilage, tendon, and bone development. Dev Biol 294:292–302
Lintas V, Fioretta ES, Motta SE et al (2018) Development of a novel human cell-derived tissue-engineered heart valve for transcatheter aortic valve replacement: an in vitro and in vivo feasibility study. J Cardiovasc Transl Res. https://doi.org/10.1007/s12265-018-9821-1
Lisy M, Kalender G, Schenke-Layland K et al (2017) Allograft heart valves: current aspects and future applications. Biopreserv Biobanking 15:148–157. https://doi.org/10.1089/bio.2016.0070
Liu AC, Joag VR, Gotlieb AI (2007) The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol 171:1407–1418. https://doi.org/10.2353/ajpath.2007.070251
Loerakker S, Ristori T, Baaijens FPTT (2016) A computational analysis of cell-mediated compaction and collagen remodeling in tissue-engineered heart valves. J Mech Behav Biomed Mater 58:173–187. https://doi.org/10.1016/j.jmbbm.2015.10.001
Mack MJ, Leon MB, Thourani VH et al (2019) Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 380:1695–1705. https://doi.org/10.1056/NEJMoa1814052
Mackay TG, Wheatley DJ, Bernacca GM et al (1996) New polyurethane heart valve prosthesis: design, manufacture and evaluation. Biomaterials 17:1857–1863. https://doi.org/10.1016/0142-9612(95)00242-1
Manji RA, Lee W, Cooper DKC (2015) Xenograft bioprosthetic heart valves: past, present and future. Int J Surg 23:280–284. https://doi.org/10.1016/j.ijsu.2015.07.009
Marelli AJ, Mackie AS, Ionescu-Ittu R et al (2007) Congenital heart disease in the general population: changing prevalence and age distribution. Circulation 115:163–172. https://doi.org/10.1161/CIRCULATIONAHA.106.627224
Mathieu P, Bouchareb R, Boulanger M-C (2015) Innate and adaptive immunity in calcific aortic valve disease. J Immunol Res 2015:851945. https://doi.org/10.1155/2015/851945
Maurer G (2006) Aortic regurgitation. Heart 92:994. https://doi.org/10.1136/HRT.2004.042614
Mayer JE, Shin’oka T, Shum-Tim D (1997) Tissue engineering of cardiovascular structures. Curr Opin Cardiol 12:528–532. https://doi.org/10.1097/00001573-199711000-00005
McCarthy KP, Ring L, Rana BS (2010) Anatomy of the mitral valve: understanding the mitral valve complex in mitral regurgitation. Eur J Echocardiogr 11:i3–i9
Meijer FMM, Kies P, Jongbloed MRM et al (2019) Excellent durability of homografts in pulmonary position analysed in a predefined adult group with tetralogy of Fallot†. Interact Cardiovasc Thorac Surg 28:279–283. https://doi.org/10.1093/icvts/ivy242
Mendirichaga R, Singh V, Blumer V et al (2017) Transcatheter mitral valve repair with mitraclip for symptomatic functional mitral valve regurgitation. Am J Cardiol 120:708–715
Merryman WD, Youn I, Lukoff HD et al (2006) Correlation between heart valve interstitial cell stiffness and transvalvular pressure: implications for collagen biosynthesis. Am J Physiol Heart Circ Physiol 290. https://doi.org/10.1152/ajpheart.00521.2005
Miller DV, Edwards WD, Zehr KJ (2006) Endothelial and smooth muscle cell populations in a decellularized cryopreserved aortic homograft (SynerGraft) 2 years after implantation. J Thorac Cardiovasc Surg 132:175–176. https://doi.org/10.1016/j.jtcvs.2006.02.038
Millington-Sanders C, Meir A, Lawrence L, Stolinski C (1998) Structure of chordae tendineae in the left ventricle of the human heart. J Anat 192:573–581. https://doi.org/10.1046/j.1469-7580.1998.19240573.x
Misfeld M, Sievers HH (2007) Heart valve macro- and microstructure. Philos Trans R Soc Lond B Biol Sci 362:1421–1436
Miyazaki Y, Soliman O, Abdelghani M et al (2017) Acute performance of a novel restorative transcatheter aortic valve: preclinical results. EuroIntervention 13:e1410–e1417. https://doi.org/10.4244/EIJ-D-17-00554
Mohty D, Pibarot P, Després J-P et al (2008) Association between plasma LDL particle size, valvular accumulation of oxidized LDL, and inflammation in patients with aortic stenosis. Arterioscler Thromb Vasc Biol 28:187–193. https://doi.org/10.1161/ATVBAHA.107.154989
Mol A, Smits AIPM, Bouten CVC, Baaijens FPT (2009) Tissue engineering of heart valves: advances and current challenges. Expert Rev Med Devices 6:259–275. https://doi.org/10.1586/erd.09.12
Morris PD, Narracott A, von Tengg-Kobligk H et al (2016) Computational fluid dynamics modelling in cardiovascular medicine. Heart 102:18–28. https://doi.org/10.1136/heartjnl-2015-308044
Motta SE, Fioretta ES, Dijkman PE et al (2018) Development of an off-the-shelf tissue-engineered sinus valve for transcatheter pulmonary valve replacement: a proof-of-concept study. J Cardiovasc Transl Res 11:182–191. https://doi.org/10.1007/s12265-018-9800-6
Motta SE, Lintas V, Fioretta ES et al (2019) Human cell-derived tissue-engineered heart valve with integrated Valsalva sinuses: towards native-like transcatheter pulmonary valve replacements. npj Regen Med 4:14. https://doi.org/10.1038/s41536-019-0077-4
Muylaert DEP, van Almen GC, Talacua H et al (2016) Early in-situ cellularization of a supramolecular vascular graft is modified by synthetic stromal cell-derived factor-1α derived peptides. Biomaterials 76:187–195. https://doi.org/10.1016/j.biomaterials.2015.10.052
Nakayama Y, Takewa Y, Sumikura H et al (2015) In-body tissue-engineered aortic valve (Biovalve type VII) architecture based on 3D printer molding. J Biomed Mater Res B Appl Biomater 103:1–11. https://doi.org/10.1002/jbm.b.33186
Niklason LE, Gao J, Abbott WM et al (1999) Functional arteries grown in vitro. Science 284:489–493
Nishimura RA, Warnes CA (2015) Anticoagulation during pregnancy in women with prosthetic valves: evidence, guidelines and unanswered questions: Table 1. Heart 101:430–435. https://doi.org/10.1136/heartjnl-2014-306500
Nistal F, Garcia-Martinez V, Arbe E et al (1990) In vivo experimental assessment of polytetrafluoroethylene trileaflet heart valve prosthesis. J Thorac Cardiovasc Surg 99(6):1074–1081
O’Brien MF, Goldstein S, Walsh S et al (1999) The SynerGraft valve: a new acellular (nonglutaraldehyde-fixed) tissue heart valve for autologous recellularization first experimental studies before clinical implantation. Semin Thorac Cardiovasc Surg 11:194–200
Ota T, Sawa Y, Iwai S et al (2005) Fibronectin-hepatocyte growth factor enhances reendothelialization in tissue-engineered heart valve. Ann Thorac Surg 80:1794–1801. https://doi.org/10.1016/j.athoracsur.2005.05.002
Parikh KN, Shah NC, Clark JB, Myers JL (2017) Pulmonary valve restitution following transannular patch repair of tetralogy of fallot. Interact Cardiovasc Thorac Surg 25:985–986. https://doi.org/10.1093/icvts/ivx215
Patterson RE, Kirk ES (1983) Analysis of coronary collateral structure, function, and ischemic border zones in pigs. Am J Phys 244:H23–H31. https://doi.org/10.1152/ajpheart.1983.244.1.H23
Perri G, Polito A, Esposito C et al (2012) Early and late failure of tissue-engineered pulmonary valve conduits used for right ventricular outflow tract reconstruction in patients with congenital heart disease. Eur J Cardiothorac Surg 41:1320–1325. https://doi.org/10.1093/ejcts/ezr221
Piatti F, Sturla F, Marom G et al (2015) Hemodynamic and thrombogenic analysis of a trileaflet polymeric valve using a fluid-structure interaction approach. J Biomech 48:3641–3649. https://doi.org/10.1016/j.jbiomech.2015.08.009
Pohl M, Wendt MO, Werner S et al (1996) In vitro testing of artificial heart valves: comparison between Newtonian and non-Newtonian fluids. Artif Organs 20:37–46
Popma JJ, Deeb GM, Yakubov SJ et al (2019) Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. https://doi.org/10.1056/NEJMoa1816885
Quinn RW, Bert AA, Converse GL et al (2016) Performance of allogeneic bioengineered replacement pulmonary valves in rapidly growing young lambs. J Thorac Cardiovasc Surg 152:1156–1165.e4. https://doi.org/10.1016/j.jtcvs.2016.05.051
Rabkin-Aikawa E, Mayer JE, Schoen FJ (2005) Heart valve regeneration. Adv Biochem Eng Biotechnol 94:141–179
Rajamannan NM, Evans FJ, Aikawa E et al (2011) Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the national heart and lung and blood institute aortic stenosis working group. Circulation 124:1783–1791. https://doi.org/10.1161/CIRCULATIONAHA.110.006767
Ram-Liebig G, Bednarz J, Stuerzebecher B et al (2015) Regulatory challenges for autologous tissue engineered products on their way from bench to bedside in Europe. Adv Drug Deliv Rev 82:181–191. https://doi.org/10.1016/j.addr.2014.11.009
Rashid ST, Salacinski HJ, Hamilton G, Seifalian AM (2004) The use of animal models in developing the discipline of cardiovascular tissue engineering: a review. Biomaterials 25:1627–1637
Reardon MJ, Van Mieghem NM, Popma JJ et al (2017) Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 376:1321–1331. https://doi.org/10.1056/NEJMoa1700456
Rehman I, Rehman A (2018) Anatomy. Thorax, Heart
Reimer JM, Syedain ZH, Haynie BHT, Tranquillo RT (2015) Pediatric tubular pulmonary heart valve from decellularized engineered tissue tubes. Biomaterials 62:88–94. https://doi.org/10.1016/j.biomaterials.2015.05.009
Reimer J, Syedain Z, Haynie B et al (2017) Implantation of a tissue-engineered tubular heart valve in growing lambs. Ann Biomed Eng 45:439–451. https://doi.org/10.1007/s10439-016-1605-7
Retta SM, Kepner J, Marquez S et al (2017) In-vitro pulsatile flow measurement in prosthetic heart valves: an inter-laboratory comparison. J Heart Valve Dis 26:72–80
Roberts WC, Ko JM (2005) Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 111:920–925. https://doi.org/10.1161/01.CIR.0000155623.48408.C5
Rodés-Cabau J, Webb JG, Cheung A et al (2012) Long-term outcomes after transcatheter aortic valve implantation. J Am Coll Cardiol 60:1864–1875. https://doi.org/10.1016/j.jacc.2012.08.960
Roe BB (1969) Late follow-up studies on flexible leaflet prosthetic valves. J Thorac Cardiovasc Surg 58:59–61
Roh JD, Sawh-Martinez R, Brennan MP et al (2010) Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci U S A 107:4669–4674. https://doi.org/10.1073/pnas.0911465107
Ross DN (1962) Homograft replacement of the aortic valve. Lancet 280:487. https://doi.org/10.1016/S0140-6736(62)90345-8
Rossi F, Van Griensven M (2014) Polymer functionalization as a powerful tool to improve scaffold performances. Tissue Eng A 20:2043–2051
Rüffer A, Purbojo A, Cicha I et al (2010) Early failure of xenogenous de-cellularised pulmonary valve conduits – a word of caution! Eur J Cardiothorac Surg 38:78–85. https://doi.org/10.1016/j.ejcts.2010.01.044
Russo M, Taramasso M, Guidotti A et al (2017) The evolution of surgical valves. Cardiovasc Med 20:285–292. https://doi.org/10.4414/cvm.2017.00532
Rutkovskiy A, Malashicheva A, Sullivan G et al (2017) Valve interstitial cells: the key to understanding the pathophysiology of heart valve calcification. J Am Heart Assoc 6:e006339. https://doi.org/10.1161/JAHA.117.006339 (available here: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5634284/)
Sabbah HN, Hamid MS, Stein PD (1985) Estimation of mechanical stresses on closed cusps of porcine bioprosthetic valves: effects of stiffening, focal calcium and focal thinning. Am J Cardiol 55:1091–1096
Sacks MS, David Merryman W, Schmidt DE (2009) On the biomechanics of heart valve function. J Biomech 42:1804–1824
Said SM, Mainwaring RD, Ma M et al (2016) Pulmonary valve repair for patients with acquired pulmonary valve insufficiency. Ann Thorac Surg 101:2294–2301. https://doi.org/10.1016/j.athoracsur.2016.01.035
Sanders B, Loerakker S, Fioretta ESES et al (2016) Improved geometry of decellularized tissue engineered heart valves to prevent leaflet retraction. Ann Biomed Eng 44:1061–1071. https://doi.org/10.1007/s10439-015-1386-4
Sarikouch S, Horke A, Tudorache I et al (2016) Decellularized fresh homografts for pulmonary valve replacement: a decade of clinical experience. Eur J Cardiothorac Surg 50:281–290. https://doi.org/10.1093/ejcts/ezw050
Scherman J, van Breda B, Appa H et al (2018) Transcatheter valve with a hollow balloon for aortic valve insufficiency. Multimed Man Cardiothorac Surg 2018. https://doi.org/10.1510/mmcts.2018.012
Schmidt D, Dijkman PE, Driessen-Mol A et al (2010) Minimally-invasive implantation of living tissue engineered heart valves. J Am Coll Cardiol 56:510–520. https://doi.org/10.1016/j.jacc.2010.04.024
Schmitt B, Spriestersbach H, H-Icí DO et al (2016) Percutaneous pulmonary valve replacement using completely tissue-engineered off-the-shelf heart valves: six-month in vivo functionality and matrix remodelling in sheep. EuroIntervention 12:62–70. https://doi.org/10.4244/EIJV12I1A12
Schoen FJ (2008) Evolving concepts of cardiac valve dynamics: the continuum of development, functional structure, pathobiology, and tissue engineering. Circulation 118:1864–1880
Schoen FJ (2012) Mechanisms of function and disease of natural and replacement heart valves. Annu Rev Pathol Mech Dis 7:161–183. https://doi.org/10.1146/annurev-pathol-011110-130257
Schoen FJ (2018) Morphology, clinicopathologic correlations, and mechanisms in heart valve health and disease. Cardiovasc Eng Technol 9:126–140. https://doi.org/10.1007/s13239-016-0277-7
Schoen FJ, Gotlieb AI (2016) Heart valve health, disease, replacement, and repair: a 25-year cardiovascular pathology perspective. Cardiovasc Pathol 25:341–352. https://doi.org/10.1016/j.carpath.2016.05.002
Shah M, Jorde UP (2019) Percutaneous mitral valve interventions (repair): current indications and future perspectives. Front Cardiovasc Med 6:88. https://doi.org/10.3389/fcvm.2019.00088
Shinoka T, Breuer CK, Tanel RE et al (1995) Tissue engineering heart valves: valve leaflet replacement study in a lamb model. Ann Thorac Surg 60:S513–S516. https://doi.org/10.1016/0003-4975(95)00733-4
Simon P, Kasimir MT, Seebacher G et al (2003) Early failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patients. Eur J Cardiothorac Surg 23:1002–1006; discussion 1006
Simon HA, Ge L, Sotiropoulos F, Yoganathan AP (2010) Simulation of the three-dimensional hinge flow fields of a bileaflet mechanical heart valve under aortic conditions. Ann Biomed Eng 38:841–853. https://doi.org/10.1007/s10439-009-9857-0
Soares ALF, Oomens CWJ, Baaijens FPT (2014a) A computational model to describe the collagen orientation in statically cultured engineered tissues. Comput Methods Biomech Biomed Engin 17:251–262. https://doi.org/10.1080/10255842.2012.680192
Soares ALF, van Geemen D, van den Bogaerdt AJ et al (2014b) Mechanics of the pulmonary valve in the aortic position. J Mech Behav Biomed Mater 29:557–567. https://doi.org/10.1016/j.jmbbm.2013.07.009
Soliman OI, Miyazaki Y, Abdelghani M et al (2017) Midterm performance of a novel restorative pulmonary valved conduit: preclinical results. EuroIntervention 13:e1418–e1427. https://doi.org/10.4244/EIJ-D-17-00553
Sripathi VC, Kumar RK, Balakrishnan KR (2004) Further insights into normal aortic valve function: role of a compliant aortic root on leaflet opening and valve orifice area. Ann Thorac Surg 77:844–851. https://doi.org/10.1016/S0003-4975(03)01518-2
Sumikura H, Nakayama Y, Ohnuma K et al (2015) In vitro hydrodynamic evaluation of a biovalve with stent (tubular leaflet type) for transcatheter pulmonary valve implantation. J Artif Organs 18:307–314. https://doi.org/10.1007/s10047-015-0851-z
Suzuki Y, Yeung AC, Ikeno F (2011) The representative porcine model for human cardiovascular disease. J Biomed Biotechnol 2011:1–10. https://doi.org/10.1155/2011/195483
Syedain ZH, Meier LA, Lahti MT et al (2014) Implantation of completely biological engineered grafts following decellularization into the sheep femoral artery. Tissue Eng Part A 20:1726–1734. https://doi.org/10.1089/ten.tea.2013.0550
Syedain Z, Reimer J, Schmidt J et al (2015) 6-Month aortic valve implantation of an off-the-shelf tissue-engineered valve in sheep. Biomaterials 73:175–184. https://doi.org/10.1016/j.biomaterials.2015.09.016
Syedain Z, Reimer J, Lahti M et al (2016) Tissue engineering of acellular vascular grafts capable of somatic growth in young lambs. Nat Commun 7:1–9. https://doi.org/10.1038/ncomms12951
Tabata N, Sugiura A, Tsujita K et al (2019) Percutaneous interventions for mitral and tricuspid heart valve diseases. Cardiovasc Interv Ther. https://doi.org/10.1007/s12928-019-00610-z
Takewa Y, Yamanami M, Kishimoto Y et al (2013) In vivo evaluation of an in-body, tissue-engineered, completely autologous valved conduit (biovalve type VI) as an aortic valve in a goat model. J Artif Organs 16:176–184. https://doi.org/10.1007/s10047-012-0679-8
Tao G, Kotick JD, Lincoln J (2012) Heart valve development, maintenance, and disease. The role of endothelial cells. In: Current topics in developmental biology. Academic Press, London, pp 203–232
Taramasso M, Emmert MY, Reser D et al (2015) Pre-clinical in vitro and in vivo models for heart valve therapies. J Cardiovasc Transl Res 8:319–327. https://doi.org/10.1007/s12265-015-9631-7
Terazawa T, Furukoshi M, Nakayama Y (2019) One-year follow-up study of iBTA-induced allogenic biosheet for repair of abdominal wall defects in a beagle model: a pilot study. Hernia 23:149–155. https://doi.org/10.1007/s10029-018-1866-1
Toninato R, Salmon J, Susin FM et al (2016) Physiological vortices in the sinuses of Valsalva: an in vitro approach for bio-prosthetic valves. J Biomech 49:2635–2643. https://doi.org/10.1016/j.jbiomech.2016.05.027
Tudorache I, Horke A, Cebotari S et al (2016) Decellularized aortic homografts for aortic valve and aorta ascendens replacement. Eur J Cardiothorac Surg 50:89–97. https://doi.org/10.1093/ejcts/ezw013
Underwood MJ, El Khoury G, Deronck D et al (2000) The aortic root: structure, function, and surgical reconstruction. Heart 83:376–380
Vesely I, Boughner D, Song T (1988) Tissue buckling as a mechanism of bioprosthetic valve failure. Ann Thorac Surg 46:302–308. https://doi.org/10.1016/S0003-4975(10)65930-9
Voges I, Bräsen JH, Entenmann A et al (2013) Adverse results of a decellularized tissue-engineered pulmonary valve in humans assessed with magnetic resonance imaging. Eur J Cardiothorac Surg 44:272–279. https://doi.org/10.1093/ejcts/ezt328
Weber B, Scherman J, Emmert MY et al (2011) Injectable living marrow stromal cell-based autologous tissue engineered heart valves: first experiences with a one-step intervention in primates. Eur Heart J. https://doi.org/10.1093/eurheartj/ehr059
Weber B, Dijkman PE, Scherman J et al (2013) Off-the-shelf human decellularized tissue-engineered heart valves in a non-human primate model. Biomaterials 34:7269–7280. https://doi.org/10.1016/j.biomaterials.2013.04.059
Wiegerinck EMA, Van Kesteren F, Van Mourik MS et al (2016) An up-to-date overview of the most recent transcatheter implantable aortic valve prostheses. Expert Rev Med Devices 13:31–45. https://doi.org/10.1586/17434440.2016.1120665
Wissing TB, Bonito V, Bouten CVC, Smits AIPM (2017) Biomaterial-driven in situ cardiovascular tissue engineering – a multi-disciplinary perspective. npj Regen Med 2:18. https://doi.org/10.1038/s41536-017-0023-2
Woo JS, Fishbein MC, Reemtsen B (2016) Histologic examination of decellularized porcine intestinal submucosa extracellular matrix (CorMatrix) in pediatric congenital heart surgery. Cardiovasc Pathol 25:12–17. https://doi.org/10.1016/j.carpath.2015.08.007
Yacoub MH, Cohn LH (2004) Novel approaches to cardiac valve repair: from structure to function: part II. Circulation 109:1064–1072
Yacoub MH, Takkenberg JJM (2005) Will heart valve tissue engineering change the world? Nat Clin Pract Cardiovasc Med 2:60–61. https://doi.org/10.1038/ncpcardio0112
Ye J, Cheung A, Lichtenstein SV et al (2006) Transapical aortic valve implantation in humans. J Thorac Cardiovasc Surg 131:1194–1196. https://doi.org/10.1016/j.jtcvs.2006.01.026
Yip CYY, Chen JH, Zhao R, Simmons CA (2009) Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler Thromb Vasc Biol 29:936–942. https://doi.org/10.1161/ATVBAHA.108.182394
Yoganathan AP, Chandran KB, Sotiropoulos F (2005) Flow in prosthetic heart valves: state-of-the-art and future directions. Ann Biomed Eng 33:1689–1694. https://doi.org/10.1007/s10439-005-8759-z
Zafar F, Hinton RB, Moore RA et al (2015) Physiological growth, remodeling potential, and preserved function of a novel bioprosthetic tricuspid valve: tubular bioprosthesis made of small intestinal submucosa-derived extracellular matrix. J Am Coll Cardiol 66:877–888. https://doi.org/10.1016/j.jacc.2015.06.1091
Zakaria MS, Ismail F, Tamagawa M et al (2017) Review of numerical methods for simulation of mechanical heart valves and the potential for blood clotting. Med Biol Eng Comput 55:1519–1548. https://doi.org/10.1007/s11517-017-1688-9
Zehr KJ, Yagubyan M, Connolly HM et al (2005) Aortic root replacement with a novel decellularized cryopreserved aortic homograft: postoperative immunoreactivity and early results. J Thorac Cardiovasc Surg 130:1010–1015. https://doi.org/10.1016/j.jtcvs.2005.03.044
Acknowledgments
S.P. Hoerstrup is a shareholder at Xeltis BV and LifeMatrix AG. M.Y. Emmert is a shareholder at LifeMatrix AG. The other authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this entry
Cite this entry
Fioretta, E.S., Motta, S.E., Gähwiler, E.K.N., Poulis, N., Emmert, M.Y., Hoerstrup, S.P. (2021). Heart Valve Bioengineering. In: Eberli, D., Lee, S.J., Traweger, A. (eds) Organ Tissue Engineering. Reference Series in Biomedical Engineering(). Springer, Cham. https://doi.org/10.1007/978-3-030-44211-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-44211-8_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-44210-1
Online ISBN: 978-3-030-44211-8
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences