Abstract
Impaired thymus function due to aging or other clinical conditions may have a number of consequences for the immune system such as an enhanced predisposition to infection and autoimmunity, slow response to vaccines with age, and possible risk of cancer development. Current approaches for exogenous thymus regeneration focus on the modulation of growth factors and hormones secreted by thymic epithelial cells. Bioengineering approach to create and use transplantable thymus tissue can offer effective regenerative strategy. This chapter aims to describe the cellular architecture and function of primary lymphoid organ thymus and discuss the current and potential bioengineering approaches to regenerate the thymus. Continued research to understand the mechanisms that regulate thymic recovery after injuries and strategies that can boost its endogenous repair is essential for furthering current and developing new promising regenerative technologies.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
1 Introduction
Throughout our lives, we need an internal system to fight off infections. Our immune system protects us from pathogens and abnormal cells such as cancer cells using a specialized type of white blood cell, the T cell, which is produced in the thymus. The thymus is a primary lymphoid organ located in the mediastinum (the midline above the heart) and is composed of two identical lobes (Fig. 1a; Safieddine and Keshavjee 2011). During the neonatal and pre-adolescent periods, the thymus is at its largest and most active phase, after which it gradually decreases in size and function (von Gaudecker 1991) and is replaced by fat (Fig. 1b; Jackson et al. unpublished). The thymus is responsible for the production of all the T cells which is involved in cellular immunity (Pearse 2006). The epithelial cells of the thymus support the proliferation and maturation of T cells as well as smaller numbers of other lymphoid cells. The specificity of T cells that are released into the systemic circulation is also under thymic control.
(a) Schematic representation of human thymus anatomy. (b) Histology of adult human thymus stained with hematoxylin and eosin. Thymus is enclosed by a thin connective tissue capsule. Numerous septa extend into the thymus subdividing the two lobes; two lobes divided into numerous lobules (about 0.5–2 mm in diameter). Blood vessels enter and leave the thymus via the connective tissue septa. Each lobulus is divided into thymus cortex – a darker peripheral zone – and thymus medulla, a lighter central zone. Medullary tissue is continuous from lobule to lobule throughout each lobe. After puberty much of the parenchyma of the thymus is replaced by adipose tissue (adipocytes), particularly cortical lymphoid tissue. This process is called involution which is under the control of both sexual hormones and stress hormones. (Figure 1a is reprinted from Safieddine and Keshavjee (2011), with permission from Elsevier)
Early phases of thymus development are characterized by rapid growth. The thymus is one of the first organs to degenerate postnatally, beginning early in life. This process called thymus involution (Steinmann et al. 1985) significantly impacts the immune system and diminished its capacity through a reduced output of naive T cells (Rudd et al. 2011). Structural changes that happen within the thymus include loss of tissue structure, fibro-adipogenetic transformation, and decline of naive T-cell export. The consequential changes in the peripheral T-cell compartment are believed to be the clinical signs of immunosenescence . Thymus involution differs from the normal aging process in other organs because of its early initiation of atrophy that continues during the life of the individual. Furthermore, the involution process also impairs the ability of the thymus to regenerate from acute damage. Analysis of thymus from various vertebrates including avian, amphibian, and teleost reveals that it undergoes age-associated involution, suggesting that this process is an evolutionary conserved event which appears to occur early in life (Shanley et al. 2009). One evolution theory called the disposable soma theory which is based on the allocation of resources by an organism between maintenance and repair and other functions to maximize Darwinian fitness appears to be the most compatible with regression of the thymus (Kirkwood 1977).
The thymus is particularly sensitive to endogenous and exogenous insults such as infection, chemotherapies or radiation therapies, shock, sex steroids, and graft-versus-host disease (GVHD). Of prime concern are chemotherapy and radiation therapy. For example, the process of hematopoietic stem cell transplantation (HSCT) can acutely damage the thymus through the treatment of tumors via chemotherapy, radiotherapy, or antibody therapy. While the early thymus has a remarkable capacity for endogenous regeneration, this capacity declines considerably with age. Age-related thymic involution may not represent a problem in healthy individuals; however, it becomes a significant clinical issue for sustaining immune competence after insult or injury that leads to immune depletion. For example, insufficient recovery of thymic activity has been directly linked to adverse clinical outcome and opportunistic infections in recipients of allo-HSCT therapy (Wils et al. 2011). In some circumstances, such as a genetic condition called DiGeorge syndrome, the thymus is either very small or absent altogether. This leads to severe immunodeficiency and autoimmune diseases (Davies 2013).
The adult thymus has a limited capacity for regeneration (endogenous thymus regeneration). This endogenous repair may not be sufficient to fully restore the function of the thymus, particularly in the face of injuries or age-related degeneration. In such cases, exogenous interventions will be required to regenerate or even replace lost thymic function. Such strategies include use of hormonal modulation such as sex steroid inhibition or growth hormone administration; use of cytokines (IL-7 and IL-22); and growth factors (like keratinocyte growth factor (KGF)) (Chaudhry et al. 2016), Other novel approaches being tested include use of precursor T cells (Dudakov and van den Brink 2011). Regenerative medicine is showing great promise in repair of a variety of tissues and organs in the body. Bioengineering of the thymus is one of them. This is an exciting intersection of regenerative medicine, tissue engineering, and immune biology, which may be beneficial in the treatment for disorders of the immune system, such as DiGeorge syndrome and age-related immunodeficiency. This chapter will describe the current state of bioengineering of the thymus.
2 Structure and Function of the Thymus
2.1 Structure and Histology
The thymus is an epithelial organ. The mammalian thymus is located in the pericardial mediastinum, anterior to the major vessels of the heart, and ventral to the base of the heart and aortic arch, with variable extension of one or both lobes into the cervical region in the rat (Haley 2003). In the guinea pig, it is located in the neck region (Dijkstra and Sminia 1990). The thymus consists of two distinct lobes connected by a connective tissue isthmus (Fig. 1a; Safieddine and Keshavjee 2011). It is also histologically consistent across different species. Each lobe of the bi-lobed thymus can be divided into morphologically distinct regions referred to as the cortex and the medulla, both of which are separated by an intermediate cortico-medullary zone (von Gaudecker 1991). In adults, thymus gets involuted due to invasion of excessive adipose tissue (Fig. 1b).
Capsule: The capsule is a thin connective tissue layer that surrounds each lobe. The capsule can further be divided into an outer and inner layer of collagen and reticular fibers, between which occasional clusters of lymphocytes can be found. The inner capsule layer gives rise to interlobular septum that partially subdivides the thymus into inter-connecting lobules of variable size and orientation. Fine trabeculae also extend from the capsule or the septum into the center of the lobules.
Epithelial Stroma: A network of epithelial reticular cells form the bulk of the supporting framework in the thymus (Banks 1993), while epithelium-free areas (known as EFAs or holes) present in the sub-capsular area extending deep into the cortex (Bruijntjes et al. 1993; Elmore 2006). Epithelial cells in the sub-capsular region form a layer one or two cells deep. The cell morphology is usually thin and sheet-like (also in the outer cortex and areas surrounding blood vessels), but elsewhere, they assume a stellate appearance. These epithelial cells form an open framework that predominantly has T lymphocytes, but smaller populations of plasma cells, B lymphocytes, and other cells such as neuroendocrine cells can also be seen.
Epithelium-Free Area (EFA): The epithelium-free areas (EFAs) in the thymus are lymphocyte-rich regions, devoid of stromal elements, non-vascularized, and with unknown function (Bruijntjes et al. 1993). It is postulated that they may be lymphocyte reservoirs (Van Ewijk 1984), proliferation sites of lymphocytes (Duijvestijn et al. 1981), or a specific intra-thymic pathway for T lymphocytes (Bruijntjes et al. 1993). These EFAs are located in the sub-capsular region, and serial sections show that they run from the sub-capsular area to deep in the cortex, often bordering the medulla (Bruijntjes et al. 1993).
It is hypothesized that the “EFAs” offer T lymphocytes a separate intra-thymic pathway where immature lymphocytes can move between the cortex and medulla and avoid contact with the stromal elements that are associated with selection (Bruijntjes et al. 1993; Elmore 2006).
Cortex: The cortex region mostly contains small, immature, and densely packed thymocytes, in addition to a transient bone marrow-derived population of predominantly phagocytic macrophages. Mitotically active thymocytes can be found in the sub-capsular cortex. Also seen from the outer cortex to the cortico-medullary junction are a gradient of small, less mitotically active cells. In the sub-capsular region and cortex region, large apoptotic bodies can also be seen. These apoptotic bodies are derived from short-lived, rapidly dividing lymphocytes that undergo apoptosis. The developing thymocytes are in contact with cortical epithelial cells that direct their growth and maturation.
Medulla: The medulla forms a significant portion of the thymus and can form small buds that reach deep into the cortex or even to the capsule in some places. It has a more elaborate cellular composition, consisting of mature T cells, epithelial cells, B lymphocytes, dendritic cells (bone marrow-derived, non-phagocytic cells), Hassall’s corpuscles (mainly in primates and humans), and mixed macrophages. The medullary T lymphocytes are larger, paler-staining and have more cytoplasm than cortical lymphocytes. Hassall’s corpuscles are rare in rodent species when compared with humans and primates. Component cells are polygonal with a large nucleus and pale, occasionally granular cytoplasm with variable cystic degeneration and dystrophic calcification (Pearse 2006). Neuroendocrine cells occur in low numbers. Their physiological function is not understood, but they can give rise to carcinoid tumors. Mast cells and eosinophils are also variably present.
Cortico-medullary Junction: The cortico-medullary junction is the region between the cortex and the medulla and is characterized by presence of abundant blood vessels. Mature and immature T lymphocytes are present, along with variable numbers of plasma cells and perivascular B lymphocytes. The number of plasma and B lymphocytes increases with increasing age of the individual.
2.2 Function
The thymus functions as a primary lymphoid organ. T-cell precursors (hematopoietic precursors) enter the thymus at the cortico-medullary junction and then undergo stages of highly ordered differentiation. During intra-thymic migration, the thymocytes (developing T cells) proliferate and differentiate and also express interleukin receptors and differentiation antigens (Van Ewijk et al. 1988). This process occurs in four different subdivisions of the thymus cortex, referred to as regions 1–4 (Fig. 2). Region 1 is the cortico-medullary junction, which contains the T-cell precursors, called CD4−CD8− double-negative 1 (DN1) cells at this stage. In region 2, these cells differentiate to the double-negative 2 (DN2) stage and undergo a proliferative expansion. In region 3, they transform into double-negative 3 (DN3) cells, where T-cell lineage commitment occurs, and the cells undergo T-cell receptor (TCR) β-chain rearrangement. The transition from double-negative to double-positive (DP) CD4+CD8+ status happens in region 4. The DP cells migrate through the cortex and into the medulla where they now differentiate into either CD4+ or CD8+ single-positive (SP) cells. During the cortico-medullary transition, these cells are also subjected to positive and negative selection. While positive selection happens mainly in the cortex and involves cortical TECs, negative selection occurs mainly in the medulla, where thymic dendritic cells (DCs) and medullary TECs mediate this process. Finally, after the differentiation and maturation within the thymic microenvironment, the mature SP cells are released into the circulation as functional naive T cells (Abbas 2004; Brown et al. 2002; Tizard 2004) (Fig. 2).
The process of formation of functional T cells from hematopoietic precursors within the thymus. Circulating T-lymphoid progenitor cells migrate into the thymic parenchyma through the vasculatures around the cortico-medullary junction in the sub-capsular region of postnatal thymus. T-cell differentiation is characterized by ordered expression of various CD surface molecules. Cells bearing a T-cell receptor that recognizes self MHC are positively selected in the cortex and pass into the cortico-medullary junction. Here, T cells that react with self-antigens undergo apoptosis by the process of negative selection. Double-positive (CD4+CED8+) thymocytes differentiate into single-positive cells either CD4+CD8− or CD4−CD8+ and exit the thymus into the blood and lymphoid tissues. (Image sourced from Parkin and Cohen 2001)
The thymic epithelial cells (TECs) are involved in positive and negative selection, while the dendritic cells (DC) are more efficient than epithelial cells in mediating negative selection. Thymic nurse cells, which are specialized epithelial cells present in the sub-capsular region, are important in early T-cell differentiation. Sub-capsular and medullary epithelial cells produce the thymic hormones, such as Thymosin that is involved in T-cell maturation. Other factors such as thymic humoral factor, serum thymic factor, and thymopoietin are also produced in the thymus which enhance lymphocyte responsiveness (Banks 1993) .
3 Embryology and Development
In mammals, the endoderm of the third and fourth pharangeal pouches and surrounding mesenchyme give rise to the thymus (Dijkstra and Sminia 1990). As development progresses, the thymus (along with the parathyroid and thyroid) migrates caudally. They separate around day 15 when the thymus migrates into the thorax. After migration is complete, the epithelial cells organize into a loose meshwork separated by the developing vasculature. Lymphocyte precursors from developing hematopoietic populate the developing thymus, which now is a lympho-epithelial organ. Immediately after birth, the thymus grows considerably in response to stimulation from postnatal antigen and the demand for large numbers of mature T cells. It is now known that genetic factors can also influence its age of onset, rate of development, and the magnitude of immunological function. In a newborn child, the thymus is more of a tri-lobular structure than bi-lobular, whereas with advancement of age, the shape and then relative size of the thymus decrease progressively compared to the surrounding tissues (Fig. 1b).
The epithelial microenvironment of the thymus develops from endodermal cells of the third pharyngeal pouch, which is located in close apposition to the parathyroid primordium. The establishment of the thymic fate of the pharyngeal epithelium is believed to be controlled by the FOXN1 gene expression (Bleul et al. 2006; Garfin et al. 2013). The mechanisms by which FOXN1 expression is initially induced in the pharyngeal endoderm are not clear, but are thought to involve interaction between multiple signaling and growth factors, including sonic hedgehog (SHH), bone morphogenetic proteins (BMPs), and the WNT signaling pathways (Osada et al. 2006; Rodewald 2008; Manley and Condie 2010). Bipotent thymic epithelial cell (TEC) progenitors develop from the endoderm of the third pharyngeal pouch and express the transcription factor FOXN1. Based on functional studies, there is evidence for the presence of bipotent progenitors that gives rise to cortical TECs (cTECs) and medullary TECs (mTECs) through compartment-specific intermediate precursor cells (Rodewald et al. 2001; Bleul et al. 2006; Rossi et al. 2006) (Fig. 3). There have been attempts to derive this cell type using the in vitro differentiation of embryonic stem and induced pluripotent stem (iPS) cells. Cortical TECs-like environments have been generated in vivo and in vitro by transgenic reprogramming using specific factors, such as CXC chemokine ligand 12 (CXCL12), Delta-like ligand 4 (DLL4), and Notch ligand 1.
Developmental lineage of thymic epithelial cell (TEC). Third pharyngeal pouch gives rise to bipotent thymic epithelial cell (TEC) progenitors that express the transcription factor forkhead box protein N1 (FOXN1). These progenitors generate mature cortical TECs (cTECs) and medullary TECs (mTECs) via compartment-specific progenitor cells
The thymus generates and supplies native T cells and a broad TCR repertoire to the body. The process of T-cell development is tightly regulated and involves a bi-directional crosstalk between the developing thymocytes and the thymus stroma (Takahama 2006). Within the thymus stroma, the thymic epithelial cells (TECs), fibroblasts, endothelial cells, and dendritic cells guide the differentiation of bone marrow-derived T-cell progenitors that ultimately lead to the formation of mature T cells (CD4+ or CD8+) expressing an MHC-restricted, antigen-specific TCR. The events that occur during this process involve the following: (i) immature thymocytes derived from blood-borne progenitor cells accumulate beneath the thymus capsule and then move inward through the cortex and the cortico-medullary junction (CMJ) and finally toward the medulla; (ii) thymocytes undergo multiple rounds of proliferation and differentiation as they progressively encounter a variety of specialized stromal cells along this pathway; and finally (iii) the thymocytes mature into T lymphocytes. The differential expression of chemokines and chemokine receptors on the surface of stromal cells and thymocytes, respectively, are now known to be involved in the trafficking of thymocytes (Norment and Bevan 2000). The mature T cells are attracted to the endothelium of blood vessels in the CMJ and medullary areas, from where the self-tolerant, MHC-restricted T cells are finally exported into the circulation (Rossi and Zlotnik 2000; Ueno et al. 2002).
4 Thymus Dysfunction and Damage
The thymus is extremely sensitive to insults such as toxins, stress hormones, and drugs and can be easily damaged. It can regenerate on its own to a certain extent, but this ability is severely reduced with increasing age. The functional capacity of the thymus is gradually reduced due to age-related degeneration, referred to as thymus involution. Damage to the thymus can result from inborn conditions, infection, or cytoablative therapies or because of graft-versus-host disease following allogeneic transplantation. Some of these conditions are discussed here with the aim to understand the molecules, cells, and processes that can be targeted for repair and regeneration of thymus structure and function.
4.1 Thymus Involution
Unlike other tissues or organs of the body, the thymus exhibits a unique pattern of aging. First, thymus involution starts much earlier, beginning in early childhood and accelerating at puberty (Steinmann et al. 1985). It is estimated that after the initial phase, approximately 3% of the thymus tissue is lost per year until middle age and approximately 1% per year subsequently. This process is called thymus involution. In addition to individual variations, a sexual dimorphism in the rate of involution exists, and it is found to be greater in males than in females (Gui et al. 2012). Some of the etiological factors that can affect the pattern of involution include genetic polymorphisms and hormones (sex steroids such as androgens) (Hsu et al. 2003; Gui et al. 2012). The greatest rate of age-related thymus involution is observed at puberty, and a decline in growth hormone (GH) concentration with age has been shown to play a role in thymus involution (Boehm et al. 2013; Griffith et al. 2012). Another factor playing a key role in aging of the thymus is damage from oxygen free radicals, particularly accumulated damage later in life. This is supported by studies showing that reducing metabolic activity (through caloric restriction or modulation of IGF signaling) can reduce involution of the thymus (Yang et al. 2009; Vallejo et al. 2009). Other evidence that supports free radical-related damage is the lack of enzyme catalase in thymus stromal cells, making these cells more sensitive to damage by oxidative by-products (Griffith et al. 2015).
Immunosenescence is a term used to describe the array of defects seen in adaptive and innate immunity with advancing age (Goronzy and Weyand 2013). This impairs the aging body’s ability to respond to vaccinations and pathogens, resulting in higher mortality from diseases in comparison to younger individuals. Immunosenescence can also lead to a loss of tumor immune surveillance and higher incidence of autoimmune disease in the elderly. Involution of the thymus is a part of immunosenescence where a decrease in cellularity and a loss of tissue organization lead to profound age-related defects in both quality and quantity of T cell produced (lynch et al. 2009). The involution of the thymus due to aging is a major reason for the decreased production of naive T cells and reduced immunity. Changes in the thymus microenvironment here include the loss of thymic epithelial cell (TEC) numbers and function, fibro-adipogenetic transformation, and a decline in crosstalk between developing thymocytes and thymic stromal cells. This crosstalk is very important for thymus homeostasis. This communication is however disrupted during aging, resulting in depletion of cells both in epithelial and lymphoid compartments.
The process of fibro-adipogenesis transformation needs a special mention with respect to thymus involution. In an aging thymus, the TECs decline, while the thymic adipocyte and fibroblast numbers increase. An expansion of lipid-bearing cells within the thymic medulla follows the adipocyte accumulation, while further adipocyte infiltration is observed in several regions such as the capsular region, sub-capsular cortex, and the perivascular space (Fig. 1b) (Yang et al. 2009; Dixit 2010). Dedifferentiation of thymic epithelial cells triggers EMT (epithelial to mesenchymal transition) first, and then the resulting fibroblast cells undergo the conventional route of differentiation program toward adipocyte lineage (Yang et al. 2009; Youm et al. 2009). This loss of TECs and rise of TEC-derived adipocytes and fibroblasts directly compromises thymopoiesis (differentiation of thymocytes into mature T lymphocytes) during aging. Also, the age-related decrease in cellular communications within the cortical and medullary thymic microenvironments results in the decreased T-cell receptor rearrangement, failure of the selection processes, and loss of self versus non-self recognition (Aspinall 1997). From a clinical perspective, elderly individuals (or individuals with highly reduced thymus function) will not only show decreased immune responsiveness but will also have severely delayed immune recovery following cytoablative treatments that can lead to increased morbidity and mortality because of opportunistic infections (Mackall et al. 1995). Additionally, age-induced alterations in the bone marrow (BM) and intrinsic defects in BM-derived hematopoietic stem cells (HSCs) can affect their capacity to generate functional lymphoid progenitors. This can further contribute to the thymus involution.
One consequence of thymus involution is the development of autoimmune disease in elderly individuals. As the T-cell negative selection is lost with age, the ability to mediate self-tolerance is impaired, and the chances of auto-reactive T cells being released into the periphery become greater (Prelog 2006; Coder et al. 2015). However, it is important to note that despite this degenerative process, some residual thymus function does persist into old age. This residual function suggests that instead of becoming a vestigial organ later in life, the thymus continues to function at a lower efficiency. This lower function can easily be lost in the face of the acute insults that are more common in the elderly population. This compounded by the reduced ability of the aged thymus to endogenously regenerate presents several clinical challenges. Therefore, strategies to bioengineer or regenerate the thymus are particularly relevant to the elderly population .
4.2 Infection
The thymus was previously thought to be an immune-privileged organ. However, increasing scientific evidence over the past decade has shown that like other organs, the thymus can get infected by bacteria, fungi, parasites, and viruses (Savino 2006). Damage to the thymus can be caused either directly by the pathogen infecting the organ or by the systemic effects that result from an infection. In a direction infection scenario, lympho-stromal disruption can negatively affect the export of newly generated naive T cells, which can further impair the immune responses against the pathogen (Nunes-Alves et al. 2013). Additionally, infection of the thymus can accelerate its involution. The thymus can be directly infected by pathogens. The human immunodeficiency virus (HIV) infection is a classic example, where the virus infects the T-cell progenitors and thymocytes (CD4+ SP) and induces apoptosis of uninfected thymocytes through secretion of viral products (Douek et al. 1998; Dion et al. 2004; Fang et al. 2008). The HIV also infects dendritic cells and TECs, leading to degradation of the thymus microenvironment (Stanley et al. 1993; Rozmyslowicz et al. 2010). Similarly, infection of the thymus by cytomegalovirus (CMV) and parasites such as Trypanosoma cruzi disrupts its environment by increasing deposition of laminin and fibronectin and increases expression of chemokine ligands (such as CCL4 and CXCL12) by stromal cells. Such changes also promote premature release of DP thymocytes from the thymus and further enhance thymic involution (Mendes-da-Cruz et al. 2006).
Factors produced by the systemic pathogens can also damage the thymus. One such notable factor is the bacterial lipopolysaccharide (LPS), which is released from bacteria such as Escherichia coli. Acute thymic atrophy can be seen due to LPS, which can lead to loss of DP thymocytes (Hick et al. 2006). Stress response caused by other infections can trigger a surge in glucocorticoid levels, which can in turn lead to apoptosis of thymocytes (Gruver and Sempowski 2008). Damage to the thymus via depletion of the DP population can happen due to other inflammatory mediators that are produced during infection. Such mediators include interferon-γ released by Salmonella infection (Deobagkar-Lele et al. 2013) and tumor necrosis factor-α (TNF-α) released by Trypanosoma cruzi (Pérez et al. 2007). Thymus atrophy seen across a broad range of pathogen infections might be a virulence strategy employed by pathogens to survive within the host by deliberately destabilizing the immune responses. In contrast, studies from mycobacterial infection of the thymus suggest that infection in the absence of atrophy can result in generation of naive T cells that are tolerant to the pathogen (Nobrega et al. 2010). Taken together, it seems that the thymic atrophy seen as a result of infection could be a deliberate strategy employed by the host so that generation of an immune repertoire tolerant to certain pathogens can be avoided. Further investigation in this direction will be necessary not only to understand this phenomenon but to also develop potential therapies that can rejuvenate a damaged thymus .
4.3 Cytoablative Therapies
Cytoablative therapies (including chemotherapies and radiation therapies) that are primarily directed against malignant cells in a patient can also target the hematopoietic system (Mackall 1999). Alkylating agents (such as cyclophosphamide) have been shown to deplete all thymocyte subsets within the thymus (Goldberg et al. 2010), while chemotherapy or radiation can also damage the thymic stroma (Fletcher et al. 2009). Thymic epithelial cells (TECs) located within the medulla (mTECs) are particularly vulnerable to the effects of chemotherapy (Fletcher et al. 2009). Since these cells are known to play a key role in negative selection, it is possible that this depletion of mTECs can have profound negative implications for development of tolerance to self-antigens following such treatments. Following chemotherapy, recovery of thymic function in younger patients can be augmented by peripheral expansion of T cells; however, similar recovery can take years in older patients (Storek et al. 1995). Similar to chemotherapy, radiation treatment can cause acute thymic damage, and the double-positive (DP) thymocyte population are particularly sensitive to this damage (Williams et al. 2009; Gentil Dit Maurin et al. 2015). Considerable reduction in TEC numbers is also seen due to stromal damage, while notably, innate lymphoid cells (ILCs) and endothelial cells have been found to be relatively resistant to radiation exposure (Dudakov et al. 2012; Zhang et al. 2014). These two cell types play an important role in endogenous thymus regeneration.
During the postnatal development of the thymus, increased levels of steroid hormones such as the glucocorticoids induce apoptosis of double-positive (DP) thymocytes that leads to thymus involution (Dooley and Liston 2012). Interestingly, some glucocorticoids are also produced by the thymus epithelium itself (Vacchio et al. 1994), which can inhibit T-cell receptor (TCR)-mediated deletion of DP thymocytes. This modulates positive or negative selection of T-cell repertoire (Mittelstadt et al. 2012). Sex steroid hormones such as estrogen, progesterone, and testosterone also affect the state of the thymus. They can reduce the lymphocyte pool within the thymus and directly induce apoptosis of thymocytes (Patiño et al. 2000). Increased levels of these hormones result in thymus involution, primarily through their effects on non-hematopoietic stromal cells (Olsen et al. 1991). The rate of thymus involution increases rapidly during puberty (Steinmann et al. 1985), and acute transient involution of the thymus can also be seen during pregnancy (Dixit et al. 2003). Thymic epithelial cells (TECs), which express a functional androgen receptor, are important targets of androgens. Recently it has been shown that androgens directly inhibit thymopoietic factors in TECs (Williams et al. 2008; Velardi et al. 2014), while other sex steroids show their effect on HSCs and the bone marrow microenvironment. Other effects of sex steroids on thymus include reduction in the number of available T-cell progenitors and decreased lymphoid differentiation (Dudakov et al. 2009).
4.4 Graft-Versus-Host Disease
The graft-versus-host disease (GVHD) arises as a complication of allogeneic hematopoietic stem cell transplantation (HSCT). There are three distinct phases of this disease that include damage to the tissue resulting from conditioning therapy, allo-reactive donor T-cell activation by host antigen-presenting cells, and soluble effectors-mediated target tissue damage (Ferrara et al. 2009; Blazar et al. 2012). Traditionally, GVHD has been viewed as a disease of the skin, liver, and gut. However, recent studies have provided evidence that it also targets the thymus via allo-reactive T cells (Krenger et al. 2000; Krenger and Holländer 2008). In the thymus, GVHD-related changes to both the stromal and lymphocyte compartments can lead to acute involution. Adverse effects include loss of TECs, loss of cortical and medullary thymocytes, and disruption of the gland architecture. This leads to distorted TCR repertoire and, consequently, decreased output of naive T cells (Lapp et al. 1985; Przybylski et al. 2007). Recent studies using mouse models have shown that TECs are not only direct targets for allo-reactive T cells, but these cells can also act as antigen-presenting cells to prime allo-reactive T cells (Hauri-Hohl et al. 2007). Other events that happen during this process include impairment of thymocyte negative selection and Treg development, which not only results in thymic damage but also the development of chronic GVHD and autoimmunity (Hollander et al. 2010; Wu et al. 2013; Dertschnig et al. 2015). It is likely that during sub-clinical GVHD, the damage to the thymus has already started, which may not be detected in the clinical setting. However, it can begin to have detrimental consequences for T-cell reconstitution. Further complicating this situation could be the use of corticosteroids to treat GVHD. Corticosteroids are popular first line of treatment for GVHD and are also known to induce thymic involution (Blazar et al. 2012). Therefore, alternate therapeutic approaches will be required to treat GVHD of the thymus.
5 Endogenous Thymus Regeneration
It was previously thought that the thymus, once damaged, does not have the capacity to regenerate itself (endogenous regeneration). However, now it is known that this is not the case. Endogenous thymus regeneration is actually a crucial function that allows for renewal of immune competence after the efficiency of the immune system is affected due to disease, infection, stress, chemotherapy/radiation therapy, etc. While most of these insults target the CD4+CD8+ DP thymocytes (T-cell progenitors), TECs are also targets of chemotherapy and irradiation therapy. The number of TECs in the thymus also shows variation during the lifetime of an individual, where they initially increase and then gradually decrease over time. At the molecular level, downregulation of transforming growth factor-β receptor 2 (TGFβR2) and increased expression of forkhead box protein N1 (FOXN1) contribute to the life-long regulation of TEC numbers. The TEC numbers decline after an insult to the thymus, followed by recovery where endogenous regeneration induces an increase of TEC progenitors. During this stage, a premature depletion of the progenitor cell pool can also happen where the TEC numbers fall more rapidly than normal, and the recovery is probably transient. Therapeutic interventions, such as fibroblast growth factor 7 (FGF7) treatment, sex hormone ablation, etc., can lead to a boost in thymic function. However, long-term studies will be needed to determine if this boost is due to increased TEC progenitors or increase in the rate of progenitor cell differentiation.
The thymus has a remarkable capacity to regenerate itself after injury. While the mechanisms underlying this regeneration remain poorly understood, studies in the past few years have revealed several pathways. One of the first studies that identified a potential pathway of endogenous thymus regeneration used mouse models, where fibroblast growth factor 7 (FGF7) was found to be crucial for regeneration after total body irradiation (TBI) injury (Alpdogan et al. 2006). Another study identified a second pathway of endogenous thymus regeneration that involved innate lymphoid cells (ILCs) in the thymus (Dudakov et al. 2012). Here, it was found that production of IL-22 by intra-thymic ILCs directly leads to survival and proliferation of TECs, which in turn supports thymopoiesis (differentiation of thymocytes into mature T lymphocytes) through regeneration of the supporting microenvironment. Another study showed a correlation between increased expressions of Foxn1 (a molecule important for thymic maintenance and regeneration) and increased expression of IL-22 after damage (Pan et al. 2014). Therefore, activation of Foxn1 after damage could represent another potent pathway of endogenous thymus regeneration. Other molecules that might be involved in endogenous thymus regeneration are those that also play important roles in steady-state thymopoiesis. These include CCL25, CXCL12, DLL4, Notch ligand, IL-7, SCF, and VEGF.
In addition to the acute damage, thymus involution drastically reduces the regenerative capacity of the thymus. Endogenous regeneration is a critical process to maintain immune competence following insults. However, chronic infection and repeated rounds of chemotherapy or radiation therapy, compounded by age-related involution, can be a significant clinical challenge that can lead to decreased capacity for immune surveillance, reduced response to vaccines, and increase in opportunistic infections. According to some studies, BMP4 has been found to drive thymic regeneration after injury (Tsai et al. 2003). This happens primarily through the upregulation of FOXN1 (the key TEC transcription factor) and its downstream targets. It is now known that in addition to TEC maintenance, FOXN1 likely contributes to age-related thymic involution when its expression declines (Chen et al. 2009; Nowell et al. 2011; Rode et al. 2015). The regulation of the Notch ligand DLL4 has also been shown to be relevant for steady-state thymus function and also for T-cell development and thymus size (Velardi et al. 2014).
6 Exogenous Thymus Regeneration
Regeneration of the immune function remains a prominent unmet need in many clinical situations. Intense research to understand the mechanisms that regulate thymic recovery after injuries and strategies that can boost its endogenous repair have resulted in advancement of knowledge that will help further exogenous regeneration strategies. At present, thymus transplantation is used as a therapeutic intervention conditions where the thymus is either absent (as in DiGeorge syndrome) or non-functional. Thymus transplantation in other types of patients is risky because the implant would be attacked by the patient’s own T cells. For transplantation, thymus fragments from very young patients that are obtained as a by-product of heart surgery are generally used.
6.1 Use of Cytokines and Growth Factors
Exogenous regeneration strategies for thymus regeneration include application of cytokines and growth factors such as interleukin-7 (IL-7), interleukin-22 (IL-22), growth hormones (GH), insulin-like growth factor-1 (IGF-1), keratinocyte growth factor (KGF), and sex steroid inhibitors (SSI). In the thymus, IL-7 is primarily produced by cTECs and to a lesser extent by fibroblasts. In the thymus, stimulation of the IL-7 receptor (IL-7R) promotes proliferation, differentiation, and survival of the developing thymocytes. Due to its critical role in promoting pro-survival signals to peripheral lymphocytes and in thymopoiesis, IL-7 has been extensively studied for its potential to enhance recovery after immune insults (Mackall et al. 2011). Studies have also shown that exogenous administration of IL-7 enhances thymopoiesis and increases the proliferation of mature peripheral T cells (Alpdogan and van den Brink 2005; van Lent et al. 2009). A phase I clinical trial involving the use of recombinant human IL-7 (hIL-7) in patients that had allogeneic hematopoietic stem cell transplantation (HSCT) post-T-cell depletion showed increased CD3, CD4, and CD8 counts after hIL-7 treatment, along with a broader TCR beta repertoire diversity compared to untreated patients (Perales et al. 2012; clinical trial No. NCT00684008).
IL-22 has a role in mediating endogenous recovery of thymus function after acute damage (Dudakov et al. 2012). In mice studies where the animals were subjected to sub-lethal irradiation, exogenous administration of IL-22 promoted accelerated thymic recovery. It is now known that IL-22 can act directly on TECs, promoting their proliferation and survival, which ultimately leads to the rejuvenation of thymopoiesis. These findings suggest that exogenous application of IL-22 represents a novel therapeutic strategy for thymus regeneration and immune function recovery. Currently, clinical trials are being conducted to evaluate the safety and pharmaco-kinetic profile of IL-22 treatment in healthy volunteers, as well as a phase IIa study where the safety of human recombinant IL-22 (hrIL-22) in combination with systemic corticosteroids is being evaluated in the treatment of gastrointestinal acute GVHD in patients receiving HSCT (Study No. NCT02406651). If there are positive outcomes from such studies, IL-22 may be part of an immune-boosting therapy approach.
Several neuroendocrine hormones have been shown to have important effects on the immune system. This includes growth hormone (GH) and insulin-like growth factor-1 (IGF-1). Using GH-deficient mice (having defects in T-cell development), the effects of GH on thymus function have been extensively investigated. Also, in pre-clinical studies using mouse models, exogenous administration of recombinant GH was shown to promote thymus regrowth (Murphy et al. 1992). Results from other studies show that use of GH can increase TCR diversity, promote better thymic cellularity, and improve recovery of the hematopoietic compartment in aged and immune-compromised animals (Chen et al. 2003; Redelman et al. 2008). The mode of action of GH with respect to thymus regeneration includes enhanced proliferation of TECs and trafficking of common lymphoid progenitors (CLPs) into the thymus (Knyszynski et al. 1992; Tsuji et al. 1994). Insulin-like growth factor-1 (IGF-1) is known as a principal mediator of the biological effects of GH (Montecino-Rodriguez et al. 1998; Alpdogan et al. 2003). In the thymus, IGF-1 has been implicated as a positive thymic regulator based on early observations that age-related declines in thymic function paralleled declines in plasma concentrations of IGF-1. IGF-1 receptor (IGF-1R) is expressed on thymocytes and peripheral T cells (Chu et al. 2008). Since, GH and IGF-1 are connected through the same pathway (Chu et al. 2008), it is expected that using IGF-1 for exogenous thymus regeneration may have similar effects as that for GH.
Keratinocyte growth factor (KGF) is another growth hormone that is being used for exogenous thymus regeneration. In the thymus, a splice variant of the fibroblast growth factor receptor 2 (FgfR2-IIIb) expressed on TECs is known to bind KGF. Considering this association, exogenous administration of recombinant KGF has been tested for enhancing thymus regeneration, particularly with reference to HSCT. Results from such studies show that KGF can protect TECs from different types of thymic injuries (Min et al. 2002; Rossi et al. 2002). TEC proliferation leading to an increase in thymocyte expansion and enhanced T-cell export was also observed. Pre-clinical studies in non-human primates have been conducted, including one where KGF treatment after an autologous HSCT in adult rhesus macaques improved thymopoiesis, enhanced naive T-cell recovery, and also accelerated hematopoietic recovery after the transplant (Wils et al. 2012). However, more studies in non-human primates and human subjects will be needed to determine the therapeutic efficacy and safety of this approach. Palifermin (recombinant human keratinocyte growth factor) is an FDA-approved drug that is used for the prevention of mucositis in recipients of high-dose chemotherapy. However, more clinical studies will be needed to evaluate the efficacy of KGF in directly enhancing T-cell reconstitution and restoring thymus function in immune-compromised patients (Min et al. 2002).
Sex steroid inhibition (SSI) using chemical or surgical approach is a well-described method to promote thymus growth (Sutherland et al. 2005; Hince et al. 2008). Using mouse models, several studies have demonstrated thymus enlargement and accelerated thymic recovery after immune insults (Sutherland et al. 2008; Goldberg et al. 2009, 2010). In the past years, several pharmacological treatments that have been developed to suppress the release of androgens from the gonads or block the androgen receptor on the target cells are now being investigated for their potential to reversibly inhibit sex steroid hormones and promote thymic regrowth and immune reconstitution. The exact mechanisms underlying SSI-based thymus regenerative effects are still not completely understood. Recent studies have shown that SSI can directly promote the expression of Notch ligand DLL4 (Velardi et al. 2014) and CCL25 (Williams et al. 2008). The function of hematopoietic stem and progenitor cells is also promoted using this treatment (Khong et al. 2015). Since, transient ablation of sex steroids represents a promising regenerative strategy for the immune system, further work will be needed to understand the different effector mechanisms so that clinically relevant therapies can be developed. Currently, two ongoing clinical trials are testing the effects of leuprolide acetate (an LHRH agonist) with palifermin (recombinant KGF) in promoting immune recovery after allogenic HSCT transplants in human patients (Study No. NCT01746849 and NCT01338987).
Another molecule that can have a key role in exogenous thymus regeneration is the RANK ligand. The RANK ligand is a TNF family member, which has emerged as an important regulator of epithelial cell growth and differentiation in different tissues (Duheron et al. 2011). It has been shown that ex vivo administration of RANKL substantially enhances the cellularity of cTEC and mTEC subsets within the thymus. It also supports T-cell progenitor homing, TEC recovery, and de novo thymopoiesis and thus represents a new therapeutic strategy to boost thymic regeneration. More importantly, RANKL administration was also shown to be efficient for T-cell regeneration and TEC recovery in older individuals with highly diminished thymic function due to natural involution (Gray et al. 2006; Tomimori et al. 2009; Chinn et al. 2012).
6.2 Use of Stem Cells, Progenitor Cells, and Induced Pluripotent Stem Cells
Stem cells and progenitor cells are considered as a promising therapeutic option for thymus repair and regeneration. Regeneration of a functional thymus was demonstrated when thymus stem or progenitor cells from a donor mouse were transplanted into a recipient mouse that lacked a thymus (Fan et al. 2015). Although, this approach is very promising, many challenges still remain that include growing these cells in the lab, expanding enough cells for transplantation, and studying their regenerative capacity in humans. These challenges will have to be overcome if thymic stem or progenitor cells are being considered for clinical use. Use of pluripotent stem cells (such as the induced pluripotent stem cells or iPSCs) is another promising avenue for exogenous thymus regeneration. Researchers have successfully created thymus-like cells from mouse and human pluripotent cells (Lai and Jin 2009; Sun et al. 2013). In order to overcome the challenge of immune rejection and create de novo human thymus tissue for transplantation, use of patient’s own cells to create iPSCs is a practical option.
The generation of thymus-derived T cells can take several months after hematopoietic stem cell transplantation (HSCT), and this process can be delayed even further by situations such as aging and GVHD (Bosch et al. 2012). The use of precursor T (pre-T) cell infusion during HSCT is one of the therapeutic approaches to accelerate immune recovery in these immune-compromised patients. For cell therapy, pre-T cells can be generated using ex vivo co-culture of HSCs with OP9 cells that have been ectopically transduced with two critical factors required for thymocyte proliferation, commitment, and differentiation, such as Delta-like 1 (Dll1) and Delta-like 4 (Dll4) (De Smedt et al. 2004; La Motte-Mohs et al. 2005; Holmes and Zúñiga-Pflücker 2009). The OP9-DL1 cells are a bone marrow-derived stromal cell line that ectopically expresses the Notch ligand, Delta-like 1 (Dll1). This simple and efficient culture system for ensuring proliferation, differentiation, and commitment of T-lineage cells from different sources of stem cells is promising. It can be used to shorten the duration of immunodeficiency in patients that have a dysfunctional thymus. The mechanism by which pre-T cells support immune system recovery is also being explored. Studies have shown that pre-T cells help T-cell reconstitution by providing a ready source of T-cell progenitors. A recently study also reported that the pre-T cells can also have a secondary effect, where through crosstalk with other cellular components, the pre-T cells can enhance thymic stromal function long after these transplanted cells have transited through the thymus (Awong et al. 2013). Another advantage of using pre-T cells is that they can be used as an “off-the-shelf” therapeutic strategy that can be administered across MHC barriers. They can also be used as vehicles for chimeric antigen receptors during immunotherapy.
Pluripotent stem cells hold great promise in the field of regenerative medicine. The selective generation of thymic epithelial progenitor cells (TEPCs) from ESCs or iPSCs in vitro for transplantation may have important implications for treatment of T-cell immune deficiencies in clinical settings. When TEPCs that have been derived from mESC were placed in vivo, they are self-renewed, develop into TECs, and reconstitute the normal thymic architecture. After bone marrow transplantation, these mESC-derived TEPCs caused an increase in the number of functional naive T cells and enhanced thymocyte regeneration (Sun et al. 2013). In a different study, transplantation of mESC-derived TEPCs resulted in the efficient generation of naive T cells in both young and old recipients following allogeneic BM transplantation (Lai et al. 2011). Hence, in a clinical setting, use of autologous pluripotent stem cell-derived TEPC grafts may lead to broad applications for restoring immune damage resulting from dysfunction of the thymus.
6.3 Targeting Cellular Pathways Within the Thymus
Understanding and exploiting the pathways underlying endogenous thymic regeneration can help us develop novel therapeutic strategies for exogenous thymus regeneration. One such insight can be obtained from study of endothelial cells (ECs). It is now known that ECs can have an active role in tissue repair via production of angiocrine factors (Rafii et al. 2016). Angiocrine growth factors are molecules produced by tissue-/organ-specific blood vessel endothelial cells that can stimulate organ-specific repair activities in diseased or damaged tissue/organs. In one study regeneration within the thymus was observed using Akt-activated ex vivo propagated ECs (Zhang et al. 2004). While it is clear that ECs can promote TEC function and regeneration after damage, further studies will be required to investigate the role that TECs play in guiding EC function with respect to thymus regeneration.
FoxN1 is a transcription factor mainly expressed in the thymic epithelium and skin. FOXN1 plays a critical role in the maturation of the thymus and skin epithelial cells essential for its development (Nehls et al. 1994). In human patients, mutations in the FoxN1 gene result in an athymic condition where thymopoiesis is completely blocked, leading to severe primary T-cell immune deficiency (Frank et al. 1999). This severe immune deficiency can often lead to death in early childhood because of severe infections. Studies have shown that expression of FoxN1 can drive differentiation of the whole TEC network from TEPC and also lead to successful colonization of thymic rudiment by bone marrow-derived precursors (Nowell et al. 2011). This knowledge can be used to develop therapeutic interventions using FoxN1 expression as a driving force for thymus regeneration.
Age-induced thymic involution is multifactorial. Therefore, targeting thymus regeneration using isolated pathways would be transitory and incomplete. One approach to achieve sustained rejuvenation of the thymus would be to target both TECs and lymphoid cells, along with other means such as activating endogenous thymic epithelial progenitor cells (TEPCs), sustained expression of forkhead box N1 (a critical TEC transcription factor), and generating TEPCs from pluripotent stem cells. Thymus epithelial stem/progenitor cells from early embryonic thymus are able to differentiate into the full range of thymic epithelial subsets (Rossi et al. 2006; Baik et al. 2013). Recently, an adult thymic epithelial progenitor population within the TEC subset has also been identified and characterized with respect to their self-renewal, colony-forming potential and generation of mature cTEC and mTEC (Wong et al. 2014). This may allow for the development of new therapies targeting thymus rejuvenation (Ventevogel et al. 2013).
Multiple strategies have been proposed and evaluated to boost thymus regeneration. However, many of these have failed to generate compelling effectiveness in clinical studies. The reason for this can be attributed to the fact that thymus requires a reciprocal cellular communication between the hematopoietic and stromal compartments for its development, maintenance, and function. This fact needs to be incorporated when designing and administering therapies aimed at thymus rejuvenation and regeneration. One approach would be a combination strategy such as administering KGF along with pre-T cells to have a synergistic effect (Zakrzewski et al. 2006) or combining sex steroid ablation (SSA) with p53 inhibition (Kelly et al. 2008).
6.4 External Modulation of T-Cell Response Within the Thymus
The role of the thymus in the establishment of the T-cell receptor repertoire is considered as an important area of intervention related to thymus regeneration. Also, a long-pursued goal in the field of transplantation has been to develop strategies to induce immune tolerance through the direct introduction of antigens into the thymus (Posselt et al. 1990). Intra-thymic injection of antigens using lentiviral vectors is one of the methods being used to modulate specific T-cell responses exogenously (Gottrand et al. 2012). Even though a majority of such studies about external modulation of T-cell response have been conducted in mouse models, there is an immense translational potential in the context of organ transplantation to induce tolerance this way. Recently, a double-positive stage of thymocyte development was identified, which could be a potential target for the modulation of FOXP3+ regulatory T cells (Nunes-Cabaco et al. 2011). As a strategy to overcome the delay in immune reconstitution following HSCT (a major cause or morbidity and mortality in such cases), intra-thymus delivery of thymocyte progenitor cells is also being explored. In one study using mouse models, it was shown that delivery of semi-allogeneic progenitor cells within the thymus results in sustained T-cell development, even across histocompatibility barriers (de Barros et al. 2013).
6.5 Thymus Transplantation
Thymus transplantation has been long used as a treatment option in cases of severe immunodeficiency that result from either mutation in the genes essential for TEC differentiation (such as FOXN1) or absence of thymus in conditions such as DiGeorge syndrome. Thymus transplantation aims for more complete reconstitution to produce naive T cells that show a broad T-cell receptor (TCR) repertoire. Postnatal thymic tissue can be readily available because it is routinely removed from infants undergoing open heart surgery through a median sternotomy. A clinical study showed approximately 75% long-term survival in 60 patients under 2 years with a complete DiGeorge anomaly after transplantation of postnatal allogeneic cultured thymus tissue (Markert et al. 2010). There was evidence of thymopoiesis, and a diverse repertoire of naive circulating T-cell responses was seen in survivors. Xenotransplantation has also been considered as an option for thymus regeneration (Zhou et al. 2012). Combined use of hematopoietic stem cell transplantation (HSCT) along with thymus transplantation is being evaluated to facilitate long-term reconstitution of immune function in cases with severe combined immune deficiencies (SCIDs) (Hu and Yang 2012).
6.6 Artificial Thymopoietic Environments
Reconstitution of certain features of the thymus environments by artificial means has also been attempted as an alternative to de novo generation of TEC progenitors. These artificial thymopoietic environments have been generated both in vitro (Beaudette-Zlatanova et al. 2011) and in vivo (Calderón and Boehm 2012). In such studies, the Delta-like ligand 4 (DLL4) was found to be a key determinant of T-cell lineage induction (Hozumi et al. 2008). In in vivo reconstitution experiments, the CXC chemokine ligand 12 (CXCL12) was found to be conducive to the generation of T cells (CD4+CD8+) (Calderón and Boehm 2012). It was also found that in individuals where the lympho-stromal crosstalk was absent during the early stages of development, functional thymopoietic environments can be successfully established at later stages of life using exogenous application of selected cytokines or growth factors, including FOXN1 (Bleul et al. 2006; Corbeaux et al. 2010).
7 Thymus Bioengineering
The advancements in regenerative medicine technologies, particularly tissue engineering, have enabled development of many tissue and organs outside the body. We know that the capacity to replace thymus function therapeutically will be beneficial in a variety of clinical settings, including improving recovery following bone marrow transplantation and supporting tolerance to transplanted cells, tissue, and organs. A fully functional bioengineered thymus can help achieve these goals. This would also be a practical therapeutic option for patients that have complete athymia due to congenital immune deficiencies, such as that seen in DiGeorge syndrome. In the past decade, several efforts have been made to characterize the complex microenvironment of the thymus and also to rebuild the complex 3D structure of this organ in vitro. To obtain full functionality of bioengineered thymus, not only will the spatial organization of the native thymus need to be recreated, but full TEC functionality will be required in each compartment. This would include cTEC and mTEC. For in vivo use, particularly in human patients, bioengineering of a thymus structure would require using artificial matrices. A particularly important area of study has been the identification and use of biomaterials to create an artificial 3D matrix that can support cell-to-cell interactions of thymus-derived cells. An example of such study is the demonstration that thymic stromal cells can support T-cell development from precursor hematopoietic cells in artificial 3D matrices (Pinto et al. 2013).
As for the cell source, the normal thymus tissue-derived cells would still represent the best source for tissue engineering. However, this would require large numbers of cells to be used in the bioengineered construct (Fig. 4). The in vitro expansion of adult thymic epithelial cells (TECs) is challenging because they tend to lose their epithelial phenotype and show diminished growth. Other cell sources for thymus tissue engineering would include endogenous thymic epithelial progenitor cells (TEPCs) or TEPC derived from differentiation of embryonic stem cells or iPSs (Sun et al. 2013; Bredenkamp et al. 2014; Su et al. 2015) and transdifferentiation of some cell lineages into TEC-like cells. A novel approach for generation of new TECs was carried out by direct reprogramming of mouse embryonic fibroblasts (MEFs) using the forced expression of FOXN1 [Bredenkamp et al. 2014]. These TECs (referred to as iTECs) were shown to promote T-cell development both in vitro and in vivo after transplantation into nude mice.
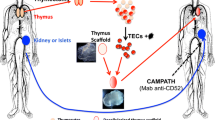
Using thymus bioengineering to introduce donor-specific allograft tolerance. Thymus gland is obtained by thymectomy from a cadaver donor along with the organ to be transplanted (like kidney or pancreatic islets). The thymic epithelial cells (TECs) will be isolated from dissociated thymus and transduced with lentivirus particles expressing the recipient’s MHC molecules. These cells will be used to reconstruct thymus organoids with surrogate thymus scaffold. Shown here are representative images of decellularized thymus scaffold (mouse) and a bioengineered thymus organoid (mouse). This bioengineered thymus organoid will be transplanted into the recipient at the time of organ transplantation. The transplant will be received by a recipient who is preconditioned with Campath (anti-CD52) or ATG (anti-thymocyte immunoglobulin). TEC, Thymic epithelial cells; MHCs, major histocompatibility complex; CAMPATH, anti-CD52 monoclonal antibody. (Image used with permission from Tajima et al. 2016)
The scaffold forms a very important part of many tissue engineering strategies. Use of decellularized tissue scaffolds ensures that the bioengineered tissue or organ retains the structural and ECM compositions of the native tissue (Fig. 5; Jackson et al. unpublished). The same strategy has been used for the thymus. In a study by Fan et al. (2015), three-dimensional thymus organoids were created using decellularized scaffolds from mouse thymus and thymic epithelial cell. When transplanted in vivo, these organoids supported thymopoiesis and also homing of lymphocyte progenitor cells. Transplanting these thymus organoids also resulted in development of immune tolerance to skin allografts in these mice, showing that it is feasible to restore certain thymus functions using bioengineered thymus organoids. The clinical implications of this approach are also being investigated. Bioengineered thymus organoids are also being used for understanding and modulating thymic negative selection. Negative selection is a key checkpoint in the T-cell development pathway where T-cell clones with high affinity to self MHC complexes (pMHCs) and self-peptides are eliminated. This is also referred to as clonal deletion, and both thymic antigen-presenting cells (such as B cells, macrophages, and thymic dendritic cells) and medullary thymic epithelial cells (mTECs) contribute to this process. Using islet cell autoantigen 69 (ICA69) as a model antigen, it was demonstrated that ICA69-reactive T cells can escape clonal deletion when thymus organoids constructed with ICA69-deficient mTECs were transplanted in athymic nude mice (Pradhan et al. 2018).
Rat thymus before and after decellularization (a, b) represents thymus anatomy before and after decellularization process. (c, d) The histology using hematoxylin and eosin staining suggests the removal of cells from the decellularized thymus scaffold. (e, f) SEM images of native and decellularized thymus showing structural preservation thymus. Extracellular matrix scaffold after decellularization (Jackson et al. unpublished)
Using thymus bioengineering, it might be possible to modulate the adaptive immune system of recipients so that donor-specific immune tolerance of allogeneic grafts can be achieved without using any mmunosuppressive drugs (Fan et al. 2015). In one such strategy described by Tajima et al. (2016), the thymus gland is harvested along with the needed (transplantable) organ from a cadaver donor and dissociated into single cell suspension. Then, TECs and other thymic mesenchymal cells are enriched and used to populate the decellularized thymus scaffolds (Fig. 4). These scaffolds can be of either allogeneic or even xenogeneic origins as the ECM components normally are not antigenic. Using lentiviral vectors, the recipient’s MHC molecules can be introduced and expressed in donor TECs, followed by culturing of the TEC-populated scaffold (bioengineered thymus organoids) to enable colonization and expansion of the cells. The graft (like kidney or pancreatic islets, etc.) is transplanted into the patient preconditioned with either anti-CD52 monoclonal antibody (CAMPATH) or anti-thymocyte immunoglobulin (ATG) so as to deplete the T cells (cause immunosuppression) within the graft (Fig. 4). Now the bioengineered thymus organoids are engrafted into the thoracic cavity of the recipient, which will facilitate the generation of a new repertoire of tolerant naive T cells. These new T cells would not respond to the donor cells, but exert adaptive immune function, resulting in the long-term survival of the transplant without the need for immunosuppression. In spite of the abovementioned progress, engineering a full thymus organ de novo still remains a challenge. This is mainly because of the highly complex structural and functional compartmentalization of this organ. Additionally, reconstitution of all the cell types that contribute to the main thymus function (differentiation and maturation of T cells) further adds to the complexity of the system. To fabricate such a complex structure having multiple cell types, use of advanced biofabrication technologies such as 3D bioprinting can offer a solution.
8 Current Challenges
Due to the nature and function of the thymus, both endogenous and exogenous regeneration strategies face many challenges. The thymic stroma is essential for the survival and function of TECs. However, recapitulating this unique 3D architecture in vitro has been a major challenge. Additionally, growing adult TECs in culture is difficult. This is in contrast to epithelial cells from other organs that easily form 2D sheets on artificial substrates. It is now known that the expression of genes critical for TEC proliferation, specification, and function is dependent on the 3D configuration of TECs within the thymus stroma (Novell et al. 2011). Therefore, when TECs are separated from their natural microenvironment and cultured in vitro, most of them either express markers of terminally differentiated epithelial cells (Saunders et al. 1995) or even transdifferentiate into skin cells (Bonfanti et al. 2010). Although we now have 2D culture systems to propagate TECs in vitro, suitable 3D culture systems will still be needed to generate sufficient TECs for therapeutics and tissue engineering. Toward this goal, TECs seeding onto Matrigel or other collagen-based 3D matrices have been explored but with limited success. An encouraging development worth mentioning is the observation that when artificial 3D matrices with mTECs were supplemented with dermal fibroblasts, some of the key molecular factors required for negative selection within the thymus (such as TRAs) were expressed in the mTECs (Pinto et al. 2013).
A big challenge toward exogenous thymus regeneration using cell or bioengineered approaches is the limited number of TECs that can be harvested from the adult thymus. Additionally, as mentioned above, their culture and expansion ex vivo are also a challenge. The proliferation of thymic epithelial progenitor cells (TEPCs) drops drastically within the first week after birth, and the TEC repertoire starts to decrease at around first year after birth in human. Preventing this early loss of TEPCs/TECs and successfully expanding them in culture remains a challenging task. When TECs are isolated and cultured as single cells, they lose cell-cell contact and geometrical organization when placed in an artificial environment. In fact, in an intact thymus, the mTECs and cTECs are compartmentalized to support specific stages of thymocyte development. To recreate this in a bioengineered environment (such as organoid, synthetic scaffold) can be challenging. However, use of 3D biofabrication tools (such as 3D bioprinting) can be utilized to achieve this compartmentalization. Another way to achieve aggregation of TECs within a specific region of an artificial matrix is by using immobilized TEC-specific antibodies, similar to what Tajima et al. (2015) have used. They successfully generated mini aggregates of TECs by incorporating TEC-specific antibodies in a polypeptide-based, self-assembling hydrogel system. These 3D aggregates of TECs that could support T lymphogenesis were transplanted into athymic nude mice. Similar results can be achieved by using 3D bioprinting to create a bioengineered thymus having TEC compartmentalization that is similar to native thymus.
Currently, transplantation of thymus tissue is primarily used as an investigational treatment for infants who have congenital anomalies, such as the DiGeorge syndrome. In such cases, donor thymus tissues are obtained from infants undergoing unrelated surgeries (such as treatment of congenital heart diseases). Tissues obtained from donors under 9 months of age are normally used for transplantation. Therefore, in the absence of a bioengineered thymus tissue or whole organ, treatment of patients with congenital anomalies or the ones who have lost most of their thymus function is a big challenge. Aging-associated progressive degeneration of the thymus and immune function (immune senescence) leads to increased susceptibility to opportunistic infections, incidence of cancer, and autoimmunity, among other issues. Preventing or reversing this immunosenescence in aged individuals so that they can lead a healthy, disease-free life is an idealistic but practical outlook for medicine. However, achieving this is challenging. This problem is significant considering the fact that more than 20% of US population is projected to be aged 65 or older by the year 2030. Also, much of the current research using stem cells as a potential tool to regenerate and repair the thymus has been performed in mice. Translating these findings to humans will be a big challenge both for researchers and clinicians.
9 Conclusions
Thymic involution occurs with age and is central to the decline in immune system function. Current approaches for exogenous thymus regeneration focus on the modulation of growth factors and hormones secreted by TECs. However, such efforts can only lead to transient and partial thymus recovery. For complete recovery, new strategies such as TEPC activation, TEC recovery using induction of FoxN1 expression, de novo generation of TEPC from stem cells, etc. can be used. Targeting pathways like BMP4 to modulate expression of key transcription factors necessary for TEC and thymocyte development such as FoxN1 or Dll4 has also opened up new possibilities for exogenous thymus regeneration. Another promising approach for boosting immune functions in patients whose thymus has been severely damaged is through the administration of RANKL that can be beneficial at several levels, including thymus homing of T-cell progenitors, TEC regeneration, de novo thymopoiesis, etc. Further, use of bioengineering approach using decellularized thymus scaffolds or functional thymus organoids can not only support regeneration of a functional T-cell compartment but also help to introduce donor-specific immune tolerance.
A major challenge to recreate the thymus microenvironment is the complex composition and organization of its extracellular matrix (ECM). Using biological scaffolds generated from decellularized can be an effective strategy to reproduce its in vivo-like ECM microenvironment. As we obtain greater understanding of the molecular mechanisms involved in thymus development, function, and involution, we move forward in developing new therapeutic tools for the repair, regeneration, or replacement of this critical organ. For treating aging-associated progressive immunosenescence, successful creation and use of transplantable thymus tissue using bioengineering approaches seems like the best option. Another solution would be to transplant artificial thymus organoids that have been engineered with host MHC-expressing TEPCs (derived either from genetically modified hESCs or iPSCs) so as to rejuvenate T-cell immunity and also treat aged-related immune disorders. The challenge for the future of thymus regeneration will be to continue the development of current promising technologies and develop new ones. Those technologies with highest potential should then be subjected to rigorous evaluation in a clinical environment.
Despite many encouraging improvements, use of current methods (including pharmacological interventions) has not been able to achieve physiologically relevant and long-term improvements in decreased thymus function. Advances in cell biology, stem cell technology, and cellular engineering are making it possible to use tissue-resident epithelial stem cells, stem cell-derived TECs, and even in vitro generated thymus tissue for thymus regeneration and/or replacement. For using thymus tissue-resident epithelial stem cells, a sufficient lack of knowledge about the compartment-specific intermediate cells and identity of bipotent TEC progenitors are the major roadblocks for endogenous thymus regeneration. A better understanding about the behavior of these cells during tissue maintenance and after injury would be valuable to devise strategies where activation of quiescent endogenous stem cells or introduction of reprogrammed cells with thymopoietic fate can achieve clinically significant effects for restoring failing thymus function.
The bone morphogenetic protein 4 (BMP4) has been shown to be critical for promoting TEC regeneration after injury. After peaking early after damage, BMP4 expression by ECs is seen to return to baseline levels by day 21. Additionally, BMP4 is also known to inhibit the differentiation of T cells. Taken together, these mechanisms point toward potential therapeutic strategies for regenerating the immune system in patients whose thymus has been irrevocably damaged. This direction needs to be further explored. The proliferation of TECs can be limited by TGF-β signaling, while the rate of TEC proliferation increases due to FGF7 signaling both during the steady state and after insult to the thymus. Contrary to this, an increase in IL-22 signaling seems to have little effect on steady-state thymopoiesis. Therefore, a long-term strategy to induce and support thymus recovery would be to provide exogenous FGF7 and IL-22 while blocking the effects of TGF-β.
The presence of a thymus is characteristic of vertebrate immune systems, with commonalities and differences seen over the course of evolution. Therefore, a comparative analysis of thymopoiesis between evolutionarily distant organisms can probably provide important insights into the factors and lesser-known pathways involved in thymus generation and regeneration. Such a comparative analysis can also provide important insights into developing allogenic or even xenogeneic transplant therapies (Yamada et al. 2012). The ECM environment of a decellularized thymus can provide the appropriate microenvironment for the long-term survival of adult TECs and can be used to create thymus organoids and tissues in vitro. Since there is no cellular component in a decellularized tissue scaffold, allogeneic or even xenogeneic rejection should not be a concern. Additionally, since decellularized thymus scaffold from mouse can be stored at 4oC for up to 1 month before use (Fan et al. 2015), it is expected that decellularized thymus scaffold from humans can be handled the same way, providing an off-the-shelf option for creating thymus organoids and tissues for regenerative applications.
A major challenge in the success of solid organ transplantation is the induction of donor-specific immune tolerance (Sachs et al. 2011). Even though the use of latest immunosuppressive drugs has caused the rates of acute rejection to decrease significantly over the past decade, the long-term survival statistics of allograft have not improved correspondingly. Use of modern immunomodulatory protocols (depletion of mature T cells, promotion of regulatory T cells, blockage of costimulatory molecules, etc.) is not helping much. One strategy to overcome this limitation is by introducing allogenic donor TECs into bioengineered thymus organoids so that the need for immunosuppressive drugs to maintain allograft survival is reduced or eliminated. Use of gene editing and delivery technologies can also be valuable in terms of thymus regeneration and bioengineering. One way of using this would be to create patient-specific thymus organoids where the antigen presentation properties of TECs are fine-tuned. This can be used either to create self-tolerance to specific tissues in treating autoimmune disorders or in case of clinical transplantation to have long-term donor-specific immune unresponsiveness. In the future, it is conceivable that patients with thymus dysfunction can be transplanted with bioengineered thymus organoids or tissues that are constructed from either cryopreserved donor TECs or TEC-like cells derived from the patient’s own cells (e.g., from iPSCs). It has been seen in DiGeorge syndrome (DGS) patients treated with bioengineered thymus organoids that around 70% of them show successful thymopoiesis and many of them can avoid immunoglobulin replacement therapy. This indicates the clinical feasibility of the approach for regenerating adaptive immunity.
As in other bioengineered solid tissues or organs, vascularization will be a critical component in the success of thymus bioengineering. Vascularization will be needed not only to prevent ischemia-induced cell death but also to transport cytokines, growth factors, hormones, and cells to and from this bioengineered organ to support normal immune function. While decellularized scaffold can be used for preserving the ECM of the vascular framework, it has been seen that there is a significant loss of factors that support angiogenesis. Studies in NOD scid gamma humanized mice have shown that human TECs and VEGF-expressing thymic mesenchyme can recruit human hematopoietic progenitor cells and promote their differentiation into T cells (Chung et al. 2014), while in other studies, VEGF produced by the thymus stroma was critical for its growth and induction of angiogenesis (Cuddihy et al. 2009). Therefore, incorporating angiogenesis-inducing factors within biomaterials of in vitro generated thymus tissues can be an effective way to promote vascularization post-implantation. There is no doubt that thymus bioengineering will form a critical component of strategies that will be used for restoration or replacement of lost thymus function and immune competence. However, more research and clinical studies will be needed for this to be translated into the clinic.
References
Abbas A (2004) Diseases of immunity. In: Kumar V, Abbas A, Fausto N (eds) Robbins and Cotran, pathologic basis of disease. W. B. Saunders, Philadelphia, pp 193–267
Alpdogan O, van den Brink M (2005) IL-7 and IL-15: therapeutic cytokines for immunodeficiency. Trends Immunol 26:56
Alpdogan O et al (2003) Insulin-like growth factor-I enhances lymphoid and myeloid reconstitution after allogeneic bone marrow transplantation. Transplantation 75:1977–1983
Alpdogan O et al (2006) Keratinocyte growth factor (KGF) is required for postnatal thymic regeneration. Blood 107:2453–2460
Aspinall R (1997) Age-associated thymic atrophy in the mouse is due to a deficiency affecting rearrangement of the TCR during intrathymic T cell development. J Immunol 158:3037–3045
Awong G et al (2013) Human proT-cells generated in vitro facilitate hematopoietic stem cell-derived T-lymphopoiesis in vivo and restore thymic architecture. Blood 122:4210–4219
Baik S, Jenkinson EJ, Lane PJ, Anderson G, Jenkinson WE (2013) Generation of both cortical and Aire + medullary thymic epithelial compartments from CD205 + progenitors. Eur J Immunol 43:589–594
Banks W (1993) Applied veterinary histology. Mosby, St. Louis
Beaudette-Zlatanova BC et al (2011) A human thymic epithelial cell culture system for the promotion of lymphopoiesis from hematopoietic stem cells. Exp Hematol 39:570–579
Blazar BR, Murphy WJ, Abedi M (2012) Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol 12:443–458
Bleul CC et al (2006) Formation of a functional thymus initiated by a postnatal epithelial progenitor cell. Nature 441:992–996
Boehm T, Swann JB (2013) Thymus involution and regeneration: two sides of the same coin? Nat Rev Immunol 13:831–838
Bonfanti P, Claudinot S, Amici AW, Farley A, Blackburn CC, Barrandon Y (2010) Microenvironmental reprogramming of thymic epithelial cells to skin multipotent stem cells. Nature 466(7309):978–982
Bosch M, Khan FM, Storek J (2012) Immune reconstitution after hematopoietic cell transplantation. Curr Opin Hematol 19:324–335
Bredenkamp N, Ulyanchenko S, O’Neill KE, Manley NR, Vaidya HJ, Blackburn CC (2014) An organized and functional thymus generated from FOXN1-reprogrammed fibroblasts. Nat Cell Biol 16:902–908
Brown T Jr, Suter M, Slauson D (2002) Immunopathology. In: Slauson D, Cooper B (eds) Mechanisms of disease. A textbook of comparative general pathology. Mosby, St. Louis, pp 247–297
Bruijntjes JP, Kuper CF, Robinson JE, Schuurman HJ (1993) Epithelium-free area in the thymic cortex of rats. Dev Immunol 3:113–122
Calderón L, Boehm T (2012) Synergistic, context-dependent and hierarchical functions of epithelial components in thymic microenvironments. Cell 149:159–172
Chaudhry MS, Velardi E, Dudakov JA, van den Brink MR (2016) Thymus: the next (re)generation. Immunol Rev 271(1):56–71
Chen BJ, Cui X, Sempowski GD, Chao NJ (2003) Growth hormone accelerates immune recovery following allogeneic T-cell-depleted bone marrow transplantation in mice. Exp Hematol 31:953–958
Chen L, Xiao S, Manley NR (2009) Foxn1 is required to maintain the postnatal thymic microenvironment in a dosage-sensitive manner. Blood 113:567–574
Chinn IK, Blackburn CC, Manley NR, Sempowski GD (2012) Changes in primary lymphoid organs with aging. Semin Immunol 24:309–320
Chu YW et al (2008) Exogenous insulin-like growth factor 1 enhances thymopoiesis predominantly through thymic epithelial cell expansion. Blood 112:2836–2846
Chung B, Montel-Hagen A, Ge S, Blumberg G, Kim K, Klein S et al (2014) Engineering the human thymic microenvironment to support thymopoiesis in vivo. Stem Cells 32(9):2386–2396
Coder BD, Wang H, Ruan L, Su D-M (2015) Thymic involution perturbs negative selection leading to autoreactive T cells that induce chronic inflammation. J Immunol 194:5825–5837
Corbeaux T et al (2010) Thymopoiesis in mice depends on a Foxn1 positive thymic epithelial cell lineage. Proc Natl Acad Sci U S A 107:16613–16618
Cuddihy AR, Ge S, Zhu J, Jang J, Chidgey A, Thurston G et al (2009) VEGF-mediated cross-talk within the neonatal murine thymus. Blood 113(12):2723–2731
Davies EG (2013) Immunodeficiency in DiGeorge syndrome and options for treating cases with complete athymia. Front Immunol 4:322
de Barros SC, Vicente R, Chebli K, Jacquet C, Zimmermann VS, Taylor N (2013) Intrathymic progenitor cell transplantation across histocompatibility barriers results in the persistence of early thymic progenitors and T-cell differentiation. Blood 121(11):2144–2153
De Smedt M, Hoebeke I, Plum J (2004) Human bone marrow CD34+ progenitor cells mature to T cells on OP9-DL1 stromal cell line without thymus microenvironment. Blood Cells Mol Dis 33:227–232
Deobagkar-Lele M, Chacko SK, Victor ES, Kadthur JC, Nandi D (2013) Interferon-γ- and glucocorticoid-mediated pathways synergize to enhance death of CD4(+) CD8(+) thymocytes during Salmonella enterica serovar typhimurium infection. Immunology 138:307–321
Dertschnig S, Hauri-Hohl MM, Vollmer M, Holländer GA, Krenger W (2015) Impaired thymic expression of tissue-restricted antigens licenses the de novo generation of autoreactive CD4+ T cells in acute GVHD. Blood 125:2720–2723
Dijkstra C, Sminia T (1990) Normal anatomy, histology, immunohistology, ultrastructure, rat. In: Jones T, Ward J, Mohr U, Hunt R (eds) Hematopoietic system. Monographs on pathology of laboratory animals. Springer, Berlin, pp 249–256
Dion M-L, Poulin J-F, Bordi R et al (2004) HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity 21:757–768
Dixit VD (2010) Thymic fatness and approaches to enhance thymopoietic fitness in aging. Curr Opin Immunol 22:521–528
Dixit VD, Sridaran R, Edmonsond MA, Taub D, Thompson WE (2003) Gonadotropin-releasing hormone attenuates pregnancy-associated thymic involution and modulates the expression of antiproliferative gene product prohibitin. Endocrinology 144:1496–1505
Dooley J, Liston A (2012) Molecular control over thymic involution: from cytokines and microRNA to aging and adipose tissue. Eur J Immunol 42:1073–1079
Douek DC, McFarland RD, Keiser PH et al (1998) Changes in thymic function with age and during the treatment of HIV infection. Nature 396:690–695
Dudakov JA, van den Brink MR (2011) Greater than the sum of their parts: combination strategies for immune regeneration following allogeneic hematopoietic stem cell transplantation. Best Pract Res Clin Haematol 24(3):467–476
Dudakov JA, Goldberg GL, Reiseger JJ, Vlahos K, Chidgey AP, Boyd RL (2009) Sex steroid ablation enhances hematopoietic recovery following cytotoxic antineoplastic therapy in aged mice. J Immunol 183:7084–7094
Dudakov JA et al (2012) Interleukin-22 drives endogenous thymic regeneration in mice. Science 336:91–95
Duheron V, Hess E, Duval M, Decossas M, Castaneda B, Klopper JE, Amoasii L, Barbaroux JB, Williams IR, Yagita H et al (2011) Receptor activator of NFkappaB (RANK) stimulates the proliferation of epithelial cells of the epidermo-pilosebaceous unit. Proc Natl Acad Sci U S A 108:5342–5347
Duijvestijn AM, Hoefsmit ECM (1981) Ultrastructure of the rat thymus: the micro-environment of T-lymphocyte maturation. Cell Tissue Res 218(2):279–292
Elmore S (2006) Enhanced histopathology evaluation of thymus. Toxicol Pathol 34:656–665
Fan Y, Tajima A, Goh SK, Geng X, Gualtierotti G, Grupillo M, Coppola A, Bertera S, Rudert WA, Banerjee I, Bottino R, Trucco M (2015) Bioengineering thymus organoids to restore thymic function and induce donor-specific immune tolerance to allografts. Mol Ther 23(7):1262–1277
Fang RHT, Colantonio AD, Uittenbogaart CH (2008) The role of the thymus in HIV infection: a 10 year perspective. AIDS 22:171–184
Ferrara JLM, Levine JE, Reddy P, Holler E (2009) Graft-versus-host disease. Lancet 373:1550–1561
Fletcher AL et al (2009) Ablation and regeneration of tolerance-inducing medullary thymic epithelial cells after cyclosporine, cyclophosphamide, and dexamethasone treatment. J Immunol 183:823–831
Frank J, Pignata C, Panteleyev AA, Prowse DM, Baden H, Weiner L et al (1999) Exposing the human nude phenotype. Nature 398:473–474
Garfin PM et al (2013) Inactivation of the RB family prevents thymus involution and promotes thymic function by direct control of Foxn1 expression. J Exp Med 210:1087–1097
Gentil Dit Maurin A, Lemercier C, Collin-Faure V, Marche PN, Jouvin-Marche E, Candéias SM (2015) Developmental regulation of p53-dependent radiation-induced thymocyte apoptosis in mice. Clin Exp Immunol 179:30–38
Goldberg GL et al (2009) Luteinizing hormone-releasing hormone enhances T cell recovery following allogeneic bone marrow transplantation. J Immunol 182:5846–5854
Goldberg GL et al (2010) Sex steroid ablation enhances immune reconstitution following cytotoxic antineoplastic therapy in young mice. J Immunol 184:6014–6024
Goronzy JJ, Weyand CM (2013) Understanding immunosenescence to improve responses to vaccines. Nat Immunol 14:428–436
Gottrand G, Taleb K, Ragon I et al (2012) Intrathymic injection of lentiviral vector curtails the immune response in the periphery of normal mice. J Gene Med 14:90–99
Gray DH, Seach N, Ueno T, Milton MK, Liston A, Lew AM, Goodnow CC, Boyd RL (2006) Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood 108:3777–3785
Griffith AV, Fallahi M, Venables T, Petrie HT (2012) Persistent degenerative changes in thymic organ function revealed by an inducible model of organ regrowth. Aging Cell 11:169–177
Griffith AV et al (2015) Metabolic damage and premature thymus aging caused by stromal catalase deficiency. Cell Rep 12:1071–1079
Gruver AL, Sempowski GD (2008) Cytokines, leptin, and stress-induced thymic atrophy. J Leukoc Biol 84:915–923
Gui J, Mustachio LM, Su D-M, Craig RW (2012) Thymus size and age-related thymic involution: early programming, sexual dimorphism, progenitors and stroma. Aging Dis 3:280–290
Haley PJ (2003) Species differences in the structure and function of the immune system. Toxicology 188(1):49–71
Hauri-Hohl MM et al (2007) Donor T-cell alloreactivity against host thymic epithelium limits T-cell development after bone marrow transplantation. Blood 109:4080–4088
Hick RW, Gruver AL, Ventevogel MS, Haynes BF, Sempowski GD (2006) Leptin selectively augments thymopoiesis in leptin deficiency and lipopolysaccharide-induced thymic atrophy. J Immunol 177:169–176
Hince M, Sakkal S, Vlahos K, Dudakov J, Boyd R, Chidgey A (2008) The role of sex steroids and gonadectomy in the control of thymic involution. Cell Immunol 252:122–138
Hollander GA, Krenger W, Blazar BR (2010) Emerging strategies to boost thymic function. Curr Opin Pharmacol 10:443–453
Holmes R, Zúñiga-Pflücker JC (2009) The OP9-DL1 system: generation of T-lymphocytes from embryonic or hematopoietic stem cells in vitro. Cold Spring Harb Protoc. https://doi.org/10.1101/pdb.prot5156
Hozumi K et al (2008) Delta-like 4 is indispensable in thymic environment specific for T cell development. J Exp Med 205:2507–2513
Hsu H-C et al (2003) Age-related thymic involution in C57BL/6J × DBA/2J recombinant-inbred mice maps to mouse chromosomes 9 and 10. Genes Immun 4:402–410
Hu Z, Yang Y-G (2012) Human lymphohematopoietic reconstitution and immune function in immunodeficient mice receiving cotransplantation of human thymic tissue and CD34(þ) cells. Cell Mol Immunol 9:232–236
Parkin J, Cohen B (2001) An overview of the immune system. Lancet 357(9270):1777–1789
Kelly RM, Highfill SL, Panoskaltsis-Mortari A, Taylor PA, Boyd RL, Hollander GA, Blazar BR (2008) Keratinocyte growth factor and androgen blockade work in concert to protect against conditioning regimen-induced thymic epithelial damage and enhance T-cell reconstitution after murine bone marrow transplantation. Blood 111:5734–5744
Khong DM et al (2015) Enhanced hematopoietic stem cell function mediates immune regeneration following sex steroid blockade. Stem Cell Rep 4:445–458
Kirkwood TB (1977) Evolution of ageing. Nature 270:301–304
Knyszynski A, Adler-Kunin S, Globerson A (1992) Effects of growth hormone on thymocyte development from progenitor cells in the bone marrow. Brain Behav Immun 6:327–340
Krenger W, Holländer GA (2008) The immunopathology of thymic GVHD. Semin Immunopathol 30:439–456
Krenger W, Rossi S, Hollander GA (2000) Apoptosis of thymocytes during acute graft-versus-host disease is independent of glucocorticoids. Transplantation 69:2190–2193
La Motte-Mohs RN, Herer E, Zuniga-Pflucker JC (2005) Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood 105:1431–1439
Lai L, Jin J (2009) Generation of thymic epithelial cell progenitors by mouse embryonic stem cells. Stem Cells 27(12):3012–3020
Lai L, Cui C, Jin J, Hao Z, Zheng Q, Ying M et al (2011) Mouse embryonic stem cell-derived thymic epithelial cell progenitors enhance T-cell reconstitution after allogeneic bone marrow transplantation. Blood 118:3410–3418
Lapp WS, Ghayur T, Mendes M, Seddik M, Seemayer TA (1985) The functional and histological basis for graft-versus-host-induced immunosuppression. Immunol Rev 88:107–133
Lynch HE, Goldberg GL, Chidgey A, van den Brink MRM, Boyd R, Sempowski GD (2009) Thymic involution and immune reconstitution. Trends Immunol 30:366–373
Mackall CL (1999) T-cell immunodeficiency following cytotoxic antineoplastic therapy: a review. Oncologist 4:370–378
Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM et al (1995) Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med 332:143–149
Mackall CL, Fry TJ, Gress RE (2011) Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol 11:330–342
Manley NR, Condie BG (2010) Transcriptional regulation of thymus organogenesis and thymic epithelial cell differentiation. Prog Mol Biol Transl Sci 92:103–120
Markert ML, Devlin BH, Mccarthy EA (2010) Thymus transplantation. Clin Immunol 135:236–246
Mendes-da-Cruz DA, Silva JS, Cotta-de-Almeida V, Savino W (2006) Altered thymocyte migration during experimental acute Trypanosoma cruzi infection: combined role of fibronectin and the chemokines CXCL12 and CCL4. Eur J Immunol 36:1486–1493
Min D et al (2002) Protection from thymic epithelial cell injury by keratinocyte growth factor: a new approach to improve thymic and peripheral T-cell reconstitution after bone marrow transplantation. Blood 99:4592–4600
Mittelstadt PR, Monteiro JP, Ashwell JD (2012) Thymocyte responsiveness to endogenous glucocorticoids is required for immunological fitness. J Clin Invest 122:2384–2394
Montecino-Rodriguez E, Clark R, Dorshkind K (1998) Effects of insulin-like growth factor administration and bone marrow transplantation on thymopoiesis in aged mice. Endocrinology 139:4120–4126
Murphy WJ, Durum SK, Anver MR, Longo DL (1992) Immunologic and hematologic effects of neuroendocrine hormones. Studies on DW/J dwarf mice. J Immunol 148:3799–3805
Nehls M, Pfeifer D, Schorpp M, Hedrich H, Boehm T (1994) New member of the winged-helix protein family disrupted in mouse and rat nude mutations. Nature 372:103–107
Nobrega C et al (2010) Dissemination of mycobacteria to the thymus renders newly generated T cells tolerant to the invading pathogen. J Immunol 184:351–358
Norment AM, Bevan MJ (2000) Role of chemokines in thymocyte development. Semin Immunol 12:445–455
Nowell CS, Bredenkamp N, Tetelin S, Jin X, Tischner C, Vaidya H et al (2011) Foxn1 regulates lineage progression in cortical and medullary thymic epithelial cells but is dispensable for medullary sublineage divergence. PLoS Genet 7(11)
Nunes-Alves C, Nobrega C, Behar SM, Correia-Neves M (2013) Tolerance has its limits: how the thymus copes with infection. Trends Immunol 34:502–510
Nunes-Cabaco H, Caramalho I, Sepulveda N, Sousa AE (2011) Differentiation of human thymic regulatory T cells at the double positive stage. Eur J Immunol 41:3604–3614
Olsen NJ, Watson MB, Henderson GS, Kovacs WJ (1991) Androgen deprivation induces phenotypic and functional changes in the thymus of adult male mice. Endocrinology 129:2471–2476
Osada M et al (2006) The Wnt signaling antagonist Kremen1 is required for development of thymic architecture. Clin Dev Immunol 13:299–319
Pan B et al (2014) Acute ablation of DP thymocytes induces up-regulation of IL-22 and Foxn1 in TECs. Clin Immunol 150:101–108
Patiño JAG, Marino MW, Ivanov VN, Nikolich-Zugich J (2000) Sex steroids induce apoptosis of CD8+CD4+ double-positive thymocytes via TNF-alpha. Eur J Immunol 30:2586–2592
Pearse G (2006) Normal structure, function and histology of the thymus. Toxicol Pathol 34(5):504–514
Perales MA et al (2012) Recombinant human interleukin-7 (CYT107) promotes T-cell recovery after allogeneic stem cell transplantation. Blood 120:4882–4891
Pérez AR et al (2007) Thymus atrophy during Trypanosoma cruzi infection is caused by an immuno-endocrine imbalance. Brain Behav Immun 21:890–900
Pinto S, Schmidt K, Egle S, Stark HJ, Boukamp P, Kyewski B (2013) An organotypic coculture model supporting proliferation and differentiation of medullary thymic epithelial cells and promiscuous gene expression. J Immunol 190:1085–1093
Posselt AM, Barker CF, Tomaszewski JE et al (1990) Induction of donor-specific unresponsiveness by intrathymic islet transplantation. Science 249:1293–1295
Pradhan I, Tajima A, Bertera S, Trucco M, Fan Y (2018) Modulating thymic negative selection with bioengineered thymus organoids. In: Soboloff J, Kappes DJ (eds) Signaling mechanisms regulating T cell diversity and function. CRC Press/Taylor & Francis, Boca Raton. Chapter 4
Prelog M (2006) Aging of the immune system: a risk factor for autoimmunity? Autoimmun Rev 5:136–139
Przybylski GK, Kreuzer K-A, Siegert W, Schmidt CA (2007) No recovery of T-cell receptor excision circles (TRECs) after non-myeloablative allogeneic hematopoietic stem cell transplantation is correlated with the onset of GvHD. J Appl Genet 48:397–404
Rafii S, Butler JM, Ding BS (2016) Angiocrine functions of organ-specific endothelial cells. Nature 529:316–325
Redelman D, Welniak LA, Taub D, Murphy WJ (2008) Neuroendocrine hormones such as growth hormone and prolactin are integral members of the immunological cytokine network. Cell Immunol 252:111–121
Rode I, Martins VC, Küblbeck G, Maltry N, Tessmer C, Rodewald HR (2015) Foxn1 protein expression in the developing, aging, and regenerating thymus. J Immunol 195(12):5678–5687
Rodewald HR (2008) Thymus organogenesis. Annu Rev Immunol 26:355–388
Rodewald HR, Paul S, Haller C, Bluethmann H, Blum C (2001) Thymus medulla consisting of epithelial islets each derived from a single progenitor. Nature 414:763–768
Rossi D, Zlotnik A (2000) The biology of chemokines and their receptors. Annu Rev Immunol 18:217–242
Rossi S et al (2002) Keratinocyte growth factor preserves normal thymopoiesis and thymic microenvironment during experimental graft-versus-host disease. Blood 100:682–691
Rossi SW, Jenkinson WE, Anderson G, Jenkinson EJ (2006) Clonal analysis reveals a common progenitor for thymic cortical and medullary epithelium. Nature 441:988–991
Rozmyslowicz T, Murphy SL, Conover DO, Gaulton GN (2010) HIV-1 infection inhibits cytokine production in human thymic macrophages. Exp Hematol 38:1157–1166
Rudd BD et al (2011) Nonrandom attrition of the naive CD8+ T cell pool with aging governed by T cell receptor: pMHC interactions. Proc Natl Acad Sci U S A 108:13694–13699
Sachs DH, Sykes M, Kawai T, Cosimi AB (2011) Immuno-intervention for the induction of transplantation tolerance through mixed chimerism. Semin Immunol 23:165–173
Safieddine N, Keshavjee S (2011) Anatomy of the thymus gland. Thorac Surg Clin 21(2):191–5,viii
Saunders DJ, Georgiou HM, Wu L, Shortman K (1995) Induction of limited growth and differentiation of early thymic precursor cells by thymic epithelial cell lines. Immunol Lett 47(1–2):45–51
Savino W (2006) The thymus is a common target organ in infectious diseases. PLoS Pathog 2:e62
Shanley DP, Aw D, Manley NR, Palmer DB (2009) An evolutionary perspective on the mechanisms of immunosenescence. Trends Immunol 30(7):374–381
Stanley SK et al (1993) Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J Exp Med 178:1151–1163
Steinmann GG, Klaus B, Müller-Hermelink HK (1985) The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol 22:563–575
Storek J, Witherspoon RP, Storb R (1995) T cell reconstitution after bone marrow transplantation into adult patients does not resemble T cell development in early life. Bone Marrow Transplant 16:413–425
Su M et al (2015) Efficient in vitro generation of functional thymic epithelial progenitors from human embryonic stem cells. Sci Rep 5:9882
Sun X, Xu J, Lu H, Liu W, Miao Z, Sui X, Liu H, Su L, Du W, He Q, Chen F, Shi Y, Deng H (2013) Directed differentiation of human embryonic stem cells into thymic epithelial progenitor-like cells reconstitutes the thymic microenvironment in vivo. Cell Stem Cell 13(2):230–236
Sutherland JS et al (2005) Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol 175:2741–2753
Sutherland JS et al (2008) Enhanced immune system regeneration in humans following allogeneic or autologous hemopoietic stem cell transplantation by temporary sex steroid blockade. Clin Cancer Res 14:1138–1149
Tajima A, Liu W, Pradhan I et al (2015) Bioengineering mini functional thymic units with EAK16-II/EAKIIH6 self-assembling hydrogel. Clin Immunol 160(1):82–89
Tajima A, Pradhan I, Trucco M, Fan Y (2016) Restoration of thymus function with bioengineered thymus organoids. Curr Stem Cell Rep 2(2):128–139
Takahama Y (2006) Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol 6:127–135
Tizard I (2004) Veterinary immunology. An introduction, 7th edn. WB Saunders, Philadelphia
Tomimori Y, Mori K, Koide M, Nakamichi Y, Ninomiya T, Udagawa N, Yasuda H (2009) Evaluation of pharmaceuticals with a novel 50-hour animal model of bone loss. J Bone Miner Res 24:1194–1205
Tsai PT, Lee RA, Wu H (2003) BMP4 acts upstream of FGF in modulating thymic stroma and regulating thymopoiesis. Blood 102:3947–3953
Tsuji Y, Kinoshita Y, Hato F, Tominaga K, Yoshida K (1994) The in vitro proliferation of thymus epithelial cells stimulated with growth hormone and insulin-like growth factor-I. Cell Mol Biol (Noisy-le-Grand) 40:1135–1142
Ueno T et al (2002) Role for CCR7 ligands in the emigration of newly generated T lymphocytes from the neonatal thymus. Immunity 16:205–218
Vacchio MS, Papadopoulos V, Ashwell JD (1994) Steroid production in the thymus: implications for thymocyte selection. J Exp Med 179:1835–1846
Vallejo AN, Michel JJ, Bale LK, Lemster BH, Borghesi L, Conover CA (2009) Resistance to age-dependent thymic atrophy in long-lived mice that are deficient in pregnancy-associated plasma protein A. Proc Natl Acad Sci U S A 106:11252–11257
Van Ewijk (1984) Immunohistology of lymphoid and non-lymphoid cells in the thymus in relation to T lymphocyte differentiation. American Journal of Anatomy 170(3):311–330
van Ewijk W, Brekelmans PJ, Jacobs R, Wisse E (1988) Lymphoid microenvironments in the thymus and lymph node. Scanning Microsc 2:2129–2140
van Lent AU et al (2009) IL-7 enhances thymic human T cell development in “human immune system” Rag2-/-IL-2Rgammac-/- mice without affecting peripheral T cell homeostasis. J Immunol 183:7645–7655
Velardi E et al (2014) Sex steroid blockade enhances thymopoiesis by modulating Notch signaling. J Exp Med 211:2341–2349
Ventevogel MS, Sempowski GD (2013) Thymic rejuvenation and aging. Curr Opin Immunol 25(4):516–522
von Gaudecker B (1991) Functional histology of the human thymus. Anat Embryol 183:1–15. https://doi.org/10.1007/BF00185830
Williams KM et al (2008) CCL25 increases thymopoiesis after androgen withdrawal. Blood 112:3255–3263
Williams KM, Mella H, Lucas PJ, Williams JA, Telford W, Gress RE (2009) Single cell analysis of complex thymus stromal cell populations: rapid thymic epithelia preparation characterizes radiation injury. Clin Transl Sci 2:279–285
Wils EJ, van der Holt B, Broers AE, Posthumus-van Sluijs SJ, Gratama JW, Braakman E, Cornelissen JJ (2011) Insufficient recovery of thymopoiesis predicts for opportunistic infections in allogeneic hematopoietic stem cell transplant recipients. Haematologica 96:1846–1854
Wils EJ et al (2012) Keratinocyte growth factor and stem cell factor to improve thymopoiesis after autologous CD34+ cell transplantation in rhesus macaques. Biol Blood Marrow Transplant 18:55–65
Wong K, Liser NL, Barsanti M, Lim JM, Hammet MV, Khong DM, Siatskas C, Gray DH, Boyd RL, Chidgey AP (2014) Multilineage potential and self-renewal define an epithelial progenitor cell population in the adult thymus. Cell Rep 8:1198–1209
Wu T, Young JS, Johnston H, Ni X, Deng R, Racine J, Wang M, Wang A, Todorov I, Wang J, Zeng D (2013) Thymic damage, impaired negative selection, and development of chronic graft-versus-host disease caused by donor CD4+ and CD8+ T cells. J Immunol 191(1):488–499
Yamada K, Scalea J (2012) Thymic transplantation in pig-to-nonhuman primates for the induction of tolerance across xenogeneic barriers. Methods Mol Biol 885:191–212
Yang H, Youm YH, Dixit VD (2009) Inhibition of thymic adipogenesis by caloric restriction is coupled with reduction in age-related thymic involution. J Immunol 183:3040–3052
Youm YH, Yang H, Sun Y, Smith RG, Manley NR, Vandanmagsar B, Dixit VD (2009) Deficient ghrelin receptor-mediated signaling compromises thymic stromal cell microenvironment by accelerating thymic adiposity. J Biol Chem 284:7068–7077
Zakrzewski JL, Kochman AA, Lu SX et al (2006) Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med 12(9):1039–1047
Zhang F et al (2004) Adenovirus E4 gene promotes selective endothelial cell survival and angiogenesis via activation of the vascular endothelial-cadherin/Akt signaling pathway. J Biol Chem 279:11760–11766
Zhang SL et al (2014) Chemokine treatment rescues profound T-lineage progenitor homing defect after bone marrow transplant conditioning in mice. Blood 124:296–304
Zhou J, Wang X, Luo G et al (2012) Partial tolerance induced by transplantation of spatially separated thymuses: a cue for T cell retolerization in thymus grafts. Scand J Immunol 75:401–408
Acknowledgments
We would like to thank Dr. Prafulla Chandra (Wake Forest Institute for Regenerative Medicine) for the editorial help.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this entry
Cite this entry
Kulkarni, G., Jackson, J.D. (2021). Tissue-Engineered Thymus. In: Eberli, D., Lee, S.J., Traweger, A. (eds) Organ Tissue Engineering. Reference Series in Biomedical Engineering(). Springer, Cham. https://doi.org/10.1007/978-3-030-44211-8_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-44211-8_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-44210-1
Online ISBN: 978-3-030-44211-8
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences