Abstract
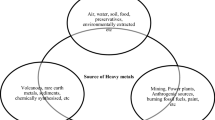
In the last decades, the worldwide industrial activity has increased exponentially and with it the destruction and pollution of the environment. Water from seas and rivers has been especially polluted due to effluents coming from several industries, such as mining, tannery, metallurgical, and electroplating. These effluents contain a great variety of heavy metals: lead, chromium, cadmium, copper, zinc, nickel, mercury, among others. Different removal methods have been used, such as ion exchange, solvent extraction, electrochemical treatment, ultrafiltration, and chemical precipitation; however, most of these are not suitable for the aqueous medium, are not cost-effective, and require high energy to be carried out. Adsorption, on the contrary, is an efficient and low cost technique that can be reversible because some of the adsorbents can be regenerated by desorption. Other eco-friendly and low-cost alternative is the bioremediation that uses material biological as adsorbent material in which a biosorption process takes place.
Within the remediation variants, there are two branches that have gained special attention in recent years: nanoremediation and nanobioremediation. The use of nanoparticles, in water treatment, provides certain advantages over traditional methods, due to its high surface area and reactivity. On the other hand, nanobioremediation is related to eco-friendly materials, which represents a big advantage over the remediation that uses oxide of metallic nanoparticles for the removal of heavy metals; however, the biological nanoparticles still present low removal efficiencies, compared with inorganic nanoparticles.
This chapter examines the advantages and disadvantages related to nanoremediation and nanobioremediation, from synthesis routes, efficiencies, and compatibility with the environment, to reuse capacity.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Speight JG (2020) Sources of water pollution. In: Speight JGBT-NWR (ed) Nat. Water Remediat. Elsevier, Oxford, United Kingdom, pp 165–198
Zheng S, Wang Q, Yuan Y, Sun W (2020) Human health risk assessment of heavy metals in soil and food crops in the Pearl River Delta urban agglomeration of China. Food Chem 316:126213. https://doi.org/10.1016/j.foodchem.2020.126213
Ma D, Su M, Qian J et al (2020) Heavy metal removal from sewage sludge under citric acid and electroosmotic leaching processes. Sep Purif Technol 242:116822. https://doi.org/10.1016/j.seppur.2020.116822
Guerra F, Attia M, Whitehead D, Alexis F (2018) Nanotechnology for environmental remediation: materials and applications. Molecules 23:1760. https://doi.org/10.3390/molecules23071760
Ossai IC, Ahmed A, Hassan A, Hamid FS (2020) Remediation of soil and water contaminated with petroleum hydrocarbon: a review. Environ Technol Innov 17:100526. https://doi.org/10.1016/j.eti.2019.100526
Gil-Díaz M, Rodríguez-Valdés E, Alonso J et al (2019) Nanoremediation and long-term monitoring of brownfield soil highly polluted with As and Hg. Sci Total Environ 675:165–175. https://doi.org/10.1016/j.scitotenv.2019.04.183
Fajardo C, Sánchez-Fortún S, Costa G et al (2020) Evaluation of nanoremediation strategy in a Pb, Zn and Cd contaminated soil. Sci Total Environ 706:136041. https://doi.org/10.1016/j.scitotenv.2019.136041
Vidmar J, Oprčkal P, Milačič R et al (2018) Investigation of the behaviour of zero-valent iron nanoparticles and their interactions with Cd2+ in wastewater by single particle ICP-MS. Sci Total Environ 634:1259–1268. https://doi.org/10.1016/j.scitotenv.2018.04.081
Fang Y, Wen J, Zhang H et al (2020) Enhancing Cr(VI) reduction and immobilization by magnetic core-shell structured NZVI@MOF derivative hybrids. Environ Pollut 260:114021. https://doi.org/10.1016/j.envpol.2020.114021
Su L, Zhen G, Zhang L et al (2015) The use of the core–shell structure of zero-valent iron nanoparticles (NZVI) for long-term removal of sulphide in sludge during anaerobic digestion. Environ Sci Process Impacts 17:2013–2021. https://doi.org/10.1039/C5EM00470E
Mu Y, Jia F, Ai Z, Zhang L (2017) Iron oxide shell mediated environmental remediation properties of nano zero-valent iron. Environ Sci Nano 4:27–45. https://doi.org/10.1039/C6EN00398B
Zhao C, Yang J, Wang Y, Jiang B (2017) Well-dispersed nanoscale zero-valent Iron supported in macroporous silica foams: synthesis, characterization, and performance in Cr(VI) removal. J Mater 2017:3094606. https://doi.org/10.1155/2017/3094606
Ou J-H, Sheu Y-T, Tsang DCW et al (2020) Application of iron/aluminum bimetallic nanoparticle system for chromium-contaminated groundwater remediation. Chemosphere 256:127158. https://doi.org/10.1016/j.chemosphere.2020.127158
Pasinszki T, Krebsz M (2020) Synthesis and application of zero-valent Iron nanoparticles in water treatment, environmental remediation, catalysis, and their biological effects. Nano 10:917. https://doi.org/10.3390/nano10050917
Zhang B-T, Zheng X, Li H-F, Lin J-M (2013) Application of carbon-based nanomaterials in sample preparation: a review. Anal Chim Acta 784:1–17. https://doi.org/10.1016/j.aca.2013.03.054
Zhu Y, Liu X, Hu Y et al (2019) Behavior, remediation effect and toxicity of nanomaterials in water environments. Environ Res 174:54–60. https://doi.org/10.1016/j.envres.2019.04.014
Berradi M, Hsissou R, Khudhair M et al (2019) Textile finishing dyes and their impact on aquatic environs. Heliyon 5:e02711. https://doi.org/10.1016/j.heliyon.2019.e02711
Ito T, Shimada Y, Suto T (2018) Potential use of bacteria collected from human hands for textile dye decolorization. Water Resour Ind 20:46–53. https://doi.org/10.1016/j.wri.2018.09.001
Jha P, Jobby R, Desai NS (2016) Remediation of textile azo dye acid red 114 by hairy roots of Ipomoea carnea Jacq. and assessment of degraded dye toxicity with human keratinocyte cell line. J Hazard Mater 311:158–167. https://doi.org/10.1016/j.jhazmat.2016.02.058
Ahsan MA, Jabbari V, Imam MA et al (2020) Nanoscale nickel metal organic framework decorated over graphene oxide and carbon nanotubes for water remediation. Sci Total Environ 698:134214. https://doi.org/10.1016/j.scitotenv.2019.134214
Jia J, Li T, Yao C et al (2020) Circulating differential miRNAs profiling and expression in hexavalent chromium exposed electroplating workers. Chemosphere 260:127546. https://doi.org/10.1016/j.chemosphere.2020.127546
Panda A, Patra DK, Acharya S et al (2020) Assessment of the phytoremediation potential of Zinnia elegans L. plant species for hexavalent chromium through pot experiment. Environ Technol Innov 20:101042. https://doi.org/10.1016/j.eti.2020.101042
Kullu B, Patra DK, Acharya S et al (2020) AM fungi mediated bioaccumulation of hexavalent chromium in Brachiaria mutica-a mycorrhizal phytoremediation approach. Chemosphere 258:127337. https://doi.org/10.1016/j.chemosphere.2020.127337
Zhong G, Huang J, Yao Z et al (2020) Intrinsic acid resistance and high removal performance from the incorporation of nickel nanoparticles into nitrogen doped tubular carbons for environmental remediation. J Colloid Interface Sci 566:46–59. https://doi.org/10.1016/j.jcis.2020.01.055
Chen Z, Mahmud S, Cai L et al (2020) Hierarchical poly(vinylidene fluoride)/active carbon composite membrane with self-confining functional carbon nanotube layer for intractable wastewater remediation. J Memb Sci 603:118041. https://doi.org/10.1016/j.memsci.2020.118041
Yin Z, Song L, Song H et al (2020) Remediation of copper contaminated sediments by granular activated carbon-supported titanium dioxide nanoparticles: mechanism study and effect on enzyme activities. Sci Total Environ 741:139962. https://doi.org/10.1016/j.scitotenv.2020.139962
Mandeep, Gulati A, Kakkar R (2020) Graphene-based adsorbents for water remediation by removal of organic pollutants: theoretical and experimental insights. Chem Eng Res Des 153:21–36. https://doi.org/10.1016/j.cherd.2019.10.013
Nhlane D, Richards H, Etale A (2020) Facile and green synthesis of reduced graphene oxide for remediation of Hg(II)-contaminated water. Mater Today Proc. https://doi.org/10.1016/j.matpr.2020.04.163
Khalifa AZ, Cizer Ö, Pontikes Y et al (2020) Advances in alkali-activation of clay minerals. Cem Concr Res 132:106050. https://doi.org/10.1016/j.cemconres.2020.106050
Phuekphong AF, Imwiset KJ, Ogawa M (2020) Designing nanoarchitecture for environmental remediation based on the clay minerals as building block. J Hazard Mater 399:122888. https://doi.org/10.1016/j.jhazmat.2020.122888
Carmona A, Malard V, Avazeri E et al (2018) Uranium exposure of human dopaminergic cells results in low cytotoxicity, accumulation within sub-cytoplasmic regions, and down regulation of MAO-B. Neurotoxicology 68:177–188. https://doi.org/10.1016/j.neuro.2018.07.019
Stojsavljević A, Borković-Mitić S, Vujotić L et al (2019) The human biomonitoring study in Serbia: background levels for arsenic, cadmium, lead, thorium and uranium in the whole blood of adult Serbian population. Ecotoxicol Environ Saf 169:402–409. https://doi.org/10.1016/j.ecoenv.2018.11.043
Diwan V, Sar SK, Biswas S, Lalwani R (2020) Adsorptive extraction of uranium(VI) from aqueous phase by dolomite. Groundw Sustain Dev 11:100424. https://doi.org/10.1016/j.gsd.2020.100424
El-Maghrabi HH, Younes AA, Salem AR et al (2019) Magnetically modified hydroxyapatite nanoparticles for the removal of uranium (VI): preparation, characterization and adsorption optimization. J Hazard Mater 378:120703. https://doi.org/10.1016/j.jhazmat.2019.05.096
Oulguidoum A, Bouyarmane H, Laghzizil A et al (2019) Development of sulfonate-functionalized hydroxyapatite nanoparticles for cadmium removal from aqueous solutions. Colloid Interface Sci Commun 30:100178. https://doi.org/10.1016/j.colcom.2019.100178
Arenas-Lago D, Abreu MM, Andrade Couce L, Vega FA (2019) Is nanoremediation an effective tool to reduce the bioavailable As, Pb and Sb contents in mine soils from Iberian Pyrite Belt? Catena 176:362–371. https://doi.org/10.1016/j.catena.2019.01.038
Liu X, Huang D, Lai C et al (2018) Recent advances in sensors for tetracycline antibiotics and their applications. TrAC Trends Anal Chem 109:260–274. https://doi.org/10.1016/j.trac.2018.10.011
Lei X, Xu T, Yao W et al (2020) Hollow hydroxyapatite microspheres modified by CdS nanoparticles for efficiently photocatalytic degradation of tetracycline. J Taiwan Inst Chem Eng 106:148–158. https://doi.org/10.1016/j.jtice.2019.10.023
Zeng Z, Fang S, Tang D et al (2019) Ultrasensitive sensor based on novel bismuth carbon nanomaterial for lead and cadmium determination in natural water, contaminated soil and human plasma. Microporous Mesoporous Mater 284:177–185. https://doi.org/10.1016/j.micromeso.2019.04.045
Zhou C, Wang X, Song X et al (2020) Insights into dynamic adsorption of lead by nano-hydroxyapatite prepared with two-stage ultrasound. Chemosphere 253:126661. https://doi.org/10.1016/j.chemosphere.2020.126661
Hayashi T, Nakamura K, Suzuki T et al (2020) OH radical formation by the photocatalytic reduction reactions of H2O2 on the surface of plasmonic excited Au-TiO2 photocatalysts. Chem Phys Lett 739:136958. https://doi.org/10.1016/j.cplett.2019.136958
Shoueir K, Kandil S, El-hosainy H, El-Kemary M (2019) Tailoring the surface reactivity of plasmonic Au@TiO2 photocatalyst bio-based chitosan fiber towards cleaner of harmful water pollutants under visible-light irradiation. J Clean Prod 230:383–393. https://doi.org/10.1016/j.jclepro.2019.05.103
Veziroglu S, Ullrich M, Hussain M et al (2020) Plasmonic and non-plasmonic contributions on photocatalytic activity of Au-TiO2 thin film under mixed UV–visible light. Surf Coatings Technol 389:125613. https://doi.org/10.1016/j.surfcoat.2020.125613
Lin C, Ma J, Yi F et al (2020) Ag NPs modified plasmonic Z-scheme photocatalyst Bi4Ti3O12/Ag/Ag3PO4 with improved performance for pollutants removal under visible light irradiation. Ceram Int 46:14650–14661. https://doi.org/10.1016/j.ceramint.2020.02.266
Wang D, Pillai SC, Ho S-H et al (2018) Plasmonic-based nanomaterials for environmental remediation. Appl Catal B Environ 237:721–741. https://doi.org/10.1016/j.apcatb.2018.05.094
Patil SS, Shedbalkar UU, Truskewycz A et al (2016) Nanoparticles for environmental clean-up: a review of potential risks and emerging solutions. Environ Technol Innov 5:10–21. https://doi.org/10.1016/j.eti.2015.11.001
Basak G, Hazra C, Sen R (2020) Biofunctionalized nanomaterials for in situ clean-up of hydrocarbon contamination: a quantum jump in global bioremediation research. J Environ Manage 256:109913. https://doi.org/10.1016/j.jenvman.2019.109913
Cecchin I, Reddy KR, Thomé A et al (2017) Nanobioremediation: integration of nanoparticles and bioremediation for sustainable remediation of chlorinated organic contaminants in soils. Int Biodeterior Biodegradation 119:419–428. https://doi.org/10.1016/j.ibiod.2016.09.027
Han B, Zhang M, Zhao D (2017) In-situ degradation of soil-sorbed 17β-estradiol using carboxymethyl cellulose stabilized manganese oxide nanoparticles: column studies. Environ Pollut 223:238–246. https://doi.org/10.1016/j.envpol.2017.01.018
Mandal SK, Ojha N, Das N (2018) Optimization of process parameters for the yeast mediated degradation of benzo[a]pyrene in presence of ZnO nanoparticles and produced biosurfactant using 3-level box-Behnken design. Ecol Eng 120:497–503. https://doi.org/10.1016/j.ecoleng.2018.07.006
Darwesh OM, Matter IA, Eida MF (2019) Development of peroxidase enzyme immobilized magnetic nanoparticles for bioremediation of textile wastewater dye. J Environ Chem Eng 7:102805. https://doi.org/10.1016/j.jece.2018.11.049
Chatterjee S, Mahanty S, Das P et al (2020) Biofabrication of iron oxide nanoparticles using manglicolous fungus Aspergillus niger BSC-1 and removal of Cr(VI) from aqueous solution. Chem Eng J 385:123790. https://doi.org/10.1016/j.cej.2019.123790
Bhattacharya P, Mukherjee D, Deb N et al (2020) Application of green synthesized ZnO nanoparticle coated ceramic ultrafiltration membrane for remediation of pharmaceutical components from synthetic water: reusability assay of treated water on seed germination. Heliyon 6:e04508. https://doi.org/10.1016/j.jece.2020.103803
Akintelu SA, Folorunso AS, Folorunso FA, Oyebamiji AK (2020) Green synthesis of copper oxide nanoparticles for biomedical application and environmental remediation. Heliyon 6:e04508. https://doi.org/10.1016/j.heliyon.2020.e04508
Libralato G, Minetto D, Lofrano G et al (2018) Toxicity assessment within the application of in situ contaminated sediment remediation technologies: a review. Sci Total Environ 621:85–94. https://doi.org/10.1016/j.scitotenv.2017.11.229
Huang Z, Zeng Z, Chen A et al (2018) Differential behaviors of silver nanoparticles and silver ions towards cysteine: bioremediation and toxicity to Phanerochaete chrysosporium. Chemosphere 203:199–208. https://doi.org/10.1016/j.chemosphere.2018.03.144
Gholami F, Mosmeri H, Shavandi M et al (2019) Application of encapsulated magnesium peroxide (MgO2) nanoparticles in permeable reactive barrier (PRB) for naphthalene and toluene bioremediation from groundwater. Sci Total Environ 655:633–640. https://doi.org/10.1016/j.scitotenv.2018.11.253
Fernando IPS, Lee W, Han EJ, Ahn G (2020) Alginate-based nanomaterials: fabrication techniques, properties, and applications. Chem Eng J 391:123823. https://doi.org/10.1016/j.cej.2019.123823
Sargin I, Baran T, Arslan G (2020) Environmental remediation by chitosan-carbon nanotube supported palladium nanoparticles: conversion of toxic nitroarenes into aromatic amines, degradation of dye pollutants and green synthesis of biaryls. Sep Purif Technol 247:116987. https://doi.org/10.1016/j.seppur.2020.116987
Nguyen NHA, Von Moos NR, Slaveykova VI et al (2018) Biological effects of four iron-containing nanoremediation materials on the green alga Chlamydomonas sp. Ecotoxicol Environ Saf 154:36–44. https://doi.org/10.1016/j.ecoenv.2018.02.027
Corsi I, Winther-Nielsen M, Sethi R et al (2018) Ecofriendly nanotechnologies and nanomaterials for environmental applications: key issue and consensus recommendations for sustainable and ecosafe nanoremediation. Ecotoxicol Environ Saf 154:237–244. https://doi.org/10.1016/j.ecoenv.2018.02.037
Zhang Q, Wang M, Gu C, Zhang C (2019) Water disinfection processes change the cytotoxicity of C60 fullerene: reactions at the nano-bio interface. Water Res 163:114867. https://doi.org/10.1016/j.watres.2019.114867
Kyzyma OA, Avdeev MV, Bolshakova OI et al (2019) State of aggregation and toxicity of aqueous fullerene solutions. Appl Surf Sci 483:69–75. https://doi.org/10.1016/j.apsusc.2019.03.167
Sánchez A, Recillas S, Font X et al (2011) Ecotoxicity of, and remediation with, engineered inorganic nanoparticles in the environment. TrAC Trends Anal Chem 30:507–516. https://doi.org/10.1016/j.trac.2010.11.011
Tang F, Yu H, Yassin Hussain Abdalkarim S et al (2020) Green acid-free hydrolysis of wasted pomelo peel to produce carboxylated cellulose nanofibers with super absorption/flocculation ability for environmental remediation materials. Chem Eng J 395:125070. https://doi.org/10.1016/j.cej.2020.125070
Choi HY, Bae JH, Hasegawa Y et al (2020) Thiol-functionalized cellulose nanofiber membranes for the effective adsorption of heavy metal ions in water. Carbohydr Polym 234:115881. https://doi.org/10.1016/j.carbpol.2020.115881
Darabitabar F, Yavari V, Hedayati A et al (2020) Novel cellulose nanofiber aerogel for aquaculture wastewater treatment. Environ Technol Innov 18:100786. https://doi.org/10.1016/j.eti.2020.100786
Abu-Danso E, Peräniemi S, Leiviskä T, Bhatnagar A (2018) Synthesis of S-ligand tethered cellulose nanofibers for efficient removal of Pb(II) and Cd(II) ions from synthetic and industrial wastewater. Environ Pollut 242:1988–1997. https://doi.org/10.1016/j.envpol.2018.07.044
Gomes MADC, Hauser-Davis RA, de Souza AN, Vitória AP (2016) Metal phytoremediation: general strategies, genetically modified plants and applications in metal nanoparticle contamination. Ecotoxicol Environ Saf 134:133–147. https://doi.org/10.1016/j.ecoenv.2016.08.024
Ebrahimbabaie P, Meeinkuirt W, Pichtel J (2020) Phytoremediation of engineered nanoparticles using aquatic plants: mechanisms and practical feasibility. J Environ Sci 93:151–163. https://doi.org/10.1016/j.jes.2020.03.034
Fernandes JP, Mucha AP, Francisco T et al (2017) Silver nanoparticles uptake by salt marsh plants – implications for phytoremediation processes and effects in microbial community dynamics. Mar Pollut Bull 119:176–183. https://doi.org/10.1016/j.marpolbul.2017.03.052
Fernandes JP, Almeida CMR, Andreotti F et al (2017) Response of microbial communities colonizing salt marsh plants rhizosphere to copper oxide nanoparticles contamination and its implications for phytoremediation processes. Sci Total Environ 581–582:801–810. https://doi.org/10.1016/j.scitotenv.2017.01.015
Hanks NA, Caruso JA, Zhang P (2015) Assessing Pistia stratiotes for phytoremediation of silver nanoparticles and Ag(I) contaminated waters. J Environ Manage 164:41–45. https://doi.org/10.1016/j.jenvman.2015.08.026
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this entry
Cite this entry
González, V. (2021). Nanoremediation and Nanobioremediation in Water Treatment: The Search for an Eco-friendly Alternative. In: Kharissova, O.V., Torres-Martínez, L.M., Kharisov, B.I. (eds) Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications. Springer, Cham. https://doi.org/10.1007/978-3-030-36268-3_28
Download citation
DOI: https://doi.org/10.1007/978-3-030-36268-3_28
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-36267-6
Online ISBN: 978-3-030-36268-3
eBook Packages: Chemistry and Materials ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics