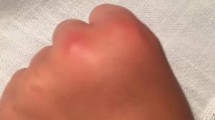
Abstract
Urticaria is a heterogeneous skin disease involving episodic wheals and/or angioedema, which occurs in 10–20% of people at some point in life. Although there are a wide array of etiologies, including infections, medications, allergic reactions, or physical stimuli, most cases remain idiopathic. Many systemic disorders are associated with urticaria, such as various forms of vasculitis, mastocytosis, or rheumatologic illnesses. Diagnosis is largely made by history and exam, at times involving a provocation test to reproduce the lesions if the history suggests inducible (aka physical) urticaria. Treatment with long-acting non-sedating H1-antihistamines is effective in over 50% of cases, but when not, other therapies such as biologics, immunosuppressive agents, or other anti-inflammatory agents may be necessary to control the hives.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
1 Introduction and Definition
Urticaria is a heterogeneous skin condition characterized by episodic appearance of wheals and/or angioedema. Wheals are cutaneous swellings of variable size, typically with reflex erythema which is usually very pruritic but could manifest as a burning sensation in some cases. Wheals manifest as a result of extravasation of fluid into epidermal spaces, with return of normal skin appearance in about 1–24 h. Angioedema, in contrast, is defined as rapid and marked extravasation of fluid into deeper dermis tissue spaces. This results in swelling which may be characterized by pain due to stretching nerve fibers, rather than pruritus, with significantly slower resolution, on the order of 1–3 days (Zuberbier et al. 2018; Godse et al. 2018; Kaplan 2002; Bernstein et al. 2014).
Acute urticaria involves episodic hives which last less than 6 weeks, whereas chronic urticaria involves symptoms on most days of the week for more than 6 weeks. The prevalence of urticaria is estimated to affect up to 20% of the general population at some point in life, but the etiology is rarely elucidated (Greaves 1995).
2 Epidemiology and Natural History
Acute urticaria is thought to affect approximately 10–20% of people at some point in life, with development of chronic spontaneous urticaria in approximately 1% of the population (Greaves 1995). Although more common in adults, it can also afflict children, but epidemiologic data on this population is lacking. Women seem to be affected about twice as frequently as men, typically starting in the third to fifth decades of life. Urticaria affects up to 1% of the general population in the United States at any particular point in time with similar prevalence described in other countries (Zuberbier et al. 2010; Gaig et al. 2004; Cooper 1991; Champion et al. 1969; Ferrer 2009; Juhlin 1981). Chronic urticaria is often a self-limited disorder, with average disease duration of 2–5 years (Greaves 2000). In patients with no clear etiology or identified underlying cause of urticaria, 30–50% will have spontaneous remission at 1 year. However, it is not uncommon for symptoms to persist for many years (Kulp-Shorten and Callen 1996; Kozel et al. 2001; Kulthanan et al. 2007; Gaig et al. 2004). A study in Spain indicated a prevalence of urticaria of 0.8% in the past year and prevalence of chronic urticaria of 0.6%. In this study, mean age of urticaria was 40 years, with disease duration of 1–5 years in 8.7% of study subjects and more than 5 years in 11.3% of study subjects (Gaig et al. 2004). Angioedema with concomitant hives is present in 40–50% of patients with chronic spontaneous urticaria. About 10% of patients experience angioedema alone without hives, while about 40% of patients exhibit hives alone (Greaves 2000; Kaplan 2002; Grattan 2004; Zuberbier et al. 2018).
3 Etiologies, Classification, and Pathophysiology
Diagnosis and classification of urticaria and angioedema are made largely by history (Charlesworth 1996; Beltrani 1996, 2004). Urticaria can be classified into various types and subtypes based on different eliciting stimuli. Most forms of urticaria follow into one of three broad categories: spontaneous urticaria, physical urticaria, or special/uncommon causes of urticaria (Sanchez-Borges et al. 2012; Lang et al. 2013; Zuberbier et al. 2018). Spontaneous urticaria includes acute spontaneous urticaria (episodic spontaneous hives and/or angioedema of less than 6 weeks duration) and chronic spontaneous urticaria (episodic hives and/or angioedema lasting more than 6 weeks duration).
The signs and symptoms of urticaria are mediated by cutaneous mast cells and basophils in the superficial dermis. Upon activation of mast cells and basophils, a variety of mediators are released, including histamine that causes the characteristic pruritus and vasodilation resulting in localized swelling in the epidermis in the case of hives and angioedema when the swelling extends to the deeper dermis/subcutaneous tissue (see Fig. 1) (Ying et al. 2002; Beck et al. 2017).
Pathogenesis of chronic urticaria (CU). CU signs and symptoms develop when skin mast cells or basophils degranulate and release histamine and other proinflammatory mediators. In chronic spontaneous urticaria, the degranulation of these cells in some patients is thought to be due to the effects of autoantibodies directed against a subunit of the high-affinity IgE receptor, FcƐRIa, or to IgE itself. Other mechanisms of mast cell or basophil activation that are potentially relevant to chronic spontaneous urticaria involve autoantigens and IgE directed against these autoantigens, as well as complement components, cytokines, and neuropeptides. TPO thyroperoxidase (Beck et al. 2017)
There are myriad of potential etiologies for urticaria. There is a greater likelihood of identifying a specific trigger for acute urticaria compared to chronic urticaria. Causes include foods; medications (Fernandez et al. 2017; Kuyucu et al. 2014; Martin-Serrano et al. 2016); envenomation due to insect stings (Matysiak et al. 2013); latex exposure through recreational, occupational, or surgical/dental application (Sussman and Beezhold 1995); and a number of contactants from plant, animal, or occupational exposures (Bourrain 2006).
Infections represent another common cause of urticaria. Viral or bacterial infections, especially in children, are a particularly common cause of urticaria, with reports of as high as 80% of acute urticaria in children being attributed to viral or bacterial infections (Sackesen et al. 2004; Mortureux et al. 1998; Minciullo et al. 2014; Imbalzano et al. 2016; Plumb et al. 2001). In studies where children were evaluated in emergency departments with urticaria in a setting of sick symptoms, viral and bacterial illness were the leading identifiable trigger for urticaria (Mortureux et al. 1998). In one study in which children with sick symptoms, also on beta-lactams, were tested for both viral illness and re-exposed to beta-lactam, roughly 66% were positive for viral illness, while only 4% had recurrence of urticaria with re-exposure to the antibiotic (Mortureux et al. 1998; Caubet et al. 2011). Mycoplasma pneumoniae infection in children has been documented to cause acute urticaria that is refractory to antihistamines but responsive to azithromycin (Wu et al. 2009; Shah et al. 2007). Parasitic infections have been well-characterized as a cause of acute, self-limited urticaria in association with peripheral eosinophilia. Examples include Strongyloides, Filaria, Echinococcus, Trichinella, and Toxocara species (Di Campli et al. 1998).
Nonsteroidal anti-inflammatory drugs (NSAIDs) are an important trigger of urticaria and angioedema. This can occur either by an immediate-type hypersensitivity or by a pharmacologic or pseudoallergic reaction, in which an agent such as ibuprofen or aspirin inhibits cyclooxygenase-1 enzyme resulting in urticaria, presumably due to that individual having an underlying anomaly in arachidonic acid metabolism (Moore-Robinson and Warin 1967; Warin 1960; Champion et al. 1969).
Another trigger of acute urticaria includes the direct activation of mast cells through specific non-IgE receptors. For example, vancomycin infusion causing “red man syndrome” is a common inpatient cause of urticaria in both children and adults. Human and animal studies of red man syndrome indicate that histamine and other vasoactive mediators are released by direct mast cell activation, with some studies indicating that degree of serum histamine release relating directly to clinical severity of disease (Healy et al. 1990). The mechanism is thought to involve non-immunologic mast cell activation of phospholipase C and phospholipase A2 pathways and may partially occur in an extracellular calcium-dependent manner (Horinouchi et al. 1993; Veien et al. 2000). Often related to the rate of infusion, the phenomenon can be ameliorated by either slowing down the infusion and/or pre-treating with antihistamines (Healy et al. 1990; Renz et al. 1998; Newfield and Roizen 1979; Veien et al. 2000; Wallace et al. 1991). Other triggers of direct mast cell activation include opiates and their derivative products, radiocontrast media, foods high in lectins and/or histamine such as strawberries and tomatoes, or the stinging nettle plant Urtica dioica , from which the disorder “urticaria” derives its name (Robledo et al. 2004; Cochran 2005; Plumb et al. 2001; Anderson et al. 2003; Cummings and Olsen 2011; Uslu et al. 2011).
Although rare, there have been several case reports of urticaria triggered by progesterone-containing oral contraceptives or progesterone-containing hormone replacement therapies (Poole and Rosenwasser 2004; Shank et al. 2009; Bernstein et al. 2011).
Several systemic syndromes where urticaria may be a prominent or presenting symptom include urticarial vasculitis, cutaneous small-vessel vasculitis, systemic mastocytosis, systemic lupus erythematosus, rheumatoid arthritis, or other autoimmune disorders (Confino-Cohen et al. 2012).
4 Chronic Urticaria
Chronic urticaria (CU) is defined as episodic hives occurring most days of the week for 6 or more weeks. Approximately 40% of patients with CU also experience angioedema (Greaves 2000). In the United States, CU has a prevalence of about 1% in the general population, with similar prevalence reported in other countries (Gaig et al. 2004; Greaves 2000; Lapi et al. 2016). CU affects both children and adults, although it is more common in adults. Women are twice as likely as men to be affected. CU can occur at any time but typically begins in the third to fifth decades of life (Confino-Cohen et al. 2012).
The diagnosis of CU is made clinically based on history and exam (see Table 1). Initial extensive laboratory work-up for CU, unless there are specific clues in the history, is not recommended as studies have demonstrated that empiric blood testing does not impact the management of disease in most cases (Tarbox et al. 2011). However, both the US and international guidelines agree that a routine complete blood count with differential, C-reactive protein and/or erythrocyte sedimentation rate should be obtained at diagnosis. A thyroid-stimulating hormone may also be appropriate in many cases (Jacobson et al. 1980; Jirapongsananuruk et al. 2010). As many as 80–90% of adults and children with CU have no specifically identified trigger and are thus diagnosed with chronic idiopathic urticaria (CIU). Skin biopsy is not routinely recommended for CU, but is indicated to exclude potentially concerning disease processes in the presence of other signs/symptoms, such as urticarial vasculitis. CU is typically a self-limited disease process. Spontaneous remission occurs in 30–50% of patients within 1 year, with an average disease duration of 2–5 years, and only 20% of patients having persistent symptoms beyond 5 years (Kulthanan et al. 2007; Harris et al. 1983). However, patients with a physical/inducible component tend to have a more protracted course (Kozel et al. 2001).
5 Antibody-Associated or Autoimmune Urticaria
Autoantibody-associated urticaria involves the presence of autoantibodies such as thyroid autoantibodies or IgE receptor autoantibodies with concomitant urticaria and is considered a subset of chronic idiopathic urticaria. A large study of nearly 13,000 patients with CU compared to over 10,000 control patients indicated increased prevalence of numerous autoimmune disorders, including thyroid disorders, celiac disease, Sjögren syndrome, systemic lupus erythematosus, dermatomyositis, polymyositis, rheumatoid arthritis, and type 1 diabetes mellitus in CU patients. In particular, in patients with CU, hypothyroidism was diagnosed in 9.8% of subjects (compared to 0.6% of controls) and hyperthyroidism in 2.6% of subjects (compared to 0.5% of controls) (Confino-Cohen et al. 2012). A study in Korea indicated that individuals with Hashimoto’s thyroiditis and Graves’ disease had higher rates of CU compared to control subjects (hazard ratio 1.5, 95% confidence interval 1.3–1.7) (Kim et al. 2017).
Thyroid autoantibodies, including thyroid peroxidase antibodies and antimicrosomal antibodies, as well as antinuclear antibodies, are more prevalent in patients with CU compared to the general population (Leznoff et al. 1983). However, the presence of autoantibodies does not necessarily correlate with autoimmune disease. For example, detection of serum thyroid autoantibodies does not necessarily correlate with thyroid dysfunction, and the majority of patients with CU and detectable thyroid autoantibodies have normal thyroid function. Furthermore, treatment with thyroid supplement in these patients has not been demonstrated to control urticaria. Thus, serology to diagnose underlying autoimmune disease in initial evaluation of CU is not warranted in the absence of additional attributes suggestive of concomitant autoimmune disease. The role of autoantibodies in CU is unclear, as it may simply reflect an underlying tendency toward the production of autoantibodies. Interestingly, patients with detectable thyroid autoantibodies who are euthyroid are often poorer responders to standard therapy for CU. The role of IgE antibodies to high-affinity IgE receptors (FcER1 alpha subunit) on mast cells and basophils is also unclear. Autologous serum skin testing and the serologic chronic urticaria index (CUI) assay are not predictive of response to therapy, and therefore, their clinical relevance is still poorly elucidated. Of note, a recent study suggests that patients with FcER1 alpha subunit antibodies refractory to high-dose H1-antihistamines may be slower to respond to omalizumab (Leznoff et al. 1983; Kaplan and Greaves 2009; Kikuchi et al. 2003; Najib et al. 2009; Greiwe and Bernstein 2017).
6 Physical or Inducible Urticarias
Physical urticaria, now referred to as inducible urticaria, is a subgroup of chronic urticaria characterized by hives that are reproducibly triggered by physical stimuli, such as scratching of the skin (dermatographism), exposure to cold, physical pressure, exercise, sunlight, heat, and rarely water or vibration (see Table 2). These same physical triggers can also provoke angioedema (Lang et al. 2013; Sanchez-Borges et al. 2012). The term inducible has replaced physical as there are cholinergic and less commonly adrenergic urticaria conditions that are induced by stimuli which provoke the autonomic nervous system such as stress and emotions.
Dermatographism is urticaria that occurs in response to stroking the skin with a firm object, such as a tongue blade or an instrument with a firm edge. Simple dermatographism is present in about 2–5% of the population, while only a minority of people have symptoms to a degree that prompts medical attention (Orfan and Kolski 1993; Kirby et al. 1971). Initially, a white line develops on the skin as a consequence of reflex vasoconstriction. This is followed by development of a linear raised swelling at the challenge site. The response typically occurs within 1–3 min and resolves in about 30 min (Orfan and Kolski 1993; Bernstein et al. 2014; Sanchez-Borges et al. 2012).
Cold urticaria involves hives elicited by cold fluids, air, wind, or contact with cold objects. Provocative cold testing, such as an ice cube challenge, can confirm diagnosis of cold urticaria. A common method is to place an ice cube (0 °C to 4 °C) contained in a plastic bag on the forearm for 5 min, followed by observing the challenge site as skin rewarms to room temperature. Development of a wheal or flare response during skin rewarming is a positive test (Wanderer et al. 1986). If, after 5 min, there is no observed reaction, the test may be repeated incrementally up to 10 min. The optimal duration of challenge testing to exclude cold urticaria has not been determined (Wanderer and Hoffman 2004). Notably, a cold stimulus should not be reapplied at a site previously challenged, as this could result in a “false-negative” result due to local desensitization of skin. Variants of acquired cold urticaria have been described in which provocative cold testing is negative. The variants include systemic atypical acquired cold urticaria, cold-dependent dermatographism, cold-induced cholinergic urticaria, acquired delayed cold urticaria, and localized cold reflex urticaria (Wanderer and Hoffman 2004).
Delayed pressure urticaria and angioedema (DPUA) involves the development of swelling in response to exposure to a pressure stimulus about 30 min to 12 h (peak of 4–6.5 h) after exposure to the stimulus (Ryan et al. 1968; Czarnetzki et al. 1984; Sussman et al. 1982; Dover et al. 1988; Warin 1989). Biopsy of angioedema lesions brought about by a pressure stimulus exhibits an intense inflammatory infiltrate characterized histologically by an infiltrate rich in both eosinophils and neutrophils in the deeper dermis and subcutaneous tissue (Winkelmann et al. 1986; Mekori et al. 1988). Diagnosis of DPUA can be confirmed by application of a pressure stimulus, such as a weight or force, to a specific area of skin, with subsequent development of angioedema at the challenge site after 4–6 h. Various published protocols indicate different pressure stimuli to be used and the challenge duration. Positive and negative values for this challenge procedure have not been determined (Estes and Yung 1981). One example of a recommended challenge involves suspension of a 15 pound weight across the patient’s shoulder for 10–15 min (Ryan et al. 1968; Sussman et al. 1982). A painful reaction at the challenge site 2–12 h later (peak swelling at 4–6.5 h) is a positive response. Other approaches include the use of calibrated dermographometer or use of weighted metal rods. The challenge procedure should only be performed if concomitant chronic idiopathic urticaria and angioedema (which may also be present in patients with DPUA) are reasonably well controlled (Estes and Yung 1981; Lawlor et al. 1989; Illig and Kunick 1969).
Exercise-induced urticaria and angioedema are forms of physical urticaria that can be confirmed by an exercise challenge in a controlled setting (Sheffer et al. 1983, 1985). As exercise increases one’s risk for anaphylaxis, this challenge should only be performed in a setting with appropriately trained personnel, supplies, and equipment to handle management and treatment of such a possibility. In patients with a specific food (i.e., celery) linked to exercise-induced urticaria and angioedema, the relevance of the specific food suspected by history can be assessed with immediate hypersensitivity skin testing if patients aren’t dermatographic or on prophylactic H1-antihistamines or by in vitro serum-specific IgE antibody. If food-associated exercise-induced urticaria and angioedema are still suspected, then a challenge procedure in a supervised setting can be performed with and without food consumption. It is important for the clinician to be mindful that urticaria may also occur during an exercise challenge in patients with cholinergic urticaria as exercise increases body temperature. In this case, the diagnosis of cholinergic urticaria can be confirmed by passive heating and/or intracutaneous injection of methacholine. Furthermore, the morphology of lesions can be used to distinguish these two conditions (Sheffer et al. 1983; Kaplan et al. 1981; Casale et al. 1986)
Solar urticaria is provoked by ultraviolet and/or visible light. The diagnosis is confirmed with photo-testing, to stimulate provocation of urticarial lesions with sunlight. Reactions are more often observed with ultraviolet (UVA) or visible wavelengths and less commonly with UVB or infrared wavelengths (Farr 2000). One common provocation test involves using a xenon arc lamp with monochromator to ascertain the minimal urticarial dose at different wavelengths of light. A non-sun-exposed portion of the skin, such as mid and lower back, is ideal for photo-testing. Other light sources, such as slide projector light bulb for physical light, fluorescent black light or fluorescent sunlamp for UVA and UVB wavelengths, or infrared lamp for infrared wavelengths can also be used if a xenon lamp with monochromator is unavailable (Roelandts 2003; Alora and Taylor 1998; Uetsu et al. 2000). For each light source or wavelength used, a positive challenge results if a pruritic erythematous wheal develops during or shortly after irradiation and fades within a few minutes after removal of the light stimulus (Roelandts 2003). It is important to distinguish solar urticaria from a polymorphous light eruption. Lesions of polymorphous light eruptions tend to last more than 24 h, in contrast to the short-lived lesions of solar urticaria. Erythropoietic protoporphyria involves lesions that are painful, rather than pruritic, and typically are associated with a positive family history and elevated protoporphyrin levels (Murphy 2003; Fesq et al. 2003).
Cholinergic urticaria is a phenomenon in which an increase in body temperature, either passively or actively, results in sweat release and subsequent provocation of urticaria. The diagnosis can be confirmed by intracutaneous injection of 0.01 mg of methacholine in 0.1 mL of saline with subsequent formation of at least one hive. Unfortunately, this technique has poor sensitivity since as little as 33% of patients with cholinergic urticaria will have a positive methacholine test response and responses that are positive are not always consistently reproducible. Therefore, this test has a poor negative predictive value, and although this test may confirm a diagnosis if positive, it cannot definitively rule out diagnosis if negative (Commens and Greaves 1978). Challenges that increase body temperature, such as hot water immersion or exercise, may have higher sensitivity. For example, partial immersion of a patient in a 42 °C bath, leading to a 0.7 °C body temperature increase, resulting in hives may have a higher sensitivity (Orfan and Kolski 1993). Finally, some patients with cholinergic urticaria may exhibit a wheal and flare response to autologous diluted sweat, suggesting that the sweat of these patients contain factors that lead to histamine release (Fukunaga et al. 2005). It has been reported in such patients that rapid desensitization to autologous sweat has been shown to be as efficacious as therapeutic intervention. However, sweat may be a different entity and not reflective of cholinergic hives (Kozaru et al. 2011).
Vibratory angioedema involves the development of angioedema after exposure to an intense vibratory stimulus. The diagnosis can be confirmed by an exaggerated reaction to the stimulation of the skin with a vortex mixer. There are currently no standardized recommendations regarding the optimal vibratory stimulus to use, duration of exposure to vibration, or grading of a positive reaction. One generally accepted challenge procedure entails supporting a patient’s forearm under the wrist and elbow, so the skin of the forearm, hand, or finger rests in the rubber cup of a vortex mixer. The mixer is vibrated at constant speed for 1–5 min. Subsequent development of erythema and edema that is sharply demarcated from normal skin within 4 min of simulation and persistent for 1 h defines a positive response. If desired, the response can be quantified by measuring the change in the forearm circumference or finger volume (Patterson et al. 1972; Metzger et al. 1976). Delayed onset of erythema and pruritus after vibratory provocation has been reported with peak symptoms occurring 4–6 h after the vibratory stimulus (Keahey et al. 1987).
Aquagenic urticaria is a water-induced etiology with diagnosis confirmed by hives following direct water exposure. One way to confirm the diagnosis is application of a water compress at 35 °C to the upper body skin for 30 min (Baptist and Baldwin 2005). The appearance of punctate 1–3 mm hives at site of application is considered a positive response. This diagnosis should be distinguished from other disorders including aquagenic pruritus, in which water exposure provokes itching but without wheal formation (Greaves et al. 1981); cold urticaria, which is induced by cold rather than water; and cholinergic urticaria, in which punctate lesions manifest in response to heat, rather than water. Notably, cases of concurrent aquagenic urticaria with cold or cholinergic urticaria have been reported (Davis et al. 1981; Mathelier-Fusade et al. 1997).
7 Treatment of Acute and Chronic Urticaria
The treatment of acute and chronic urticaria begins with the use of H1 non-sedating antihistamines which can be dosed 1–4 times the Food and Drug Administration (FDA)-approved recommended dose. Treatment begins at a step appropriate for the patient’s level of severity and previous treatment history. At each level of the stepwise algorithm, medication(s) should be assessed for patient adherence, tolerance, and efficacy. Once consistent control of urticaria/angioedema is achieved (usually 3–6 months after complete control of hives), a “step-down” approach to treatment can begin (Bernstein et al. 2014; Fine and Bernstein 2016). The US and international guideline treatment algorithms are illustrated and compared regarding similarities and differences in Fig. 2. For the US guidelines, Step 1 involves starting monotherapy with a second-generation non-sedating H1-antihistamine, such as cetirizine, in addition to strict avoidance of suspected or known triggers (such as NSAIDs) and any relevant physical factors if a form of inducible urticaria/angioedema syndrome is present. Step 2 comprises one or more of the following: increasing the dose of the second-generation antihistamine started in Step 1 to 2–4 times the original dose (maximum dose 4× the approved treatment dose), adding another second-generation antihistamine, adding an H2-receptor antagonist medication, adding a leukotriene receptor antagonist, and/or adding a first-generation antihistamine to be taken at bedtime. Recent international guidelines object to using a combination of second-generation antihistamines or a first-generation antihistamine due to the lack of scientific evidence. Concerns about first-generation antihistamines are related to their sedating effects which can affect cognition and motor coordination. Step 3 therapy includes dose advancement to a more potent combination antihistamine (such as doxepin or hydroxyzine) as tolerated. Again, this step is not recommended by the international guidelines due to sedation affecting cognition and mental performance. Finally, Step 4 therapy in the US guidelines, which is Step 3 in the international guidelines, recommends adding an alternative agent, such as cyclosporine, omalizumab, or other anti-inflammatory therapies such as hydroxychloroquine, sulfasalazine, dapsone, or colchicine. The international guidelines only recommend omalizumab as Step 3 therapy due to the strength of medical evidence supporting this treatment for hives. For the international guidelines, Step 4 involves starting cyclosporine. This treatment is recommended after omalizumab due to a less robust strength of evidence and its toxicity. Oral corticosteroids may be used short term (1–3 weeks maximum) for exacerbations of urticaria or angioedema but are not recommended on a frequent or continuous basis due to short-term and long-term side effects (Zuberbier et al. 2014). A number of therapies recommended by the US guidelines such as montelukast and H2-antihistamines for Step 1 therapy, sedating combination and/or first-generation antihistamines for Step 3 therapy, or anti-inflammatory agents for Step 4 therapy are not recommended by the international guidelines; rather they are relegated to an “alternative treatment” box because of low level of scientific evidence supporting their use (Table 3) (Zuberbier and Bernstein 2018). However, clinicians can use these agents in the proper context for the treatment of their patients unresponsive or incompletely responsive to antihistamines.
Comparison of the international and US urticaria guideline treatment algorithms (Zuberbier and Bernstein 2018). EAACI, European Academy of Allergy and Clinical Immunology; fgAH, first-generation antihistamine; LTRA, leukotriene receptor antagonist; sgAH, second-generation antihistamine; WAO, World Allergy Organization. *Different spellings as used in respective guideline. Additional comments: EAACI/WAO: A short course of corticosteroids may be considered in case of severe exacerbation. AAAAI/ACAAI: Begin treatment at step appropriate for patient’s level of severity and treatment history; “step-down” treatment is appropriate at any step, once consistent control of urticaria/angioedema is achieved. Used with permission from Zuberbier and Bernstein “A Comparison of the United States and International Perspective on Chronic Urticaria Guidelines”, Journal of Allergy and Clinical Immunology in Practice, 2018 May 18
8 Conclusions
Acute and chronic urticaria can be challenging conditions to evaluate and treat. However, if guidelines are followed in an algorithmic manner, the majority of these cases can be treated very successfully which should result in improvement in patient quality of life, decreased morbidity, and reduced health care costs. The clinician should be knowledgeable about the US urticaria guidelines as well as the recent international guidelines and how they agree and differ.
References
Alora MB, Taylor CR. Solar urticaria: case report and phototesting with lasers. J Am Acad Dermatol. 1998;38:341–3.
Anderson BE, Miller CJ, Adams DR. Stinging nettle dermatitis. Am J Contact Dermat. 2003;14:44–6.
Baptist AP, Baldwin JL. Aquagenic urticaria with extracutaneous manifestations. Allergy Asthma Proc. 2005;26:217–20.
Beck LA, Bernstein JA, Maurer M. A review of international recommendations for the diagnosis and management of chronic urticaria. Acta Derm Venereol. 2017;97:149–58.
Beltrani VS. Urticaria and angioedema. Dermatol Clin. 1996;14:171–98.
Beltrani VS. Urticaria: reassessed. Allergy Asthma Proc. 2004;25:143–9.
Bernstein IL, Bernstein DI, Lummus ZL, Bernstein JA. A case of progesterone-induced anaphylaxis, cyclic urticaria/angioedema, and autoimmune dermatitis. J Womens Health (Larchmt). 2011;20:643–8.
Bernstein JA, Lang DM, Khan DA, Craig T, Dreyfus D, Hsieh F, Sheikh J, Weldon D, Zuraw B, Bernstein DI, Blessing-Moore J, Cox L, Nicklas RA, Oppenheimer J, Portnoy JM, Randolph CR, Schuller DE, Spector SL, Tilles SA, Wallace D. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270–7.
Bourrain JL. Occupational contact urticaria. Clin Rev Allergy Immunol. 2006;30:39–46.
Casale TB, Keahey TM, Kaliner M. Exercise-induced anaphylactic syndromes. Insights into diagnostic and pathophysiologic features. JAMA. 1986;255:2049–53.
Caubet JC, Kaiser L, Lemaitre B, Fellay B, Gervaix A, Eigenmann PA. The role of penicillin in benign skin rashes in childhood: a prospective study based on drug rechallenge. J Allergy Clin Immunol. 2011;127:218–22.
Champion RH, Roberts SO, Carpenter RG, Roger JH. Urticaria and angio-oedema. A review of 554 patients. Br J Dermatol. 1969;81:588–97.
Charlesworth EN. Urticaria and angioedema: a clinical spectrum. Ann Allergy Asthma Immunol. 1996;76:484–95; quiz 495–9.
Cochran ST. Anaphylactoid reactions to radiocontrast media. Curr Allergy Asthma Rep. 2005;5:28–31.
Commens CA, Greaves MW. Tests to establish the diagnosis in cholinergic urticaria. Br J Dermatol. 1978;98:47–51.
Confino-Cohen R, Chodick G, Shalev V, Leshno M, Kimhi O, Goldberg A. Chronic urticaria and autoimmunity: associations found in a large population study. J Allergy Clin Immunol. 2012;129:1307–13.
Cooper KD. Urticaria and angioedema: diagnosis and evaluation. J Am Acad Dermatol. 1991;25:166–74; discussion 174–6.
Cummings AJ, Olsen M. Mechanism of action of stinging nettles. Wilderness Environ Med. 2011;22:136–9.
Czarnetzki BM, Meentken J, Rosenbach T, Pokropp A. Clinical, pharmacological and immunological aspects of delayed pressure urticaria. Br J Dermatol. 1984;111:315–23.
Davis RS, Remigio LK, Schocket AL, Bock SA. Evaluation of a patient with both aquagenic and cholinergic urticaria. J Allergy Clin Immunol. 1981;68:479–83.
Di Campli C, Gasbarrini A, Nucera E, Franceschi F, Ojetti V, Sanz Torre E, Schiavino D, Pola P, Patriarca G, Gasbarrini G. Beneficial effects of Helicobacter pylori eradication on idiopathic chronic urticaria. Dig Dis Sci. 1998;43:1226–9.
Dover JS, Black AK, Ward AM, Greaves MW. Delayed pressure urticaria. Clinical features, laboratory investigations, and response to therapy of 44 patients. J Am Acad Dermatol. 1988;18:1289–98.
Estes SA, Yung CW. Delayed pressure urticaria: an investigation of some parameters of lesion induction. J Am Acad Dermatol. 1981;5:25–31.
Farr PM. Solar urticaria. Br J Dermatol. 2000;142:4–5.
Fernandez TD, Mayorga C, Salas M, Barrionuevo E, Posadas T, Ariza A, Laguna JJ, Moreno E, Torres MJ, Dona I, Montanez MI. Evolution of diagnostic approaches in betalactam hypersensitivity. Expert Rev Clin Pharmacol. 2017;10:671–83.
Ferrer M. Epidemiology, healthcare, resources, use and clinical features of different types of urticaria. Alergologica 2005. J Investig Allergol Clin Immunol. 2009;19(Suppl 2):21–6.
Fesq H, Ring J, Abeck D. Management of polymorphous light eruption: clinical course, pathogenesis, diagnosis and intervention. Am J Clin Dermatol. 2003;4: 399–406.
Fine LM, Bernstein JA. Guideline of chronic urticaria beyond. Allergy Asthma Immunol Res. 2016;8: 396–403.
Fukunaga A, Bito T, Tsuru K, Oohashi A, Yu X, Ichihashi M, Nishigori C, Horikawa T. Responsiveness to autologous sweat and serum in cholinergic urticaria classifies its clinical subtypes. J Allergy Clin Immunol. 2005;116:397–402.
Gaig P, Olona M, Munoz Lejarazu D, Caballero MT, Dominguez FJ, Echechipia S, Garcia Abujeta JL, Gonzalo MA, Lleonart R, Martinez Cocera C, Rodriguez A, Ferrer M. Epidemiology of urticaria in Spain. J Investig Allergol Clin Immunol. 2004;14:214–20.
Godse K, De A, Zawar V, Shah B, Girdhar M, Rajagopalan M, Krupashankar DS. Consensus statement for the diagnosis and treatment of Urticaria: a 2017 update. Indian J Dermatol. 2018;63:2–15.
Grattan CE. The urticaria spectrum: recognition of clinical patterns can help management. Clin Exp Dermatol. 2004;29:217–21.
Greaves MW. Chronic urticaria. N Engl J Med. 1995;332:1767–72.
Greaves M. Chronic urticaria. J Allergy Clin Immunol. 2000;105:664–72.
Greaves MW, Black AK, Eady RA, Coutts A. Aquagenic pruritus. Br Med J (Clin Res Ed). 1981;282:2008–10.
Greiwe J, Bernstein JA. Therapy of antihistamine-resistant chronic spontaneous urticaria. Expert Rev Clin Immunol. 2017;13:311–8.
Harris A, Twarog FJ, Geha RS. Chronic urticaria in childhood: natural course and etiology. Ann Allergy. 1983;51:161–5.
Healy DP, Sahai JV, Fuller SH, Polk RE. Vancomycin-induced histamine release and “red man syndrome”: comparison of 1- and 2-hour infusions. Antimicrob Agents Chemother. 1990;34:550–4.
Horinouchi Y, Abe K, Kubo K, Oka M. Mechanisms of vancomycin-induced histamine release from rat peritoneal mast cells. Agents Actions. 1993;40:28–36.
Illig L, Kunick J. Clinical picture and diagnosis of physical urticaria. II. Hautarzt. 1969;20:499–512.
Imbalzano E, Casciaro M, Quartuccio S, Minciullo PL, Cascio A, Calapai G, Gangemi S. Association between urticaria and virus infections: a systematic review. Allergy Asthma Proc. 2016;37:18–22.
Jacobson KW, Branch LB, Nelson HS. Laboratory tests in chronic urticaria. JAMA. 1980;243:1644–6.
Jirapongsananuruk O, Pongpreuksa S, Sangacharoenkit P, Visitsunthorn N, Vichyanond P. Identification of the etiologies of chronic urticaria in children: a prospective study of 94 patients. Pediatr Allergy Immunol. 2010;21:508–14.
Juhlin L. Recurrent urticaria: clinical investigation of 330 patients. Br J Dermatol. 1981;104:369–81.
Kaplan AP. Clinical practice. Chronic urticaria and angioedema. N Engl J Med. 2002;346:175–9.
Kaplan AP, Greaves M. Pathogenesis of chronic urticaria. Clin Exp Allergy. 2009;39:777–87.
Kaplan AP, Natbony SF, Tawil AP, Fruchter L, Foster M. Exercise-induced anaphylaxis as a manifestation of cholinergic urticaria. J Allergy Clin Immunol. 1981;68:319–24.
Keahey TM, Indrisano J, Lavker RM, Kaliner MA. Delayed vibratory angioedema: insights into pathophysiologic mechanisms. J Allergy Clin Immunol. 1987;80:831–8.
Kikuchi Y, Fann T, Kaplan AP. Antithyroid antibodies in chronic urticaria and angioedema. J Allergy Clin Immunol. 2003;112:218.
Kim YS, Han K, Lee JH, Kim NI, Roh JY, Seo SJ, Song HJ, Lee MG, Choi JH, Park YM. Increased risk of chronic spontaneous urticaria in patients with autoimmune thyroid diseases: a nationwide, population-based study. Allergy Asthma Immunol Res. 2017;9:373–7.
Kirby JD, Matthews CN, James J, Duncan EH, Warin RP. The incidence and other aspects of factitious wealing (dermographism). Br J Dermatol. 1971;85:331–5.
Kozaru T, Fukunaga A, Taguchi K, Ogura K, Nagano T, Oka M, Horikawa T, Nishigori C. Rapid desensitization with autologous sweat in cholinergic urticaria. Allergol Int. 2011;60:277–81.
Kozel MM, Mekkes JR, Bossuyt PM, Bos JD. Natural course of physical and chronic urticaria and angioedema in 220 patients. J Am Acad Dermatol. 2001;45:387–91.
Kulp-Shorten CL, Callen JP. Urticaria, angioedema, and rheumatologic disease. Rheum Dis Clin N Am. 1996;22:95–115.
Kulthanan K, Jiamton S, Thumpimukvatana N, Pinkaew S. Chronic idiopathic urticaria: prevalence and clinical course. J Dermatol. 2007;34:294–301.
Kuyucu S, Mori F, Atanaskovic-Markovic M, Caubet JC, Terreehorst I, Gomes E, Brockow K, Pediatric Task Force of, EAACI Drug Allergy Interest Group. Hypersensitivity reactions to non-betalactam antibiotics in children: an extensive review. Pediatr Allergy Immunol. 2014;25:534–43.
Lang DM, Hsieh FH, Bernstein JA. Contemporary approaches to the diagnosis and management of physical urticaria. Ann Allergy Asthma Immunol. 2013;111:235–41.
Lapi F, Cassano N, Pegoraro V, Cataldo N, Heiman F, Cricelli I, Levi M, Colombo D, Zagni E, Cricelli C, Vena GA. Epidemiology of chronic spontaneous urticaria: results from a nationwide, population-based study in Italy. Br J Dermatol. 2016;174:996–1004.
Lawlor F, Black AK, Ward AM, Morris R, Greaves MW. Delayed pressure urticaria, objective evaluation of a variable disease using a dermographometer and assessment of treatment using colchicine. Br J Dermatol. 1989;120:403–8.
Leznoff A, Josse RG, Denburg J, Dolovich J. Association of chronic urticaria and angioedema with thyroid autoimmunity. Arch Dermatol. 1983;119:636–40.
Martin-Serrano A, Barbero N, Agundez JA, Vida Y, Perez-Inestrosa E, Montanez MI. New advances in the study of IgE drug recognition. Curr Pharm Des. 2016;22:6759–72.
Mathelier-Fusade P, Aissaoui M, Chabane MH, Mounedji N, Leynadier F. Association of cold urticaria and aquagenic urticaria. Allergy. 1997;52:678–9.
Matysiak J, Matysiak J, Breborowicz A, Kokot ZJ. Diagnosis of hymenoptera venom allergy–with special emphasis on honeybee (Apis mellifera) venom allergy. Ann Agric Environ Med. 2013;20:875–9.
Mekori YA, Dobozin BS, Schocket AL, Kohler PF, Clark RA. Delayed pressure urticaria histologically resembles cutaneous late-phase reactions. Arch Dermatol. 1988;124:230–5.
Metzger WJ, Kaplan AP, Beaven MA, Irons JS, Patterson R. Hereditary vibratory angioedema: confirmation of histamine release in a type of physical hypersensitivity. J Allergy Clin Immunol. 1976;57:605–8.
Minciullo PL, Cascio A, Barberi G, Gangemi S. Urticaria and bacterial infections. Allergy Asthma Proc. 2014;35:295–302.
Moore-Robinson M, Warin RP. Effect of salicylates in urticaria. Br Med J. 1967;4:262–4.
Mortureux P, Leaute-Labreze C, Legrain-Lifermann V, Lamireau T, Sarlangue J, Taieb A. Acute urticaria in infancy and early childhood: a prospective study. Arch Dermatol. 1998;134:319–23.
Murphy GM. Diagnosis and management of the erythropoietic porphyrias. Dermatol Ther. 2003;16:57–64.
Najib U, Sheikh J. An update on acute and chronic urticaria for the primary care provider. Postgrad Med. 2009;121:141–51.
Najib U, Bajwa ZH, Ostro MG, Sheikh J. A retrospective review of clinical presentation, thyroid autoimmunity, laboratory characteristics, and therapies used in patients with chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2009;103:496–501.
Newfield P, Roizen MF. Hazards of rapid administration of vancomycin. Ann Intern Med. 1979;91:581.
Orfan NA, Kolski GB. Physical urticarias. Ann Allergy. 1993;71:205–12; quiz 212–5.
Patterson R, Mellies CJ, Blankenship ML, Pruzansky JJ. Vibratory angioedema: a hereditary type of physical hypersensitivity. J Allergy Clin Immunol. 1972;50: 174–82.
Plumb J, Norlin C, Young PC, Utah Pediatric Practice Based Research Network. Exposures and outcomes of children with urticaria seen in a pediatric practice-based research network: a case-control study. Arch Pediatr Adolesc Med. 2001;155:1017–21.
Poole JA, Rosenwasser LJ. Chronic idiopathic urticaria exacerbated with progesterone therapy treated with novel desensitization protocol. J Allergy Clin Immunol. 2004;114:456–7.
Renz CL, Thurn JD, Finn HA, Lynch JP, Moss J. Oral antihistamines reduce the side effects from rapid vancomycin infusion. Anesth Analg. 1998;87:681–5.
Robledo T, Cimarra M, Agustin P, Martinez-Cocera C. Adverse reaction to dextromethorphan. Allergy. 2004;59:890.
Roelandts R. Diagnosis and treatment of solar urticaria. Dermatol Ther. 2003;16:52–6.
Ryan TJ, Shim-Young N, Turk JL. Delayed pressure urticaria. Br J Dermatol. 1968;80:485–90.
Sackesen C, Sekerel BE, Orhan F, Kocabas CN, Tuncer A, Adalioglu G. The etiology of different forms of urticaria in childhood. Pediatr Dermatol. 2004;21:102–8.
Sanchez-Borges M, Asero R, Ansotegui IJ, Baiardini I, Bernstein JA, Canonica GW, Gower R, Kahn DA, Kaplan AP, Katelaris C, Maurer M, Park HS, Potter P, Saini S, Tassinari P, Tedeschi A, Ye YM, Zuberbier T, WAO Scientific and Clinical Issues Council. Diagnosis and treatment of urticaria and angioedema: a worldwide perspective. World Allergy Organ J. 2012;5: 125–47.
Shah KN, Honig PJ, Yan AC. “Urticaria multiforme”: a case series and review of acute annular urticarial hypersensitivity syndromes in children. Pediatrics. 2007;119:e1177–83.
Shank JJ, Olney SC, Lin FL, McNamara MF. Recurrent postpartum anaphylaxis with breast-feeding. Obstet Gynecol. 2009;114:415–6.
Sheffer AL, Soter NA, McFadden ER Jr, Austen KF. Exercise-induced anaphylaxis: a distinct form of physical allergy. J Allergy Clin Immunol. 1983;71:311–6.
Sheffer AL, Tong AK, Murphy GF, Lewis RA, McFadden ER Jr, Austen KF. Exercise-induced anaphylaxis: a serious form of physical allergy associated with mast cell degranulation. J Allergy Clin Immunol. 1985;75:479–84.
Sussman GL, Beezhold DH. Allergy to latex rubber. Ann Intern Med. 1995;122:43–6.
Sussman GL, Harvey RP, Schocket AL. Delayed pressure urticaria. J Allergy Clin Immunol. 1982;70:337–42.
Tarbox JA, Gutta RC, Radojicic C, Lang DM. Utility of routine laboratory testing in management of chronic urticaria/angioedema. Ann Allergy Asthma Immunol. 2011;107:239–43.
Uetsu N, Miyauchi-Hashimoto H, Okamoto H, Horio T. The clinical and photobiological characteristics of solar urticaria in 40 patients. Br J Dermatol. 2000;142:32–8.
Uslu S, Bulbul A, Diler B, Bas EK, Nuhoglu A. Urticaria due to Urtica dioica in a neonate. Eur J Pediatr. 2011;170:401–3.
Veien M, Szlam F, Holden JT, Yamaguchi K, Denson DD, Levy JH. Mechanisms of nonimmunological histamine and tryptase release from human cutaneous mast cells. Anesthesiology. 2000;92:1074–81.
Wallace MR, Mascola JR, Oldfield EC 3rd. Red man syndrome: incidence, etiology, and prophylaxis. J Infect Dis. 1991;164:1180–5.
Wanderer AA, Hoffman HM. The spectrum of acquired and familial cold-induced urticaria/urticaria-like syndromes. Immunol Allergy Clin N Am. 2004;24: 259–86, vii.
Wanderer AA, Grandel KE, Wasserman SI, Farr RS. Clinical characteristics of cold-induced systemic reactions in acquired cold urticaria syndromes: recommendations for prevention of this complication and a proposal for a diagnostic classification of cold urticaria. J Allergy Clin Immunol. 1986;78:417–23.
Warin RP. The effect of aspirin in chronic urticaria. Br J Dermatol. 1960;72:350–1.
Warin RP. Clinical observations on delayed pressure urticaria. Br J Dermatol. 1989;121:225–8.
Winkelmann RK, Black AK, Dover J, Greaves MW. Pressure urticaria–histopathological study. Clin Exp Dermatol. 1986;11:139–47.
Wu CC, Kuo HC, Yu HR, Wang L, Yang KD. Association of acute urticaria with Mycoplasma pneumoniae infection in hospitalized children. Ann Allergy Asthma Immunol. 2009;103:134–9.
Ying S, Kikuchi Y, Meng Q, Kay AB, Kaplan AP. Th1/Th2 cytokines and inflammatory cells in skin biopsy specimens from patients with chronic idiopathic urticaria: comparison with the allergen-induced late-phase cutaneous reaction. J Allergy Clin Immunol. 2002;109: 694–700.
Zuberbier T, Bernstein JA. A comparison of the United States and international perspective on chronic urticaria guidelines. J Allergy Clin Immunol Pract. 2018;6:1144.
Zuberbier T, Balke M, Worm M, Edenharter G, Maurer M. Epidemiology of urticaria: a representative cross-sectional population survey. Clin Exp Dermatol. 2010;35:869–73.
Zuberbier T, Aberer W, Asero R, Bindslev-Jensen C, Brzoza Z, Canonica GW, Church MK, Ensina LF, Gimenez-Arnau A, Godse K, Goncalo M, Grattan C, Hebert J, Hide M, Kaplan A, Kapp A, Abdul Latiff AH, Mathelier-Fusade P, Metz M, Nast A, Saini SS, Sanchez-Borges M, Schmid-Grendelmeier P, Simons FE, Staubach P, Sussman G, Toubi E, Vena GA, Wedi B, Zhu XJ, Maurer M, European Academy of Allergy and Clinical Immunology, Global Allergy and Asthma European Network, European Dermatology Forum, World Allergy Organization. The EAACI/Ga(2) LEN/EDF/WAO guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014;69:868–87.
Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, Bernstein JA, Bindslev-Jensen C, Brzoza Z, Buense Bedrikow R, Canonica GW, Church MK, Craig T, Danilycheva IV, Dressler C, Ensina LF, Gimenez-Arnau A, Godse K, Goncalo M, Grattan C, Hebert J, Hide M, Kaplan A, Kapp A, Katelaris CH, Kocaturk E, Kulthanan K, Larenas-Linnemann D, Leslie TA, Magerl M, Mathelier-Fusade P, Meshkova RY, Metz M, Nast A, Nettis E, Oude-Elberink H, Rosumeck S, Saini SS, Sanchez-Borges M, Schmid-Grendelmeier P, Staubach P, Sussman G, Toubi E, Vena GA, Vestergaard C, Wedi B, Werner RN, Zhao Z, Maurer M. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. The 2017 revision and update. Allergy. 2018;73:1393.
Author information
Authors and Affiliations
Corresponding author
Section Editor information
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this entry
Cite this entry
Lavery, W.J., Bernstein, J.A. (2019). Acute and Chronic Urticaria. In: Allergy and Asthma. Springer, Cham. https://doi.org/10.1007/978-3-030-05147-1_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-05147-1_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-05146-4
Online ISBN: 978-3-030-05147-1
eBook Packages: MedicineReference Module Medicine