Abstract
Chronic rhinosinusitis (CRS) is a common disease, affecting up to 10% of the population at some time. Symptoms alone do not define the disease; objective evidence of inflammation by nasal endoscopy and/or sinus CT scan is also required. In the USA alone, the estimated annual direct and indirect costs exceed $30 billion. There are two subtypes, depending upon whether nasal polyps (NP) are present: CRSw(with)NP and CRSs(without)NP. A variety of risk factors and comorbidities have been described; in most cases, an aeroallergen evaluation should be performed, and, in recalcitrant cases, an immunodeficiency evaluation should be considered. The pathogenesis is unclear; a variety of factors have been implicated as contributory. They include impaired antimicrobial responses, ciliary abnormalities, epithelial dysfunction, microbial dysbiosis, autoantibodies, and S. aureus enterotoxins acting as allergens and/or superantigens. Maximal medical therapy, often including corticosteroids, antibiotics, and saline irrigations, is the initial treatment. Only those who fail are considered for surgical treatment.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
1 Introduction
Rhinosinusitis is a significant health issue that appears to be increasing in frequency. Rhinosinusitis is generally divided into acute or chronic based on whether the requisite signs and symptoms have been going on for more than 12 weeks. Chronic rhinosinusitis (CRS) is associated with poor quality of life, absenteeism, presenteeism, and a large financial burden in both direct and indirect medical expenditures. Recent estimates of the indirect costs of CRS in the USA, $12.8 billion, are thought to exceed direct costs (DeConde and Soler 2016). There are two forms of CRS, one with nasal polyps (CRSwNP) and one without (CRSsNP). While the focus of this review is on CRSwNP, for contrast, information on CRSsNP is included as well. In the past decade, there have been several documents published relative to CRS including practice parameters, position papers, and guidelines (Scadding et al. 2008, Fokkens et al. 2012, Kaplan 2013, Peters et al. 2014, Orlandi et al. 2014, Bachert et al. 2014, Hellings et al. 2017).
The inflammation of CRSsNP can be any combination of T helper type 1 (Th1), Th2, and/or Th17 (Tan et al. 2017). The inflammation of CRSwNP tends to be Th2, with eosinophilia. However, the NP of some ethic groups, for example, Asians, is less likely to be eosinophilic; in addition the NP of certain disease states, like cystic fibrosis (CF), is less likely to be eosinophilic (Zhang et al. 2017).
The inflammation of CRS can last for decades; glucocorticoids and antibiotics are the most common medical treatments. As they are unsatisfactory in some patients, approximately 300,000 surgeries are performed every year in the USA for CRS; the most common procedure is functional endoscopic sinus surgery (FESS), but other procedures such as balloon sinuplasty (BSP) are also performed. BSP catheters were approved by the FDA more than a decade ago. While there is literature that BSP can be a useful technique (Chandra et al. 2016), there are reports of failure rates as high as 66% (Tomazic et al. 2013).
The nasal and sinus microbiomes of CRS patients are different than normals. Whether that is causal or an epiphenomenon is unknown. There are a variety of other alterations in CRS, including decreased epithelial barrier integrity, altered levels of cytokines, decreased antimicrobial peptides produced in the sinonasal mucosa, changes of the epithelium toward mesenchymal transition, and mucociliary dysfunction. What role those and other described alterations play in the pathogenesis of CRS is unclear (Schleimer 2017).
2 Epidemiology and Risk Factors
CRS is estimated to affect 5–15% of the population in Europe and North America; however, doctor diagnosed CRS estimates are in the 2–4% range (Fokkens et al. 2012; Orlandi et al. 2014). A systematic review of 2014 costs associated with adult CRS in the USA estimated the direct costs to be $6.9–$9.9 billion and the indirect costs to be $13 billion (Smith et al. 2015). In that same study, annual medication costs prior to FESS ranged from $1547 to $2700 per patient; costs of medications were reduced after outpatient FESS which ranged in price from $8200 to $10,500. A study of insurance claims data also concluded that the costs of CRS were reduced after FESS (Bhattacharyya et al. 2011); in this study the reduction was approximately $885 in year 1 and $1331 in year 2. Another study of claims data also reported that costs of CRS were reduced after FESS (Purcell et al. 2015); the reported reduction averaged $600/year for each of the 3 years of follow-up. In addition, they found that disease-specific costs for conditions often associated with CRS such as depression, allergy, and asthma also decreased as did antibiotic use (28.2 days vs. 15.9 days per year). A retrospective database analysis of 35.5 million covered lives has reported that FESS within 1 year of diagnosis of CRS reduces both cost and healthcare utilization as compared to FESS which occurred after >5 years of medical management (Benninger et al. 2015). There are no long-term follow-up studies to determine whether the cost of surgery is eventually paid for by reduction of postoperative costs of CRS.
CRSsNP is more prevalent than CRSwNP. Men are more likely to have CRSwNP than women. The most common age of onset is in the third or fourth decade of life. A number of diseases are associated with CRS. As those diseases often predate the CRS, it is generally accepted that they are predisposing or risk factors. Details can be found in a recent practice parameter publication (Peters et al. 2014).
Multiple studies of allergic rhinitis (AR) and CRS report association in both children and adults. In adults with CRS, 40–84% have AR (Van Lancker et al. 2005). One study reported that there is a correlation between extensive sinus disease on CT and AR (Ramadan et al. 1999). Surgical outcomes, corticosteroid use, and symptomatology, in those with CRSwNP, do not seem to be influenced by AR (Bonfils and Malinvaud 2008). There are also multiple studies of nasal lavage that implicate allergic responses in CRS; specifically, CRS patients have higher levels than normal individuals of allergic mediators such as leukotrienes, histamine, and Th2 cytokines (Peters et al. 2014).
Immunodeficiency can contribute to CRS and should especially be considered and evaluated in CRS patients that are resistant to medical and/or surgical treatments. Just as patients with recurrent acute sinusitis or recurrent pneumonia should be evaluated for immunodeficiency, so should recalcitrant CRS patients, in whom the prevalence of immunodeficiency has been reported to be about 15% (Carr et al. 2011). The American Red Cross and the Jeffery Modell Foundation both consider at least two serious sinus infections per year as a warning sign of primary immunodeficiency (PID) (Jeffery Modell Foundation 2012). While humoral PID is the most likely cause of recalcitrant CRS, other deficiencies including complement and cellular may play a role (Cunningham-Rundles and Bodian 1999). Prior to highly active antiretroviral therapy (HAART), the prevalence of CRS was significant in the HIV-infected population. However, the prevalence of CRS in that population receiving HAART is only 3–6%, similar to the general population (Campanini et al. 2005).
In a study of 446,480 electronic health records of individuals with and without CRS, several associations were reported. Compared to CRSsNP and control subjects, those with CRSwNP were more likely to be older and male. Prior to CRS diagnosis, those with CRS had a higher prevalence of a number of diseases including AR, asthma, gastroesophageal reflux, sleep apnea, anxiety, and headaches (Tan et al. 2013). Other risk factors reportedly associated with CRS include bronchiectasis (Bose et al. 2016), ciliary impairment, aspirin sensitivity, biofilms (layers of bacteria and their extruded polysaccharide matrix adherent to a biologic or non-biologic surface), and cigarette smoking (Fokkens et al. 2012; Bachert et al. 2014). Smoking cessation reduces corticosteroid use and improves CRS symptoms as well as quality of life scores (Phillips et al. 2017). A recent systematic review of the environmental and occupational literature related to CRS was unable to identify occupational or environmental exposures that play a role in CRS (Sundaresan et al. 2015). Table 1 enumerates factors associated with CRS as well as the references for those associations.
3 Pathogenesis
While the pathogenesis of CRS remains unclear, a variety of factors may be contributory; all described factors occur locally in the sinonasal tissue. Among them are epithelial dysfunction, epithelial to mesenchymal transition (EMT), mucociliary impairment, decreased innate antimicrobial responses, increased innate type 2 lymphoid cells (ILC2s), increased B cells and plasmablasts, increase in type 2 cytokines, alterations of the clotting pathway, autoantibodies, and staphylococcus enterotoxins acting as allergens or superantigens. Table 2 is a partial compilation of factors reported to be different in CRS compared to normal, healthy individuals without sinonasal disease.
Potential contributing epithelial dysfunctions in CRS include acantholysis (loss of intercellular connections), acanthosis (diffuse epidermal hyperplasia), and EMT (Schleimer 2017). In addition, proteins such as periostin, laminin, and vimentin, known to be associated with EMT, are increased in the sinonasal tissue (Zhang et al. 2016). Mucociliary dysfunction in CRS has been reported for many years; the severity of CRS likely correlates with the amount of dysfunction (Chen et al. 2006). Some bacteria produce toxins that cause ciliary damage; among them are bacteria that are associated with CRS: Streptococcus pneumoniae, Haemophilus influenzae, and Pseudomonas aeruginosa (Brook 2016). Numerous studies have reported that microbial dysbiosis, particularly a decrease in diversity compared to normal subjects, occurs in CRS (Psaltis and Wormald 2017). However, whether microbial dysbiosis is a cause, an association or an epiphenomenon of CRS is not clear.
Another epithelial abnormality that commonly occurs in CRS is changes in local, sinonasal antimicrobial responses. For example, multiple proteins of the innate immune system that are important for pathogen recognition and destruction tend to be increased in CRS. However, some innate molecules are reduced in CRS. In some cases, such as with toll-like receptors (TLRs), it is unclear which ligands and receptors are increased, decreased, or unchanged compared to controls (Hamilos 2014). A genetic polymorphism in the bitter taste receptors, e.g., T2R38, can contribute to CRSsNP (Lee and Cohen 2015). In normal people, when those receptors are engaged by molecules produced by bacteria, the epithelial cells respond by producing antimicrobial molecules to kill the bacteria. This response is abrogated in those with certain polymorphisms. A group of antimicrobial peptides, the S100 proteins, including psoriasin and calprotectin, may be reduced in CRS (Tieu et al. 2010). Enzymatic antimicrobial molecules such as lactoferrin and lysozyme also may be reduced in CRS (Psaltis et al. 2008). Complement deficiency, specifically, mannose-binding lectin deficiency, has been reported in some CRS patients. There are multiple studies that conclude that humoral immunodeficiency, both specific antibody deficiency (SAD) and common variable immunodeficiency (CVID), contributes to CRS in some patients (Chiarella and Grammer 2017).
Injured respiratory epithelium is likely to produce Th2-promoting cytokines such as thymic stromal lymphopoietin (TSLP). TSLP is elevated in CRSwNP (Miljkovic et al. 2014). That is likely contributing to the TH2 cytokines found in most European CRSwNP (Hulse et al. 2015). In addition, large numbers of B cells, plasma cells, and plasmablasts occur in mucosal tissue (Gevaert et al. 2005). There are also reports of autoantibodies, both against double-stranded DNA and the bullous pemphigoid 180 antigen, in the CRS tissue but not systemically in patients with CRSwNP (Tan et al. 2011). Enterotoxins such as staphylococcal enterotoxins A and B (SEA and SEB), from staphylococcus may drive inflammation of CRSwNP by acting as both allergens and superantigens (Seiberling et al. 2005; Bachert and Zhang 2012). Finally, macrophages and IL-13 are higher in CRSwNP than in CRSsNP or in controls. IL-13 suppresses tissue plasminogen activator (tPA) and macrophages produce factor XIIIA, resulting in cross-linked fibrin with very little fibrinolysis (Takabayashi et al. 2013).
In some diseases such as cystic fibrosis and in some populations such as CRSwNP in Asians, eosinophilic mucosal inflammation is less likely. The reasons for greater neutrophil predominance in certain diseases and populations are an area of active investigation (Zhang et al. 2017).
3.1 Genetics
There are several publications that suggest that CRS occurs more commonly in families (Fokkens et al. 2012; Rugina et al. 2002). However, when a search of the literature was performed in 2013, except for mutations in the cystic fibrosis transmembrane conductance regulator gene (CFTR), no other genetic polymorphisms were confirmed in reference populations (Hsu et al. 2013). Subsequently, a bitter taste receptor gene polymorphism (e.g., T2R38) has been associated with CRS in one US study (Lee and Cohen 2015). This finding was replicated in two Canadian populations (Mfuna Endam et al. 2014). However, the association was not replicated in an Italian population (Gallo et al. 2016). In a 2017 review of the literature, familial clustering was again confirmed. The authors concluded that there are reports of a number of discovery cohorts in which polymorphisms were associated with CRS (Cohen 2017). Information about selected genes studied in CRS can be found in Table 3. However, in attempted replication cohorts, except for CFTR and the bitter taste receptors, genetic polymorphisms associated with CRS are unconfirmed (Halderman and Lane 2017).
3.2 Diagnosis
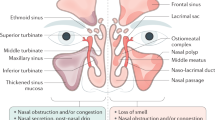
The definition of CRS has evolved over the past several decades. In more recent publications, there is a consensus about the definition (Fokkens et al. 2012; Peters et al. 2014). Table 4 shows the diagnostic criteria for CRS. First, the duration of signs and symptoms should be at least 12 weeks. Second, nasal and sinus inflammation should be present resulting in at least two symptoms, one of which must be nasal obstruction/congestion or nasal discharge (posterior or anterior rhinorrhea). Other symptoms are facial pain/pressure and reduction or loss of olfaction. In children, loss of olfaction can be replaced by cough. In addition, the sinonasal inflammation must be supported by endoscopic findings of nasal polyps, mucopurulent discharge, or edema and/or CT (computed tomography) findings compatible with CRS. Figure 1 is a sinus CT showing normal anatomy. Figure 2 is a CT scan of CRSwNP.
The timing and cost-effectiveness of imaging, in particular, sinus CT scan without contrast, has been studied. There are not studies of the cost-effectiveness of anterior rhinoscopy or nasal endoscopy. In patients with compatible symptoms for at least 12 weeks, sinus CT scans are cost effective, mostly due to reduction in antibiotic use (Leung et al. 2014; Lobo et al. 2015). In these studies, more than half of sinus CT scans were normal even though the patients had symptoms compatible with CRS for more than 12 weeks. The most common diagnoses subsequent to a normal sinus CT scan were perennial allergic rhinitis, non-allergic rhinitis, headache syndromes, and facial pain syndromes. It has been recognized for more than a decade that most patients with self-diagnosed or physician-diagnosed sinus headaches actually have migraines (Tepper 2004). Rhinorrhea and nasal congestion, two of the cardinal CRS symptoms, occur in more than half of the subjects when they experience migraines.
3.3 Prognosis
The prognosis of CRS depends upon a variety of factors including severity, treatment, and comorbidities. The initial treatment for CRS is generally medical which is covered in the next section. Prior to consideration of surgery for CRS, most would give a course of maximal medical therapy (MMT) that includes corticosteroids and antibiotics (Patel et al. 2017). There are no studies that describe the long-term outcomes of such MMT, i.e., the number and proportion of individuals who are able to maintain sufficient improvement that they do not seek a surgical option, which is generally FESS.
The surgical prognosis is influenced by several factors. It should be noted that most follow-up studies are 12–24 months, with the longest follow-up being 6 years. In CRSsNP, the T2R38 genotype that codes for a nonfunctional bitter taste receptor may have worse outcomes than other genotypes (Adappa et al. 2016). Recurrence of nasal polyps (NPs) after FESS is 35%, 38%, and 40% at 6, 12, and 18 months, respectively (DeConde et al. 2017). In a Portuguese study of CRSwNP, nonatopic asthma and exposure to occupational dust were associated with recurrence of NPs (Veloso-Teles and Cerejeira 2017). Osteitis (inflammation of the bone without invasion of bacteria or neutrophils) and biofilm formation are bad prognostic comorbidities that almost always require surgical treatment (Zhao and Wormald 2017). There are short-term (6-month follow-up) studies post FESS that report improvement of quality of life in children, even if they have CF as a comorbidity (Fetta et al. 2017).
The amount of improvement in the Sinonasal Outcome Test (SNOT-22) after FESS is variable. In a study from the UK, 66% achieved clinically relevant improvement, whereas in studies in the USA and Canada, the proportion tends to be above 80% (Hopkins et al. 2015). Outcomes such as olfaction, cognitive function, and sleep quality have also been evaluated after FESS. A meta-analysis reported that olfaction improved after FESS; this improvement was more pronounced in those with CRSwNP (Kohli et al. 2016). In another study of FESS, there was improvement in cognitive function as measured by the Cognitive Failures Questionnaire (CFQ) in CRSwNP patients; no significant improvement was found for those with CRSsNP (Alt et al. 2016). In a study in which patients chose medical or surgical treatment for CRS, those who opted for FESS had significant improvement in the Pittsburgh Sleep Quality Index (PSQI). Those who chose medical management did not improve and had PSQI scores that were worse than the control population (Alt et al. 2017).
While it is beyond the scope of this article to cover, it should be noted that there is a significant body of literature that suggests that CRS outcome has an impact on asthma; specifically, in patients who have CRS and asthma, CRS exacerbations are likely to be significantly associated with worsening asthma (Lee et al. 2017). Therefore, the CRS prognosis also affects the asthma prognosis. With an emphasis on personalized medicine, there is investigation into the endotypes of CRS. The objective is to understand the various endotypes which should allow for individualized treatment, the subject of the next section (Kim and Cho 2017).
4 Management
Recent guideline and practice parameter publications include several management scheme diagrams that illustrate an algorithmic approach to patients with CRS (Fokkens et al. 2012; Peters et al. 2014). Once the diagnosis of CRS is established, consideration should be given to determining if aeroallergens might be contributing to the inflammation. This is especially important with aeroallergens such as dust mite and animal dander for which avoidance measures could be helpful. If patients are having frequent exacerbations of CRS requiring antibiotics or if the CRS is recalcitrant to therapy, consideration of an immunodeficiency evaluation is in order. Specifically, laboratory tests that could be useful include quantitative immunoglobulins and specific antibody responses to vaccines. In those patients with CVID, immunoglobulin replacement may be useful in reducing CRS inflammation (Walsh et al. 2017). In those patients who have normal immunoglobulins but low levels of antibody against Streptococcus pneumoniae serotypes, a 23 valent pneumococcal vaccine may result in the patient developing normal amounts of protective antibody and fewer exacerbations of CRS requiring antibiotics (Kashani et al. 2015; Keswani et al. 2017). In those patients who do not respond to vaccination with increased S. pneumoniae antibody, a diagnosis of specific antibody deficiency (SAD) would be appropriate. The mainstay of therapy for patients with SAD is prophylactic antibiotics; however, there are no standardized protocols and no controlled studies of efficacy (Perez et al. 2017). Published guidelines recommend immunoglobulin therapy for SAD patients, based on retrospective studies (Perez et al. 2017). In patients with CRS and antibody deficiency, either SAD or CVID, immunoglobulin replacement may reduce Lund-Mackay CT sinus scores and frequency of CRS exacerbations (Walsh et al. 2017).
Medical management is the initial approach for patients with CRS. Many references suggest that MMT should be tried prior to consideration of FESS. MMT protocols vary widely and include the following interventions for variable amounts of time: nasal corticosteroids (91% of MMT protocols include this intervention), oral antibiotics (89%), systemic corticosteroids (61%), saline rinse irrigation (39%), oral antihistamines (11%), oral/topical decongestants (10%), and oral mucolytics (10%) (Dautremont and Rudmik 2015). Intranasal corticosteroids (INCS) are generally used on a daily basis; a 2016 Cochrane review reported that INCS results in a moderate benefit for nasal blockage and a small benefit for rhinorrhea (Chong et al. 2016a). Patients with CRSwNP often require twice daily doses of INCS. Nasal saline irrigation is useful if patients adhere to the regimen (Chong et al. 2016b). The use of antibiotics should be culture directed if possible (Fokkens et al. 2012; Peters et al. 2014); amoxicillin-clavulanic acid is a reasonable empiric antibiotic, while clindamycin would be appropriate for the penicillin allergic individual. Antibiotics are more likely to be useful in CRSsNP (Head et al. 2016b). Short-course (3–7 days) oral corticosteroids may be useful for exacerbations, particularly of CRSwNP (Head et al. 2016a); however, the risk/benefit ratio of prescribing oral corticosteroids needs to be considered as side effects can occur. A range of systemic corticosteroid prescribing options for CRS has been reported (Scott et al. 2017). Oral prednisone is the most commonly prescribed preparation; the median starting dose was 50 mg (20–80 mg), and the average duration was 5 days (1–21 days). Biologics, including omalizumab, mepolizumab, benralizumab, and dupilumab, are increasingly reported to be useful in the medical management of CRS (Bachert et al. 2015; Chiarella et al. 2017); at the time this article was written, no biologic has been approved by the FDA to treat CRS. If medical treatment is successful, the patient can use INCS and saline as maintenance therapy. Occasional use of antibiotics and/or short-course (3–7 days) oral corticosteroids may be needed for exacerbations.
However, if maintenance therapy with INCS and saline is not sufficient; if the patient requires frequent, more than twice a year, oral corticosteroids and/or antibiotics; or if the patients wants to explore a surgical option, surgery, specifically FESS in adults, should be considered. In children with CRS, there is evidence that the adenoids may serve as a reservoir for pathogenic bacteria; as a result, adenoidectomy is a surgical treatment that has been reported to be useful in the pediatric population (Mahdavinia and Grammer 2013). A prospective, non-randomized study comparing medical and surgical therapy for CRS in adults has been published (Smith et al. 2013). Patients who elected FESS had fewer course of antibiotics, fewer missed school/work days, and improved quality of life during the 2-year follow-up. In short, when aggressive medical management fails to control CRS, surgery may result in better outcomes. In a recent study of CRS patients, multivariate logistic regression was used to evaluate factors that increase the likelihood of the patient choosing FESS over continuing medical management (Chapurin et al. 2017). Those factors were CRSwNP as compared to CRSsNP odds ratio (OR) =4.28, cystic fibrosis OR = 2.42, and academic site (compared to a community site) OR = 1.86. As mentioned above, long-term follow-up studies after surgery for CRS have not been reported.
5 Special Issues
There are several aspects of CRS that require special consideration: complications, cystic fibrosis (CF), aspirin-exacerbated respiratory disease (AERD) , and allergic fungal rhinosinusitis (AFRS).
The complications of CRS are primarily due to changes in the surrounding bone in response to chronic inflammation. Among those changes are osteitis, mucoceles, metaplastic bone, bone erosion, and expansion that can damage adjacent structures resulting, for example, in optic neuropathy (Fokkens et al. 2012). Another complication is the spread of infection from the sinuses to surrounding tissues causing cellulitis or osteomyelitis, invasion of the bone by bacteria, and neutrophils as opposed to osteitis which is bone inflammation without invasion. Imaging studies are necessary to define these complications which may require urgent intervention to prevent serious sequelae like blindness. Some indications for urgent evaluation and treatment are found in Table 5.
Nasal polyps in children should raise the possibility of CF (Marshak et al. 2011). CRS may be the initial problem in those CF patients with milder CFTR gene mutations. Almost all CF patients have CRS, with about one third having CRSwNP; those NPs tend to be neutrophilic, not eosinophilic. In CF patients, the pathogens in the upper and lower airway tend to be similar. FESS tends to be useful in CF patients with refractory CRS; there have been reports of improvement in lung function after such surgery (Kovell et al. 2011). However, long-term prospective studies of lung function after FESS are not available.
There is a subset of patients with CRSwNP and asthma who have respiratory reactions after ingesting aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs); those patients have aspirin-exacerbated respiratory disease (AERD). In general, it is recommended that such patients avoid NSAIDs. In these patients, FESS has been reported to improve asthma, but long-term prospective studies have not been reported; AERD patients are more likely to experience regrowth of NP than other patients with CRSwNP (Fokkens et al. 2012). Desensitization followed by daily aspirin therapy may decrease the rate of NP recurrence (Lee et al. 2010; Kowalski et al. 2016). Other therapies that have been recommended for AERD include leukotriene-modifying drugs, saline irrigation, and nasal corticosteroids (Levy et al. 2016).
The role of fungi in the pathogenesis of CRS has been investigated in the past decade; it is generally agreed that fungi do not contribute to the pathogenesis of most CRS (Zhao et al. 2017). However, in some patients who have immediate-type hypersensitivity to fungi, eosinophilic mucin, and characteristic CT findings of high attenuation, it is thought that the fungi play a role in the CRSwNP that is termed allergic fungal rhinosinusitis (AFRS) (Fokkens et al. 2012; Peters et al. 2014). Patients with AFRS tend to require surgery as well as long-term oral and/or topical corticosteroids to maintain control. As adjunctive therapy, oral antifungals may play a role. While immunotherapy with fungal antigens initially was reported to be useful, more recent studies do not show benefit (Marple et al. 2002).
6 Conclusions
Chronic rhinosinusitis is a very common disease resulting in significant morbidity. The initial approach is medical management, but surgical intervention may be required in those whose response is suboptimal. A variety of comorbidities and subtypes are recognized that need somewhat different approaches to management: aeroallergen sensitization, immunodeficiency, bone complications, infectious complications, CF, AERD, and AFRS. There is a need for studies of long-term outcome data, especially postsurgery, to enhance clinical decision-making in CRS.
References
Adappa ND, Farquhar D, Palmer JN, Kennedy DW, Doghramji L, Morris SA, Owens D, Mansfield C, Lysenko A, Lee RJ, Cowart BJ, Reed DR, Cohen NA. TAS2R38 genotype predicts surgical outcome in nonpolypoid chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6:783–91.
Alt JA, Mace JC, Smith TL, Soler ZM. Endoscopic sinus surgery improves cognitive function in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6:1264–72.
Alt JA, Ramakrishnan VR, Platt MP, Kohli P, Storck KA, Schlosser RJ, Soler ZM. Sleep quality outcomes after medical and surgical management of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017;7:113–8.
Bachert C, Zhang N. Chronic rhinosinusitis and asthma: novel understanding of the role of IgE ‘above atopy’. J Intern Med. 2012;272:133–43.
Bachert C, Pawankar R, Zhang L, Bunnag C, Fokkens WJ, Hamilos DL, Jirapongsananruk O, Kern R, Meltzer EO, Mullol J, Naclerio R, Pilan R, Rhee CS, Suzaki H, Voegels R, Blais M. ICON: chronic rhinosinusitis. World Allergy Organ J. 2014;7:25–53.
Bachert C, Zhang L, Gevaert P. Current and future treatment options for adult chronic rhinosinusitis: focus on nasal polyposis. J Allergy Clin Immunol. 2015;136:1431–40.
Benninger MS, Sindwani R, Holy CE, Hopkins C. Early versus delayed endoscopic sinus surgery in patients with chronic rhinosinusitis: impact on health care utilization. Otolaryngol Head Neck Surg. 2015;152:546–52.
Bhattacharyya N, Orlando RR, Grebner J, Martinson M. Cost burden of chronic rhinosinusitis: a claims-based study. Otolaryngol Head Neck Surg. 2011;114:440–5.
Bonfils P, Malinvaud D. Influence of allergy in patients with nasal polyposis after endoscopic sinus surgery. Acta Otolaryngol. 2008;128:186–92.
Bose S, Grammer LC, Peters AT. Infectious chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2016;4:584–9.
Brook I. Microbiology of chronic rhinosinusitis. Eur J Clin Microbiol Infect Dis. 2016;35:1059–68.
Campanini A, Marani M, Mastroianni A, Cancellieri C, Vicini C. Human immunodeficiency virus infection: personal experiences in changes in head and neck manifestations due to recent antiretroviral therapies. Acta Otorhinolaryngol Ital. 2005;25:30–5.
Carr TF, Koterba AP, Chandra R, Grammer LC, Conley DB, Harris KE, Kern R, Schleimer RP, Peters AT. Characterization of specific antibody deficiency in adults with medically refractory chronic rhinosinusitis. Am J Rhinol Allergy. 2011;25:241–4.
Chandra RK, Kern RC, Cutler JL, Welch KC, Russell PT. REMODEL larger cohort with long-term outcomes and meta-analysis of standalone balloon dilation studies. Laryngoscope. 2016;126:44–50.
Chapurin N, Pynnonen MA, Roberts R, Schulz K, Shin JJ, Witsell DL, Parham K, Langman A, Cerpenter D, Vambutas A, Nguyen-Huynh A, Wolfley A, Lee WT. CHEER national study of chronic rhinosinusitis practice patterns: disease comorbidities and factors associated with surgery. Otolaryngol Head Neck Surg. 2017;156:751–6.
Chen B, Shaari J, Claire SE, Palmer JN, Chiu AG, Kennedy DW, Cohen NA. Altered sinonasal ciliary dynamics in chronic rhinosinusitis. Am J Rhinol. 2006;20:325–9.
Chiarella SE, Grammer LC. Immune deficiency in chronic rhinosinusitis: screening and treatment. Expert Rev Clin Immunol. 2017;13:117–23.
Chiarella SE, Hendrick S, Peters AT. Monoclonal antibody therapy in sinonasal disease. Am J Rhinol Allergy. 2017;31:93–5.
Chong LY, Head K, Hopkins C, Philpott C, Schilder AG. Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016a;4:CDC011996.
Chong LY, Head K, Hopkins C, Philpott C, Glew S, Scadding G, Burton MJ, Schilder AG. Saline irrigation for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016b;4:CDC011995.
Cohen NA. The genetics of the bitter taste receptor T2R38 in upper airway innate immunity and implications for chronic rhinosinusitis. Laryngoscope. 2017;127:44–51.
Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunologic features of 248 patients. Clin Immunol. 1999;92:34–48.
Dautremont JF, Rudmik L. When are we operating for chronic rhinosinusitis? A major systematic review of maximal medical therapy protocols prior to endoscopic sinus surgery. Int Forum Allergy Rhinol. 2015;5:1095–7.
DeConde AS, Soler ZM. Chronic rhinosinusitis: epidemiology and burden of disease. Am J Rhinol Allergy. 2016;30:134–9.
DeConde AS, Mace JC, Levy JM, Rudmik L, Alt JA, Smith TL. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope. 2017;127:550–5.
Fetta M, Tsilis NS, Segas JV, Nikolopoulos TP, Vlastarakos PV. Functional endoscopic sinus surgery improves the quality of life in children suffering from chronic rhinosinusitis with nasal polyps. Int J Pediatr Otorhinolaryngol. 2017;100: 145–8.
Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, Georgalas C, Goossens H, Harvey R, Hellings P, Hopkins C, Jones N, Joos G, Kalogjera L, Kern R, Kowalski M, Price D, Riechelmann H, Schlosser R, Senior B, Thomas M, Toskala E, Voegels R, de Wang Y, Wormald PJ. EPOS 2012: Europena position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012;23:1–298.
Gallo S, Grossi S, Montrasio G, Binelli G, Cinquetti R, Simmen D, Castelnuovo P, Campomenosi P. TAS2R38 taste receptor gene and chronic rhinosinusitis: new data from an Italian population. BMC Med Genet. 2016;17:54–9.
Gevaert P, Holtappels G, Johansson SG, Cuvelier C, Cauwenberge P, Bachert C. Organization of secondary lymphoid tissue and local IgE formation to Staphylococcus aureus enterotoxins in nasal polyp tissue. Allergy. 2005;60:71–9.
Halderman A, Lane AP. Genetic and immune dysregulation in chronic rhinosinusitis. Otolaryngol Clin N Am. 2017;50:13–28.
Hamilos DL. Host-microbial interactions in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2014;133:640–53.
Head K, Chong LY, Hopkins C, Philpott C, Schilder AG, Burton MJ. Short-course steroids as an adjunct therapy for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016a;4:CDC011992.
Head K, Chong LY, Piromchai P, Hopkins C, Philpott C, Schilder AG, Burton MJ. Systemic and topical antibiotics for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016b;4:CDC099112.
Hellings PW, Fokkens WJ, Bachert C, Akdis CA, Bieber T, Agache I, Bernal-Sprekelsen M, Canonica GW, Gevaert P, Joos G, Lund V, Muraro A, Onerci M, Zuberbier T, Pugin B, Seys SF. Positioning the principles of precision medicine in care pathways for allergic rhinitis and chronic rhinosinusitis-A EUFOREA-ARAI-EPOS-AIRWAYS ICP statement. Allergy. 2017;72:1297–305.
Hopkins C, Rudmik L, Lund VJ. The predictive value of the preoperative Sinonasal Outcome Test-22 in patients undergoing sinus surgery for chronic rhinosinusitis. Laryngoscope. 2015;125:1779–84.
Hsu J, Avila PC, Kern RC, Hayes MG, Schleimer RP, Pinto JM. Genetics of chronic rhinosinusitis; state of the field and directions forward. J Allergy Clin Immunol. 2013;131:977–93.
Hulse KE, Stevens WW, Tan BK, Schleimer RP. Pathogenesis of nasal polyposis. Clin Exp Allergy. 2015;45:328–46.
Jeffery Modell Foundation. Primary Immunodeficiency Resource Center. 2012. http://www.jmfworld.com
Kaplan A. Canadian guidelines for chronic rhinosinusitis. Can Fam Physician. 2013;59:1275–81.
Kashani S, Carr TF, Grammer LC, Schleimer RP, Hulse KE, Kato A, Kern RC, Conley DB, Chandra RK, Tan BK, Peters AT. Clinical characteristics of adults with chronic rhinosinusitis and specific antibody deficiency. J Allergy Clin Immunol Pract. 2015;3:236–42.
Keswani A, Dunn NM, Manzur A, Kashani S, Bossuyt X, Grammer LC, Conley DB, Tan BK, Kern RC, Schleimer RP, Peters AT. The clinical significance of specific antibody deficiency (SAD) severity in chronic rhinosinusitis (CRS). J Allergy Clin Immunol Pract. 2017;5:1105–11.
Kim DW, Cho SH. Emerging endotypes of chronic rhinosinusitis and its application to precision medicine. Allergy Asthma Immunol Res. 2017;9:299–306.
Kohli P, Naik AN, Farhood Z, Ong AA, Nguyen SA, Soler ZM, Schlosser RJ. Olfactory outcomes after endoscopic sinus surgery for chronic rhinosinusitis: a meta-analysis. Otolaryngol Head Neck Surg. 2016;155:936–48.
Kovell LC, Wang J, Ishman SL, Zeitlin PL, Boss EF. Cystic fibrosis and sinusitis in children: outcomes and socioeconomic status. Otolaryngol Head Neck Surg. 2011;145:146–53.
Kowalski ML, Wardzynska A, Makowska JS. Clinical trials of aspirin treatment after desensitization in aspirin-exacerbated respiratory disease. Immunol Allergy Clin N Am. 2016;36:705–11.
Lee RJ, Cohen NA. Role of the bitter taste receptor T2R38 in upper respiratory infection and chronic rhinosinusitis. Curr Opin Allergy Clin Immunol. 2015;15:14–20.
Lee RU, White AA, Ding D, Dursun AB, Woessner KM, Simon RA, Stevenson DD. Use of intranasal ketorolac and modified oral aspirin challenge for desensitization of aspirin-exacerbated respiratory disease. Ann Allergy Asthma Immunol. 2010;105:130–5.
Lee TJ, Fu CH, Wang CH, Huang CC, Huang CC, Change PH, Chen YW, Wu CC, Wu CL, Kuo P. Impact of chronic rhinosinusitis on severe asthma patients. PLoS One. 2017;12:e0171047.
Leung PM, Chandra RK, Kern RC, Conley DB, Tan BK. Primary care and upfront computed tomography scanning in the diagnosis of chronic rhinosinusitis: a cost-based decision analysis. Laryngoscope. 2014;124:12–8.
Levy JM, Rudmik L, Peters AT, Wise SK, Rotenberg BW, Smith TL. Contemporary management of chronic rhinosinusitis with nasal polyposis in aspirin-exacerbated respiratory disease: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2016;6:1273–80.
Lobo BC, Ting JY, Tan BK. Cost efficiency workup and management of patients with chronic rhinosinusitis- challenges and unmet needs. Curr Otorhinolaryngol Rep. 2015;3:94–100.
Mahdavinia M, Grammer LC. Chronic sinusitis and age: is the pathogenesis different? Expert Rev Anti-Infect Ther. 2013;11:1029–40.
Marple B, Newcomer M, Schwade N, Mabry R. Natural history of allergic fungal rhinosinusitis: a 4 to 10 year follow-up. Otolaryngol Head Neck Surg. 2002;127:361–6.
Marshak T, Rivlin Y, Bentur L, Ronen O, Uri N. Prevalence of rhinosinusitis among atypical cystic fibrosis patients. Eur Arch Otorhinolaryngol. 2011;268:519–24.
Mfuna Endam L, Filali-Mouhim A, Boisvert P, Boulet LP, Bosse Y, Desrosiers M. Genetic variation in taste receptors are associated with chronic rhinosinusitis: a replication study. Int Forum Allergy Rhinol. 2014;4:200–6.
Miljkovic D, Bassiouni A, Cooksley C, Ou J, Hauben E, Wormald PJ, Vreugde S. Association between group 2 innate lymphoid cells enrichment, nasal polyps and allergy in chronic rhinosinusitis. Allergy. 2014;69:1154–61.
Orlandi RR, Smith TL, Marple BF, Harvey RJ, Hwang PH, Kern RC, Kingdom TT, Luong A, Rudmik L, Senior BA, Toskala E, Kennedy DW. Update on evidence-based reviews with recommendation in adult chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4(Suppl 1):S1–S15.
Patel ZM, Thamboo A, Rudmik L, Nayak JV, Smith TL, Hwang PH. Surgical therapy vs continued medical therapy for medically refractory chronic rhinosinusitis: a systematic review and meta-analysis. Int Forum Allergy Rhinol. 2017;7:119–27.
Perez E, Bonilla FA, Orange JS, Ballow M. Specific antibody deficiency: controversies in diagnosis and management. Front Immunol. 2017;8:586–92.
Peters AT, Spector S, Hsu J, Hamilos DL, Baroody FM, Chandr RK, Grammer LC, Kennedy DW, Cohen NA, Kaliner MA, Wald ER, Karagianis A, Slavin RG. Diagnosis and management of rhinosinusitis: a practice parameter update. Ann Allergy Asthma Immunol. 2014;113:347–85.
Phillips KM, Hoehle L, Bergmark RW, Caradonna DS, Gray ST, Sedaghat AR. Reversal of smoking effects on chronic rhinosinusitis after smoking cessation. Otolaryngol Head Neck Surg. 2017;157(4):737. EPub ahead of print.
Psaltis AJ, Wormald PJ. Therapy of sinonasal microbiome in CRS: a critical approach. Curr Allergy Asthma Rep. 2017;17:59–65.
Psaltis AJ, Wormald PJ, Ha KR, Tan LW. Reduced levels of lactoferrin in biofilm-associated chronic rhinosinusitis. Laryngoscope. 2008;118:895–901.
Purcell PL, Beck S, Davis GE. The impact of endoscopic sinus surgery on total direct healthcare costs among patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2015;5:498–505.
Ramadan HH, Fornelli R, Ortiz AO, Rodman S. Correlation of allergy and severity of sinus disease. Am J Rhinol. 1999;13:345–7.
Rugina M, Serrano E, Klossek JM, Crampette L, Stoll D, Bebear JP, Perrahia M, Rouvier P, Peynegre R. Epidemiological and clinical aspects of nasal polyposis in France; the ORLI group experience. Rhinology. 2002;40:75–9.
Scadding GK, Durham SR, Mirakian R, Jones NS, Drake-Lee AB, Ryan D, Dixon TA, Huber PA, Nasser SM, British Society for Allergy and Clinical Immunology. BSACI guidelines for the management of rhinosinusitis and nasal polyposis. Clin Exp Allergy. 2008;38:260–75.
Schleimer RP. Immunopathogenesis of chronic rhinosinusitis and nasal polyposis. Annu Rev Pathol Mech Dis. 2017;12:331–57.
Scott JR, Ernst HM, Rotenberg BW, Rudmik L, Sowerby LJ. Oral corticosteroid prescribing habits for rhinosinusitis: the American Rhinologic society membership. Am J Rhinol Allergy. 2017;31:22–6.
Seiberling KA, Grammer L, Kern RC. Chronic rhinosinusitis and superantigens. Otolaryngol Clin N Am. 2005;38:1215–36.
Smith TL, Kern R, Palmer JN, Schlosser R, Chandra RK, Chiu AG, Conley D, Mace JC, Fu RF, Stankiewicz J. Medical therapy vs surgery for chronic rhinosinusitis: a prospective, multi-institutional study with 1-year follow-up. Int Forum Allergy Rhinol. 2013;3:4–9.
Smith KA, Orlandi RR, Rudmik L. Cost of adult chronic rhinosinusitis: a systematic review. Laryngoscope. 2015;125:1547–56.
Sundaresan AS, Hirsch AG, Storm M, Tan BK, Kennedy TL, Greene JS, Kern RC, Schwartz BS. Occupational and environmental risk factors for chronic rhinosinusitis: a systematic review. Int Forum Allergy Rhinol. 2015;5:996–1003.
Takabayashi T, Kato A, Peters AT, Hulse KE, Suh LA, Carter R, Norton J, Grammer LC, Cho SH, Tan BK, Chandra RK, Conley DB, Kern RC, Fujieda S, Schleimer RP. Excessive fibrin deposition in nasal polyps caused by fibrinolytic impairment through reduction of tissue plasminogen activator expression. Am J Respir Crit Care Med. 2013;187:49–57.
Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, Zhou J, Norton J, Carter R, Hinchcliff M, Harris K, Peters A, Grammer LC, Kern RC, Mohan C, Schleimer RP. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128:1198–206.
Tan BK, Chandra RK, Pollak J, Kato A, Conley DB, Peters AT, Grammer LC, Avila PC, Kern RC, Stewart WF, Schleimer RP, Schwartz BS. Incidence and associated premorbid diagnoses of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;131:1350–60.
Tan BK, Klinger A, Poposki JA, Stevens WW, Peters AT, Suh LA, Norton J, Carter RG, Hulse KE, Harris KE, Grammer LC, Schleimer RP, Welch KC, Smith SS, Conley DB, Kern RC, Kato A. Heterogeneous inflammatory patterns in chronic rhinosinusitis without nasal polyps in Chicago, Illinois. J Allergy Clin Immunol. 2017;139:699–703.
Tepper SJ. New thoughts on sinus headache. Allergy Asthma Proc. 2004;25:95–6.
Tieu DD, Peters AT, Carter RG, Suh L, Conley DB, Chandra R, Norton J, Grammer LC, Harris KE, Kato A, Kern RC, Schleimer RP. Evidence for diminished levels of epithelial psoriasin and calprotectin in chronic rhinosinusitis. J Allergy Clin Immunol. 2010;125:667–75.
Tomazic PV, Stammberger H, Braun H, Habermann W, Schmid C, Hammer GP, Koele W. Feasibility of balloon sinuplasty in patients with chronic rhinosinusitis: the Graz experience. Rhinology. 2013;51:120–7.
Van Lancker JA, Yarnold PA, Ditto AM, Tripathi A, Conley DB, Kern RC, Harris KE, Grammer LC. Aeroallergen hypersensitivity: comparing patients with nasal polyps to those with allergic rhinitis. Allergy Asthma Proc. 2005;26:109–12.
Veloso-Teles R, Cerejeira R. Endoscopic sinus surgery for chronic rhinosinusitis with nasal polyps: clinical outcome and predictive factors of recurrence. Am J Rhinol Allergy. 2017;31:56–62.
Walsh JE, Gurrola JG 2nd, Graham SM, Mott SL, Ballas ZK. Immunoglobulin replacement therapy reduces chronic rhinosinusitis in patients with antibody deficiency. Int Forum Allergy Rhinol. 2017;7:30–6.
Zhang N, Van Crombruggen K, Bachert C. Barrier function of the nasal mucosa in health and type-2 biased airway diseases. Allergy. 2016;71:295–307.
Zhang Y, Gevaert E, Lou H, Wang X, Zhang L, Bachert C, Zhang N. Chronic rhinosinusitis in Asia. J Allergy Clin Immunol. 2017;140:1230–9.
Zhao YC, Wormald PJ. Biofilm and osteitis in refractory chronic rhinosinusitis. Otolaryngol Clin North Am. 2017;50:49–60.
Zhao YC, Bassiouni A, Tanjararak K, Vreugde S, Wormald PJ, Psaltis AJ. Role of fungi in chronic rhinosinusitis through ITS sequencing. Laryngoscope. 2017;128(1):16. [Epub ahead of print].
Author information
Authors and Affiliations
Corresponding author
Section Editor information
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this entry
Cite this entry
Grammer, L.C. (2019). Chronic Rhinosinusitis and Nasal Polyposis. In: Allergy and Asthma. Springer, Cham. https://doi.org/10.1007/978-3-030-05147-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-05147-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-05146-4
Online ISBN: 978-3-030-05147-1
eBook Packages: MedicineReference Module Medicine