Abstract
Food allergy has become a significant public health burden over the past decades with an ever increasing prevalence. Many different pathophysiologic mechanisms have been investigated and discussed. The current consensus on development of food allergies is the alteration of clinical and immunologic tolerance to foods. Pre- and postnatal exposures and other factors both in the patient and also the environment seem to be the main drivers in this altered immune state resulting in sensitization to food proteins.
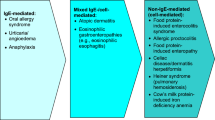
Food allergies can present as many different entities. Pure IgE-mediated allergies are IgE-mediated food allergies or pollen-food cross-reactivities, while atopic dermatitis and eosinophilic esophagitis represent a mixed IgE-/cell-mediated sensitivity to food allergens.
Symptoms of adverse reactions to food allergens manifest in most organ systems, including the lungs, gastrointestinal tract, cardiovascular system, and the skin. Often more than one organ system is affected with anaphylaxis being the most severe and potentially resulting in death.
Clinical history, specific serum IgE testing and skin prick testing are the mainstay in diagnosis of food allergies. Novel diagnostic tools utilizing advances and availability of recombinant allergens and cellular and genetic testing are being investigated.
While novel treatment approaches that are focusing on achievement of tolerance or sustained unresponsiveness are being studied on the cellular level and in clinical trials, the mainstay of management remains strict avoidance of the food allergen.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- IgE-mediated food allergy
- Dual allergen exposure hypothesis
- Anaphylaxis
- Sustained unresponsiveness
- Food challenge proven food allergy
1 Introduction
IgE-mediated food allergy has been increasing in the westernized world over the past decades. Symptoms of IgE-mediated food allergy can manifest in many organ systems, including the lungs, gastrointestinal tract, and skin. The most dramatic manifestation of an acute allergic reaction is anaphylaxis which can lead to hypotension and organ failure and may result in death. The cause of this significant increase is not known and various hypotheses regarding the underlying mechanism have been generated over the past years. Because of the prevalence of food allergy, the universality of food ingestion as a basic means for growth, development, and survival of human kind, and also the social aspect of food ingestion, not only patients and their families are affected by this epidemic. In an attempt to keeping patients safe and to decrease prevalence, recommendations regarding food allergy touch most areas of life, from food introduction in infancy, over guidelines for schools and camps, to food processing and labeling laws. The increased public awareness might also lead to self-imposed food avoidances for suspected reactions.
Extensive research in all aspects of food allergy is being conducted, and diagnosis, management, and treatment guidelines are being adjusted based on novel discoveries. The main focus remains on primary prevention and to establish therapies to achieve tolerance or sustained unresponsiveness in affected patients.
Solid knowledge about etiology, natural history, diagnosis, and management is crucial not only for allergists but also for other health care providers to ensure optimal patient care and selection of appropriate testing and guidance.
2 Epidemiology and Natural History
2.1 Prevalence and Incidence
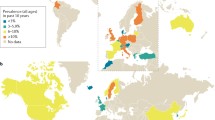
Food allergies are one of the most common medical conditions in the developed world. According to some studies’ metrics, the prevalence of IgE-mediated food allergy as diagnosed by oral food challenge is as high as approximately 3–8% of children and 1–3% of adults (Rona et al. 2007; Osterballe et al. 2005). Using peanut allergy as an example, epidemiologic studies reveal shared findings of high rates of allergies among developed nations. In the USA, the National Health and Nutrition Examination Survey (NHANES) showed a prevalence of peanut allergy of 1.8% of children (Liu et al. 2010). Similarly, peanut allergy affects 1.8% of children in Canada (Gupta et al. 2011), 2% of children in the United Kingdom (Nicolaou et al. 2010), and even as high as 3.0% of children in Australia (Osborne et al. 2011). While these estimates reflect a common prevalence of peanut allergy closer to 2.0%, a compilation of studies go on to reflect that a food allergy of some kind likely affects up to 8% of children and as many as 5% of adults (Sicherer and Sampson 2014). Interestingly however, according to a study investigating the prevalence of food allergies documented in electronic health records, about five times as many individuals will report having allergies than those who have actually undergone allergy testing. Furthermore, of the individuals who actually have undergone allergy testing only about half will test positive for at least an intermediate severity of IgE response (Acker et al. 2017). The increased rates of patient reported allergies indicate growing concern regarding allergic conditions in the developed world.
Allergic disease first came to the forefront as a public health issue in the mid-1900s with what has been described as the “first wave” – when a peak of almost 50% of the populations of westernized countries reported experiencing respiratory symptoms of allergic rhinitis at some stage of life (Prescott and Allen 2011). Over the past two to three decades, however, a “second wave” has since followed with food allergy becoming an important manifestation of allergic disease. Correspondingly, there has been an increase in the number of emergency room visits and hospitalizations for allergic conditions, namely, anaphylaxis, urticaria, and angioedema (Gupta et al. 2007; Lin et al. 2005; Poulos et al. 2007). In addition to the growing incidence of allergic conditions, there is also a decreased likelihood with which afflicted individuals are growing out of their allergies (Prescott and Allen 2011). For example, studies in Australia have shown there to not only be an increased prevalence of IgE-mediated allergic diseases but also a longer disease course associated with allergic conditions, which subsequently increases duration of disease burden and healthcare costs (Longo et al. 2013).
2.2 Risk Factors
There are numerous risk factors implicated in the development of IgE-mediated food allergy. Some of these are unmodifiable risk factors, such as gender and race, while others are modifiable risk factors such as vitamin D and dietary intake, hygiene, and certain environmental exposures. Genetic and/or endocrinologic factors may play a role in the onset of food allergy, as boys have been found to have higher rates of food allergies than girls, while women have higher rates of food allergies than men (Liu et al. 2010; Sicherer et al. 2004). Furthermore, Asian and black children in developed nations also tend to have higher rates of food allergy as compared to white children (Sicherer and Sampson 2014). Comorbid atopic conditions, such as eczema, are associated with higher rates of food allergy as well, and there is also increased likelihood of developing food allergy if a family member also has food allergies (Sicherer and Sampson 2014). This has also been shown in sibling and twin studies. A child has a sevenfold increased risk of developing a peanut allergy if a sibling has a peanut allergy (Hourihane et al. 1996). For monozygotic twins, it has been shown that the risk of peanut allergy is 64% higher if the twin sibling also has a peanut allergy (Sicherer et al. 2000).
Certain lifestyles and dietary choices also appear to predispose to food allergy. For example, studies using NHANES data have described a higher risk of food allergy in children with low vitamin D intake in the children themselves or even in the mother during pregnancy. Similarly, individuals who live farther away from the equator and are exposed to less ambient UV radiation will have decreased endogenous vitamin D production and also have higher rates of food allergy (Osborne et al. 2012; Sheehan et al. 2009). Hygiene and germ exposure may also play a role in the development of food allergies, as children born via C-section have higher rates of food allergies. Conversely, children of lower birth order who are exposed to the infections of their older siblings as well as children who attend daycare at a young age will have lower rates of food allergy (Lack 2012). Other studies have described a higher rate of shellfish allergy among inner-city children who are more frequently exposed to the cross-reactive proteins found in cockroaches (Maloney et al. 2011; Wang et al. 2011).
The Learning Early About Peanut Allergy , or LEAP, trial has triggered a fundamental change in the concept that early food allergen exposure was a risk factor for food allergy to the opposite understanding that food allergen avoidance might actually sensitize the individual to food allergens (Fleischer 2017). In the study, infants with severe eczema, egg allergy, or both were randomized to consume or avoid peanut until the age of 60 months. It was found that early introduction of peanut resulted in a decreased frequency of peanut allergy in this high risk group (Fleischer 2017). It is clear that an understanding of the risk factors that predispose to allergic conditions can provide useful information regarding possible routes to identify and manage high risk populations, provide them with preventative measures, and reduce morbidity, mortality, and healthcare burdens and costs.
2.3 Prevention
The mainstay of prevention of known food allergy remains avoidance of the allergic food trigger. However, there have been recent developments in the understanding of how to possibly lower the risk of onset of food allergy in the first place. For example, while data from CoFAR, the Consortium of Food Allergy Research, have shown that maternal ingestion of peanut during pregnancy will increase infant serum peanut IgE levels, other studies have shown there to be a subsequent decrease in the development of peanut food allergy and asthma (Maslova et al. 2012).
Other data and observations have revealed mixed effects of food allergy prevention attempts. While exclusive breast-feeding continues to be recommended in infants for at least the first 4–6 months of life, certain formulas have been found to confer a protective risk against the development of atopic disease while others have not. Extensively hydrolyzed casein formula has been found to be protective against the development of eczema (but not food allergy) as compared to soy formulas or whole milk based formulas (Des Roches et al. 2012; Kelso et al. 2013).
Numerous studies have shown that avoidance of allergenic foods at a young age may actually be a risk factor in the development of food allergy and atopy in general. Conversely, food diversity at a young age has been shown to result in a decreased risk of atopic sensitization later in life. Individuals who consume more fruits, vegetables, and a variety of home-prepared meals are less likely to develop food allergy (Joseph et al. 2011).
Finally, more recent studies are also showing the protective effects of optimizing the gut flora. Prebiotics, probiotics, synbiotics, and bacterial lysates have been increasingly studied and found to have a role in the reduction of the risk of eczema. While these studies remain inconclusive at this time, it is possible that a better understanding of bacterial diversity of the GI tract will identify another route of protection against food allergy and atopic disease (Pfefferle et al. 2013; Kuitunen 2013).
2.4 Natural History
The natural history of IgE-mediated food allergy diagnosed in childhood has traditionally carried a good prognosis, particularly for milk, egg, wheat, and soy allergies (Savage et al. 2010; Savage et al. 2007; Skripak et al. 2007). However recent studies have shown an increasing inability to tolerate allergenic foods even with increasing age. Previously, for example, about half of children who had a cow milk protein allergy would have resolution of their allergy by 1 year of age, about two-thirds would have resolution by 2 years of age, and as high as 90% of children would have resolution by 3 years of age (Høst 1994). However, more recent studies reveal that IgE-mediated cow milk protein allergy has been found to persist in 21% of children as old as 16 years of age (Skripak et al. 2007). Similar trends are observed with other allergenic foods, such as with egg, soy, wheat, peanut, fish, and shellfish allergy. In all cases, it appears to be the case that higher levels of IgE antibody confer an increased likelihood of persistence of an allergic condition into late childhood (Savage et al. 2010; Savage et al. 2007).
Attempts have been made to quantify the likelihood of resolution of allergic disease based on several factors, including serum IgE levels and skin prick testing results. One such resource is the Consortium of Food Allergy Research, or CoFAR, which has generated calculators predicting milk and egg allergy resolution based on data compiled from a large bank of documented food allergies (www.cofargroup.org). Similarly, resources exist to predict the likelihood of developing food allergy at all. It is worth noting that genetic factors play an important role in the natural history of allergic disease, and for this reason genetic testing has become a promising area for further exploration in an attempt to identify at-risk individuals before the onset of a severe reaction (Li et al. 2016). Moreover, advances have already been made in medicine’s ability to impact the prognosis of allergic conditions and facilitate individuals’ abilities to outgrow their allergies. For example, immunotherapy has yielded promising results for allergic rhinitis for years (Wood 2016). Unfortunately, the efficacy of this therapy has remained limited in regards to food allergies and for this reason allergen avoidance remains the mainstay of treatment. However, with further research and advances in immunotherapy, it is possible that over the next several years the success of immunotherapy in modulating allergic rhinitis may be able to translate to food allergies (Wood 2016).
3 Pathogenesis
3.1 Immunologic Mechanisms
All immune-mediated adverse reactions to a food are subsumed under the term food allergy. Disease entities that are included in this definition are IgE-mediated immediate hypersensitivity reactions, delayed cell-mediated reactions that are not IgE mediated, as well as a mixed presentation of both IgE and non-IgE-mediated reactions. In this chapter, we focus on IgE-mediated food allergies.
3.1.1 Sensitization to Foods
Experimental mouse models have significantly enhanced our understanding of mechanisms of food sensitization. In general, two routes of sensitization are used in mouse models investigating food allergies – sensitization via topical/epicutaneous exposure and sensitization via oral/intestinal exposure.
Early models mainly employed oral sensitization routes and it was noted that pure ingestion of allergens usually leads to oral tolerance in mice. To achieve sensitization via the oral route, adjuvants such as cholera toxin or staphylococcal enterotoxin B (SEB) were used (Ganeshan et al. 2009). As it became evident that topical/epicutaneous sensitization plays a major role in sensitization to food allergens, different mouse models employing epicutaneous sensitization have been developed (Han et al. 2014; Leyva-Castillo et al. 2013; Oyoshi et al. 2010; Tordesillas et al. 2014). Tolerance is the natural response of both mice and human to exposure with harmless food proteins.
In the mouse model, it was shown that tolerance to an orally ingested antigen is mediated by presentation to CD103+ Dendritic cells. Topical exposure to an antigen leads to tolerance via CD11b+ and Langerhans cells.
Three different pathways leading to sensitization via the epicutaneous route (usually with breached integrity of the skin) and oral route (with exogenous adjuvants to break oral tolerance) have been described.
IL-33 expression from both keratinocytes in the skin and intestinal epithelial cells has been shown to be a central cytokine in the development of sensitization and food allergy. Allergenic triggers on intestinal epithelial cells were shown to increase OX40L expression on CD103+DCs and thus causing a predominantly Th2 weighted immune response (Blázquez and Berin 2008). Similarly innate triggers on keratinocytes were shown increase IL-33 expression leading to a Th2 skewed immune response (Tordesillas et al. 2014). In addition to leading to a Th2 skewed immune response, IL-33 also stimulates group 2 innate lymphoid cells (ILC2s). The activations and proliferation of the ILC2 lead to increased production of IL-4 which results in suppressed generation of T- regulatory cells (Tregs) in the small intestine (Noval Rivas et al. 2016). IL-33 can also act directly on mast cells and augment activation in acute reactions to food allergens.
Similarly to IL-33, TSLP can increase OX40L expression on dendritic cells resulting in a Th-2 skewed immune response and recruitment of basophils (Leyva-Castillo et al. 2013). TSLP was also described to exert its effect directly on basophils (Siracusa et al. 2011) also leading to IL-4 production.
Another Th-2 inducing cytokine that plays a major role in food sensitization and allergy is IL-25 and its effect on group 2 innate lymphoid cells. IL-25 leads to release of Th-2 inducing cytokines like IL-4 from ILC2s (Lee et al. 2016).
Recently, an additional cytokine (IL-9) in the allergic response to food was described as a key player. IL-9 is a growth factor for mast cells and overexpression of IL-9 leads to intestinal mastocytosis and increased epithelial permeability (Osterfeld et al. 2010).
3.1.2 Dual Allergen Exposure Hypothesis
This intricate interplay of genetics and environment resulting in the immunopathogenesis of food allergy and the insight gained from murine models is also reflected in many clinical studies.
Most studies concur that the main site of food sensitization, especially in peanut allergy, is the skin. Therefore, atopic dermatitis is a major risk factor for food allergies. This has been described in very early studies investigating the relation of food protein in creams (Lack et al. 2003) and soaps (Fukutomi et al. 2014). The finding that mutations in the protein filaggrin which is essential for maintaining the skin barrier go along with higher rates of atopic dermatitis and also food allergies supports this concept (Brown et al. 2011). House dust which contained peanut protein was described as a major risk for the development of peanut sensitization in children with atopic dermatitis, especially in individuals with Filaggrin mutation.
Similarly, peanut-specific T cells from peanut allergic patients can be found in skin homing but not in gut homing compartments.
On the other hand studies have shown that early oral exposure to food proteins results in lower incidence of food sensitization and food allergies (Du Toit et al. 2008). This concept has been the basis of more recent large clinical trials and has changed recommendations regarding feeding practices and timing of food introduction (Du Toit et al. 2015; Fleischer et al. 2016). The LEAP trial, arguably one of the tide changing publications in food allergies in recent times, investigated if early introduction of peanut protein into the diet of at-risk-infants reduced the rates of peanut sensitization in these infants. This large trial showed significant reduction of peanut allergy in infants randomized into the group that started ingestion of peanut between the ages of 4 and 11 months. This effect was sustained even after peanut was avoided for up to 1 year (Du Toit et al. 2016). A similarly designed trial investigating early introduction of egg failed to show similarly clear results (Palmer et al. 2017). It was noted that a significant proportion of the participants was already sensitized at the age of 4–6 months and egg was tolerated poorly in this cohort.
This approach is also the basis of the EAT trial. In this trial, a group of breast fed infants was introduced to six different allergenic foods to investigate the effect on tolerance. This cohort did not include specifically selected infants with eczema or prior sensitization. The adherence to the intervention was low, but a nonsignificant tendency could be shown for egg and peanut introduction (Perkin et al. 2016).
As mentioned above these trials have led to a change in recommendations. In early 2017 the American Academy of Pediatrics revised guidelines regarding introduction of peanut to advised early introduction of peanut. Infants at risk for food allergies (atopic dermatitis or other already diagnosed sensitization to other foods) are advised to consult a specialist before introduction peanut into their diet (Togias et al. 2017, Image 1).
3.1.3 Genetic Associations
For peanut allergy, genetic risk factors have been identified. Mutation of filaggrin, the gene that promotes barrier function of the skin, has been shown to be positively correlated with peanut allergy (Brown et al. 2011). A large US-based study performed genome wide associations on 2759 patients and reported that the variants HLA-DRB1 and HLA-DQB1 showed a statistically significant association with peanut sensitization (Hong et al. 2015). More recently a multicenter analysis pooling genome-wide association studies from multiple countries, including data from the above-mentioned study, identified c11orf30/EMSY as a gene locus linked to higher risk for both peanut allergy and food allergy in general (Asai et al. 2017).
3.1.4 Hygiene Hypothesis
In 1989 the hygiene hypothesis was first postulated describing an influence of family size on the development of atopic dermatitis and allergic rhinitis (Carpenter et al. 1989). This concept of environmental factors modulating the development of atopy was further supported by several European studies reporting lower rates of allergic disease in children raised in farm environments with ingestion of raw milk and close proximity to livestock (van Neerven et al. 2012; von Mutius and Radon 2008). Many additional trials have since been conducted and report lower rates of atopic disease in children exposed to more diverse environments such as larger families, livestock exposure, or daycare attendance. Savage et al. have shown that a lack of diversity in the microbiome of 3–6 months old children is associated with a higher incidence of reported IgE-mediated food allergy and sensitization at age 3 years (Savage et al. 2017).
3.1.5 Role of Food Processing on Allergenic Properties
The allergenic properties of food are not fixed and innate to the respective food but depend on many additional physical or chemical factors. For fruits for example, it was shown that postharvest storage and ripening can change allergenicity to more allergenic in the case of apples and to less allergenic in the case of mangoes. Thermal treatment of foods has been reported to change allergenicity as well. This has been described for fruits and vegetables in pollen food syndrome where cooking denatures the protein structure and subsequently results in better tolerance of the cooked versions of the food as compared to the raw versions. Similarly, baked milk and egg products are tolerated by a subgroup of patients with milk and egg allergy, which subsequently confers a higher probability of outgrowing food allergies. The reduced allergenicity of baked milk and egg products results from partial denaturation and mainly reaction of the food with the matrix (most commonly the grain flour). However, other foods have been reported to be resistant to thermal degradation, like the major peanut protein Ara h 1 (Koppelman et al. 1999).
Biochemical treatment of food to reduce allergenicity is used in the preparation of hypoallergenic baby formula. Milk is treated with proteolytic enzymes resulting in degradation of the intact milk protein. Residual small protein strands are removed by hypofiltration.
Roasting of peanut on the other hand was found to increase the allergenicity (Gruber et al. 2005; Vissers et al. 2011). This at least partially explains higher rates of peanut sensitization in the United States and certain European countries as opposed to China, which has a similarly high per capita consumption of peanut, however, not roasted peanut.
To better study, the effect and allergenicity of food proteins a mouse model was developed by Ahrens et al. (2014).
Factors influencing sensitization versus tolerance are summarized in Image 2.
4 IgE-Mediated Food Allergy: Subforms
The NIAID-sponsored Expert Panel Report on food allergy is defining food allergy “as an adverse health effect arising from a specific immune response that occurs reproducibly on exposure to a given food” (Boyce et al. 2011). Food allergy encompasses reactions based on IgE-mediated sensitization, non-IgE-mediated processes, cell-mediated reactions, and a mixed presentation of IgE-mediated and cell-mediated reactions. The Expert Panel Report categorized celiac disease as a non-IgE-mediated disorder and allergic contact dermatitis as a cell-mediated disorder.
This book chapter is focusing on IgE-mediated reactions. Non-IgE-mediated and cell-mediated food reactions will be discussed elsewhere.
4.1 IgE-Mediated Food Allergy
Reactions caused by preformed IgE antibodies to food allergens are rapid in onset (minutes to hours) and usually present as one or more of the subforms discussed below.
Eight foods have been reported as the most common food allergens in the United States (milk, egg, wheat, soy, peanut, tree nuts, fish, and shell fish).
Of these, milk, egg, wheat, and soy are food allergies that are mainly present in childhood and are usually outgrown.
Cow’s milk allergy is the most common IgE-mediated food allergy affecting children. It is the third most common food involved in fatal or near fatal reactions (Bock et al. 2007). Symptoms consistent with cow’s milk allergy are found in 5–15% of infants (Rona et al. 2007). Cow’s milk protein is commonly the first foreign protein given to infants in developed countries. Sensitization has been reported to occur in infancy through cow’s milk-based infant formula, skin contact with milk products and even transference of cow’s milk proteins through maternal breast milk has also been reported. Genetic predisposition also plays a major role in the development of cow’s milk allergy. When making the diagnosis of cow’s milk allergy, it is important to distinguish IgE-mediated allergy from lactose intolerance, which is a completely different disease process and presents with gastrointestinal symptoms alone.
There are more than 25 different proteins in cow’s milk that can all act as allergens. 80% of those proteins are caseins and 20% are whey proteins. The caseins are αs1-, αs2-, β-, and κ-caseins (Bos d 8). The most important whey proteins are α-lactalbumin (Bos d 4) and β-lactoglobulin (Bos d 5) (Wal 2004).
Cross-reactivity has been described between cow’s milk and other mammalian milk proteins. Strong cross-reactivity has been observed between cow’s milk and milk from sheep, goat, and buffalo (>90%) and a weak cross-reactivity to mare’s and donkey’s milk (5%). Thus, affected children may react at the first exposure to goat’s or sheep’s milk (Restani et al. 2002).
Interestingly, it has been found that about 13–20% kids with cow’s milk allergy also react to beef. Conversely, about 92% of children with beef allergy have been found to having a concomitant cow’s milk protein allergy (Martelli et al. 2002).
Based on the described cross-reactivities, other mammalian milks should not be used as substitutes in cow’s milk allergic children. Soy milk can be used as a substitute but is not generally recommended as there are high rates of soy milk allergy in cow’s milk allergic children as well, with up to 10–14% of infants with cow’s milk allergy also having been reported sensitized to soy. Additionally many soy- or rice-based drinks do not have the nutritional value needed for optimal growth and development (Bhatia and Greer 2008; Allen et al. 2009).
In infants under 12 months of age, extensively hydrolyzed casein or whey based formulas are usually well tolerated. Occasionally the use of amino acid-based formulas is indicated.
Egg allergy is the second most common food allergy affecting children, after milk allergy. Prevalence of egg allergy has been reported in up to 2.5% of children (Rona et al. 2007). Interestingly egg allergy is the most common food allergy in children with atopic dermatitis (Caubet and Wang 2011). The major allergens in egg were found to be in egg white, ovomucoid, ovalbumin, ovotransferrin, and lysozyme (Leduc et al. 1999; Rupa and Mine 2003). Chicken egg yolk has also been reported to cause IgE-mediated reactions; however, the prevalence is much lower and it is more common in adult patients, opposite to egg white allergy in infants and children.
Milk and egg are unique among the major food allergens in that they can be consumed in both the natural form and in a baked form where heating has altered the allergenicity. The majority of young children can tolerate milk and egg in the baked (heat-denatured form), and it has been shown that children who are able to consume and tolerate milk and egg in the baked forms have higher rates of outgrowing their milk/egg allergy will eventually be able to consume the unaltered forms of milk and egg later in life, (Leonard et al. 2012). A population-based study investigating the resolution of milk allergy has reported close to 60% of children outgrowing their milk allergy by the age of 5 years. Factors that predicted persistence of the allergy beyond 5 years of age included reaction to a small amount of milk at the first exposure (less than 10 mL), having the first reaction at less than 30 days of age and having a large skin prick test size (Elizur et al. 2012).
Seafood allergy has increased following the increased ingestion of seafood over the past few decades, a spike that is thought to be likely secondary due to culinary preferences and the perceived nutritional value. Seafood allergy is typically lifelong as affected individuals generally do not outgrow their allergy. Reactions to seafood are not always IgE mediated, but can be elicited by toxins as described for scombroid poisoning or other toxins (Feng et al. 2016). An important hidden food allergen related to reactions to fish is the allergen derived from the nematode worm Anisakis simplex, which may be found in fish. The parasite was first described in the 1960s and human infestation and infection has been described under the term Anisakiasis. In the 1990s allergic and anaphylactic reactions to fish in nonfish-sensitized patients, initially mainly from Northern Spain have been reported. These reactions were caused by sensitization to Anisakis spp., though it is unclear if a previous infection with the parasite leads to sensitization (Audicana and Kennedy 2008). Contrary to what has initially been thought, cooking or heat treatment does not alter the allergenicity of the antigen.
Reactions to seafood are not only elicited by ingestion but can also be caused by handling seafood and vapor from cooking (James and Crespo 2007).
While usually combined due to origin from the water and also culinary habits, fish and shell fish are different species with unique antigens. Shell fish can be further divided into mollusks (mussels, etc.) and crustaceans (shrimp, lobster, etc.). Parvalbumin is the major food allergen in fish and has been described for Baltic cod (Gad c 1), carp (Cyp c 1), chub mackerel (Sco j 1), and Atlantic salmon (Sal s 1), (Perez-Gordo et al. 2012; Untersmayr et al. 2006). It has been described that the primary sequence of the allergens and resulting IgE binding epitopes are unique to the individual fish, while the secondary and tertiary protein structures are more comparable across different fish. That might explain the relatively low cross-reactivity to other fish species of only 50%.
Tropomyosin is the major allergen reported in shellfish (Hoffman et al. 1981). Tropomyosin is a heat stable pan allergen described in many invertebrates, including shellfish species (mollusks and crustaceans) and also dust mites and cockroaches. This explains the relatively high cross-reactivity of up to 75% or higher between different shell fish species, dust mites, and cockroaches. In fact, antigens to shrimp have been found in populations who do not consume shellfish for religious reasons (Fernandes et al. 2003). Cross-reactivity has also been reported with Anisakis spp. and fish.
Wheat allergy is the best described grain allergy and one of the seven most common food allergies. Allergy to wheat can manifest as an IgE-mediated food allergy, but wheat can also be the trigger for reactions of another underlying immune mechanism/disease entity like celiac disease, baker’s asthma, FPIES to wheat and exacerbation of atopic dermatitis with wheat ingestion among others. IgE-mediated wheat allergy usually starts in infancy and early childhood and is commonly outgrown by adolescence, while some cases persist into adulthood (Keet et al. 2009). While skin prick testing and specific IgE testing are readily available for wheat, the interpretation is more challenging compared to other food allergens. Based on different studies, challenge decision points range from 20 to 100 kU/L (Sampson 2001). In addition to testing, a detailed clinical history and food challenges are crucial in the management of wheat allergy. Patients with wheat allergy are often sensitized to other grains; however, testing is not always informative or available. Food challenges are helpful in these cases.
Soy allergy is more prevalent in infants and young children. The prevalence of soy allergy is thought to be about 0.7% (Zuidmeer et al. 2008). Soy bean is a legume and among the best characterized food allergens. The allergens that are responsible for the majority of the allergic reactions in infants and children are seed storage proteins, Gly m 5, Gly m 6, and Gly m 8. In adults the majority of allergic reactions to soy bean are due to sensitization to the Bet v 1 homologue Gly m 4 (Ito 2015). For all soy components, there is a high cross-sensitivity to other legumes noted; however, cross-reactivity to other legumes is not as common. Because soy bean oils and soy lecithin are common ingredients, in many food products patients require detailed instructions regarding ingestion of these products. Processed soy bean oil and also soy lecithin contain a minimal amount of soy bean protein and are generally considered safe for patients with soy bean allergy.
Peanut allergy is the most publically discussed food allergy with a high prevalence. Investigations on early introduction of peanut into infants’ diet and subsequent changes of recommendations regarding food introduction also contribute to the strong public awareness of peanut allergy (Fleischer et al. 2016; Du Toit et al. 2008, 2015, 2016).
Testing for peanut allergy is available in the form of skin prick testing and spec IgE to whole peanut and peanut components. Testing for peanut by skin prick testing and specific IgE testing has a high positive predictive value. Specific IgE levels between 13 and 15 kU/L have a 95–99 PPV for clinical reactivity in children with suggestive clinical history (Maloney et al. 2008). Similarly, wheal sizes of >8 mm were shown to have a 95–99% PPV in children with suggestive history. In children younger than 2 years, a wheal size of <4 mm was found to be predictive of sensitization.
Tolerance of peanut in patients with test results above the described cutoffs is often due to sensitization to the Bet v 1 homologue Ara h 8. The peanut components Arah h 1, Ara h 2, and Ara h 3 are linked to systemic reactions to peanut (Flinterman et al. 2008).
Tree nut allergy is one of the most common causes for an acute IgE-mediated reaction to food. A recent metaanalysis reported a prevalence of IgE positive, challenge proven tree nut allergy of about 2%. The rate for reported, not challenge confirmed tree nut allergy, was up to 4.9% in the studies included in the analysis (McWilliam et al. 2015). Hazelnut, almond, cashew, pistachio, walnut, pecan, brazil nut, macadamia nut, and pine nut are the most frequently consumed tree nuts in the United States. Based on dietary habits and environmental factors, the prevalence of sensitization to certain tree nuts shows great regional diversity. Hazelnut is the most common tree nut allergy in Europe, while walnut and cashew are responsible for most allergic reactions to tree nuts in the United States. Almost all of the tree nuts have been reported to cause severe and possibly fatal reactions. Tree nut allergy can present both in childhood and adulthood. Both acute IgE-mediated reactions and oral symptoms due to cross-reactivity to the birch pollen component Bet v 1 can be seen in adulthood and teenagers. In children, mostly direct IgE-mediated allergy to tree nuts is being seen. Little is known about the clinical course; it was reported that children who are allergic to two or more tree nuts have a lower chance of outgrowing their tree nut allergy (Fleischer et al. 2005).
Tree nut components and cross-reactivity have been well studied, and IgE testing to tree nut components is available and offered by most major laboratories (Table 1).
4.2 Pollen Food Syndrome
Pollen food syndrome , also known as oral allergy syndrome or pollen associated food allergy syndrome, is a relatively common manifestation of oral allergy symptoms caused by cross-reactivity between food proteins and pollen. Foods that are not of plant origin, such as milk or egg, do not cause pollen food syndrome. Pollen food syndrome is noted in adults with pollen allergy, but it is important to note that not all patients who report symptoms of pollen food syndrome also experience symptoms of seasonal allergy or hay fever.
Pollen food syndrome is thought to be the most common food allergy in adults and likely has become more prevalent with the increase in allergic sensitization to pollen in general (Sicherer 2001).
While pollen food syndrome is more prevalent in adults, it sometimes starts in childhood. Patients usually experience symptoms of pollen allergy first and then go on to develop the oral component. It is often noted that the number of fruits and vegetables the patient reacts to increases over time, this is especially common in children. Symptoms commonly persist lifelong. While allergy immunotherapy directed against the pollen a patient is sensitized to may alleviate the symptoms of seasonal allergies, it is not guaranteed to also affect the oral manifestation of pollen food syndrome.
It is not fully understood why some patients develop pollen food syndrome while others who are also sensitized to pollen do not, though a variety of risk factors have been identified. It was noted that sensitization to tree pollen, especially birch pollen, has been more strongly associated with the development of pollen food syndrome especially if the pollen-related IgE level was significantly elevated or pollen sensitization to more than one variety of pollen was found (90, 91). Patients are more likely to develop pollen food syndrome if they also have symptomatic seasonal allergic rhinitis as opposed to sensitization to pollen alone.
The development of pollen food syndrome is also geographically associated with patients in Northern Europe and the Northern United States, with patients presenting commonly with birch pollen associated symptoms. In comparison, patients in Japan are often sensitized to cedar and present with pollen food syndrome to tomato (Inuo et al. 2015).
The most common manifestation of pollen food syndrome is urticaria of the oral mucosa with associated pruritus and mild angioedema of the lips. Systemic symptoms are rare and have been reported in less than 10% (Ortolani et al. 1993). It is important to note that a genuine food allergy should be suspected in patients with allergic reactions to fruits and vegetables with no concomitant sensitization to pollen noted.
The association of pollen sensitization to the respective fruit is detailed in Table 1.
Patients are usually instructed to avoid the food in the form that is causing symptoms. Occasionally patients report only symptoms to the peel while tolerating the pulp. Often symptoms may vary by season with more significant symptoms noted during the height of the pollen season. Heating of any form of the food commonly results in denaturation of the protein and leads to tolerance; however, heating does not lead to tolerance of the nuts that are also associated with pollen food syndrome.
In addition patients should avoid large amounts of the food, as, for example, in smoothies or other drinkable preparations, since more allergen than can be tolerated may be ingested and will pass mucous membranes more quickly, possibly leading to systemic reactions. Ingestion of allergenic foods on an empty stomach should be avoided, as should ingestion of the food in combination with proton pump inhibitors or other medications that increase the pH of the stomach and lead to decreased destruction and digestion of the food.
Diagnosis of pollen food syndrome includes a detailed history of symptoms and past reactions. Both skin and specific IgE testing can be helpful, especially component testing to determine the degree of sensitization to pollen cross-reactive components of foods. Food challenges might be indicated on a case to case basis. Patients generally are not instructed to avoid the cross-reactive foods; however, patients should be educated about precautions and the forms of the foods that are tolerated, i.e., apple sauce or apple pie as opposed to fresh apple. An epinephrine autoinjector is prescribed for patients who are at a higher risk for systemic reactions, but it is not regularly indicated in patients with simple pollen food associated oral symptoms.
4.3 Association Between Aeroallergens of Animal or Fungal Origin and Food Allergens
Associations of environmental allergens to food allergens of nonplant origin have to be distinguished from pollen associated food allergy syndromes.
Allergic sensitization to indoor arthropods such as dust mites and cockroaches as well as house pets such as cats and dog and sensitization to mold and mold spores have been linked to associated allergic reaction to food allergens.
A cross-reactivity between Alternaria alternata and mushroom and spinach has been reported (Herrera et al. 2002). In addition sensitization to mold via the respiratory tract and subsequent ingestion of food containing mold spores has been reported. A notable case is the reported fatal anaphylaxis of a teenager with reported sensitization to mold and penicillin ingesting a pancake mix that was heavily contaminated by mold spores, resulting in fatal anaphylaxis (Bennett and Collins 2001). Similarly, allergic and anaphylactic reactions in mite sensitized patients who ingested mite containing foods, mainly wheat containing foods (also called Pancake syndrome), have been reported (Sánchez-Borges et al. 2009).
Sensitization to house dust mite has also been reported as the source of sensitization in the dust mite- mollusk-crustacean syndrome, a rare syndrome where sensitization to house dust mites can lead to anaphylactic reactions to shellfish even at the first ingestion (Kütting and Brehler 2001).
Furry pets are an important source of respiratory allergens in the United States and Europe. Cross-reactive serum albumins from mammals kept as pets or farm animals have been reported. Sensitization to the serum albumin occurs by inhalation or ingestion as they are present in all body fluids of the animals. The associated allergy syndrome has been termed pork-cat syndrome. Reactions to ingested pork meat in cat sensitized patients have been reported. The serum albumin is heat labile and therefore reactions are more common to smoked or dried or short cooked meats (Hilger et al. 1997). Cross-reactivity between the serum albumin as an inhalant allergen and ingested allergen is not limited to cat and pork but has been described for other mammal pairs as well as within one animal species. Bovine serum albumin is an important component of cow’s milk and sensitivity to cow’s milk in some cases might result in sensitivity to raw or undercooked beef. However, it is not a general recommendation that all children with cow’s milk allergy also avoid raw or undercooked beef (Vicente-Serrano et al. 2007). This cross-reactivity has to be distinguished from the delayed food-induced anaphylaxis to mammalian meats as described below.
The bird-egg syndrome involves primary sensitization to bird aeroallergens with secondary reactions to egg based on cross-reactivity between the bird allergens (feathers, droppings, serum, and meat) with the egg yolk. Interestingly, the egg-bird syndrome is connected to egg yolk sensitivity that starts in infancy with subsequent bird aeroallergen sensitivity. This is due to the presence of alpha livetin, also known as chicken serum albumin in dander and the egg yolk (Popescu 2015).
4.4 Delayed Food-Induced Anaphylaxis to Mammalian Meats
Allergy to food proteins in the cause of most food allergies and also the forms of meat allergy discussed above.
This entity described here involves sensitization to the carbohydrate epitope galactose-alpha-1,3-galactose (alpha-gal). Alpha-gal was described to be present in the digestive tract of ticks and through a tick bite can be expressed into the human host. Alpha-gal as a carbohydrate moiety is present on cells and tissues of all mammals except the higher order primates which includes humans. Through tick bites, humans can get sensitized to alpha-gal and subsequent ingestion of meat of different species including beef, pork, and lamb leads to a delayed allergic reaction. The reaction is usually delayed by 3–6 h after ingestion. Interestingly, a cluster of reactions to cetuximab, a monoclonal chimeric mouse-human IgG1 monoclonal antibody directed against human epithelial growth factor, was reported mainly in the South Eastern United States. Alpha-gal was detected in cetuximab and patients who developed allergic reactions to cetuximab were generally sensitized to alpha-gal before cetuximab was administered (Chung et al. 2008).
Primary IgE-mediated food allergy to individual meats has to be distinguished from alpha-gal sensitization. Serum IgE to individual types of meats is available as well as IgE for alpha-gal. A detailed clinical and reaction history is also important to aid in the diagnosis and management.
4.5 Food-Dependent Exercise-Induced Anaphylaxis
The term food-dependent exercise-induced anaphylaxis (FDEIA) is reserved for a specific form of anaphylaxis where ingestion of a specific food leads to anaphylaxis if food ingestion is followed by exercise. Ingestion of the food without subsequent exercise does not lead to anaphylaxis, and exercise alone without prior ingestion of the food also does not result in anaphylaxis, distinguishing it from food allergy- and exercise-induced anaphylaxis. Foods commonly involved in this FDEIA are shellfish, wheat, fruits and vegetables (celery), nuts, egg, mushroom, and meats. Rare cases have been reported where any ingestion of solid foods followed by exercise can result in anaphylaxis (Morita et al. 2013). This is an IgE-mediated process and documentation of the presence of food directed IgE antibodies in combination with a convincing clinical presentation helps in making the diagnosis. Exercise challenges after ingestion of the suspected food contrasted to ingestion without subsequent exercise can be confirmatory.
4.6 Food Allergens in Medications
Food allergens can be present in certain medications or formulations as either a contamination of a certain lot of the medication or as a component of the medication other than the active ingredient (usually called excipients).
If a certain lot of medication is contaminated by a food protein, this poses a significant risk for a food allergic patient if the contaminant is significant enough to elicit a reaction, but the medication should not generally be avoided in patients with food allergies. The susceptibility to an allergic reaction to the food component in the medication depends on the patient’s general sensitivity to the food allergen and the IgE level and also the amount of allergen present in the medication. This topic has been reviewed in detail by Kelso in 2014 (Kelso 2014).
5 Mixed IgE Antibody-/Cell-Mediated Allergies
5.1 Atopic Dermatitis
Atopic dermatitis is a chronic, pruritic, inflammatory skin condition that belongs to the family of atopic diseases such as food allergy, asthma, and allergic rhinitis. It is often associated with an increased IgE level and related atopic disorders are more common. Patients with atopic dermatitis are at a higher risk for developing food allergies. Based on the dual allergen exposure hypothesis, patients with atopic dermatitis are at an increased risk of being exposed to the food protein via the skin before oral ingestion and thus at a higher risk of becoming sensitized rather than tolerant.
Total IgE levels are often significantly elevated in patients with atopic dermatitis. Positive testing to food allergens is also very common; however, many patients that are found to be sensitized do not show clinical allergy despite positive testing. Therefore, panels of tests for allergens that are tolerated are not generally recommended in the absence of clinical reactions. In individual patients, ingestion of certain foods can exacerbate their atopic dermatitis. Trial elimination diets and avoidance of the suspected foods for a few weeks should lead to improvement of the skin condition and reintroduction should result in an exacerbation of the skin lesions. Prolonged avoidance of foods might lead to the development of acute IgE-mediated food allergies and therefore caution is warranted when foods are being reintroduced.
5.2 Eosinophilic Gastroenteropathies
Eosinophilic gastroenteropathies (EGID) are characterized by chronic eosinophilic infiltration of parts of the GI tract that lead to clinical gastrointestinal dysfunction pathologic changes of the gastrointestinal tissues. The pathophysiology is poorly understood. Patients with EGID are often also diagnosed with sensitization to food or environmental allergens. Food triggers can often be identified and elimination leads to improvement of clinical, endoscopic, and histologic symptoms. However, the pathophysiologic mechanism is not completely understood.
6 Mimics of Food Allergy
Occasionally patients are seen in the allergy office for presentations that appear to be food allergies, but upon further investigation are found to be conditions that present with similar symptoms but a very different underlying mechanism. A classic example of a disease that presents like an acute IgE-mediated reaction is scombroid fish poisoning, a toxic reaction to histamine-like toxins in spoiled dark fish meat. Occasionally patients present with clear rhinorrhea that is usually linked to food ingestion, most commonly spicy foods. If no additional symptoms are reported, no underlying sensitization is found, and the reaction is linked to ingestion of spicy or savory foods, the diagnosis of gustatory rhinitis can be made. The auriculo-temporal syndrome is another example where a neurologic response leads to increased salivation and reflexive facial vasodilatation of the lower cheek. See also Table 2.
7 Diagnosis
7.1 Clinical/Reaction History
The clinical and reaction history is an essential part of the diagnostic work-up for a suspected food allergy. A detailed history is the initial step in the evaluation of a possible food allergy. The main goal is to distinguish a food allergy from another kind of reaction that is elicited by the food, for example, the differentiation between an acute mediated milk allergy and lactose intolerance. The clinical history also helps to differentiate between the different forms of food allergy, for example, Food Protein Induced Enterocolitis Syndrome elicited by wheat ingestion versus an acute IgE-mediated food allergy to wheat. And lastly, the clinical history often helps to identify the causing food allergen or narrow down to a few possible culprits.
A routine clinical history for the diagnosis of food allergy includes questions regarding the types of food that were ingested, type of reaction with all signs and symptoms, timing of the ingestion and subsequent reaction, treatment of the reaction, and response to that treatment. In addition it is important to document any additional allergic or atopic diseases, other food allergies, or previous reactions to the same or other foods.
When discussing possible foods that might have caused the allergic reaction, it is important to note that the seven foods discussed above are responsible for the majority of the IgE-mediated allergic reactions to food. However, food that the patient consumes on a regular basis is rarely the cause for an allergic reaction, but rather foods that are rarely eaten and are consumed knowingly or as a contaminant of the patient’s food.
Contributory factors, including exercise before or after ingestion of the food, viral infections, or use of medications that might alter the gastric permeability, are important factors to note.
7.2 Signs and Symptoms
7.2.1 Urticaria/Angioedema
Localized or generalized urticaria is the most common form of an allergic reaction to a food. About 20% of cases of acute urticaria have been reported being caused by allergic reactions to food. In comparison chronic urticaria is rarely caused by food allergens. Urticaria is caused by degranulation of mast cells in the superficial dermis and also basophils resulting in mediator release leading to the characteristic symptoms. Angioedema is caused by degranulation of mast cells in deeper layers of the dermis or subcutaneous tissue. Both Urticaria and Angioedema can be the presentation of a localized reaction or be part of a systemic reaction. Generalized flushing and erythema of the skin can also be noted, often when the reaction is progressing to a more systemic form. Ocular symptoms like tearing and conjunctival injection as well as pruritus are also caused by mast cell activation and mediator release.
Acute contact urticaria can be caused by direct skin contact with the relevant food; this is commonly caused by the major allergens but can also be elicited by contact with raw meats, seafood and raw fruits and vegetables (Table 3).
7.2.2 Oropharyngeal Symptoms
As described above for urticaria and angioedema, oropharyngeal symptoms can represent a mild localized reaction or be a prodrome or part of a systemic reaction. Oropharyngeal symptoms as part of the oral allergy syndrome are considered a contact reaction to the profilins of the fruits and vegetables that cross-react with the pollen proteins mainly of tree, grass, and weed pollen (Refer to Sect. 4.2).
7.2.3 Airway Symptoms (Rhinitis/Asthma/Laryngeal Edema)
Allergic rhinitis and asthma in general are common conditions in patients with food allergy because of the shared underlying mechanism and co-presentation of atopic diseases. Additionally rhinitis, rhinorrhea, wheezing, and coughing are common presentations of acute allergic reactions to food allergens. Patients can also present with laryngeal edema and voice changes. They might report a sense of choking or difficulty swallowing their saliva. These symptoms, especially symptoms involving the lungs or larynx, are usually part of a systemic reaction and do not present as isolated symptoms of an acute allergic reaction.
Isolated asthma exacerbations by inhalation of foodstuff, especially flour in a condition called Baker’s Asthma, have been described. However, this form of occupational asthma is caused by the irritation of the lungs by the food product and the food can be ingested without problems.
7.2.4 Gastrointestinal Symptoms
Gastrointestinal symptoms such as nausea, vomiting, abdominal pain, cramping, and diarrhea are common features in anaphylaxis. Isolated nausea can be considered a mild symptom; however, often it progresses to more significant symptoms as vomiting or abdominal cramping. The term gastrointestinal anaphylaxis can be used for severe symptoms that are limited to the GI tract. Nausea and vomiting tend to be early signs of anaphylaxis occurring within a few minutes to 1–2 h, while diarrhea might also present later in the course of the allergic reaction.
7.2.5 Anaphylaxis
Anaphylaxis is an acute allergic reaction that is acute in onset and can progress to death (Sampson et al. 2006). While it is the most severe and also most discussed presentation of a food allergic reaction, it is relatively uncommon with a recent meta-analysis reporting an incidence of 0.14 events per 100 patients years in patients with a diagnosed food allergy (Umasunthar et al. 2015). The rate of fatal anaphylaxis was reported to be 1.81 per one million patient years in patients with a diagnosed food allergy.
Anaphylaxis caused by food ingestion is often noted within minutes of ingestion and characterized by multiple, severe, progressive symptoms. All symptoms and combinations of symptoms described above can be present in anaphylaxis. Gastrointestinal symptoms are often a leading presentation. In addition the reaction can include cardiovascular collapse and may result in death. Patients sometimes describe a feeling of impending doom at the start of the allergic reaction. Cutaneous and gastrointestinal symptoms are more common in children and development of shock is more common in adult patients.
Early signs of anaphylaxis can be variable and it might not be immediately obvious that the reaction will develop into an anaphylactic reaction. The reaction can then progress in a uniphasic fashion with symptom resolution after adequate treatment or may evolve to a biphasic or protracted reaction. Biphasic reactions are characterized by recurrence of symptoms within 1–4 h after apparent resolution of symptoms. About 20% of anaphylactic reactions progress to a biphasic reaction. Protracted reactions are characterized by persistence of symptoms for hours or even days despite treatment.
Several factors influence the development of an anaphylactic reaction and also the severity of the reaction. The amount of the food that was ingested shows a positive correlation with the severity of the symptoms. Ingestion of fatty foods often results in lowered absorption of the allergen and might result in a milder reaction. Concomitant ingestion of alcohol or non-steroidal anti-inflammatory drugs can increase the gastric permeability and lead to more pronounced or rapid symptoms. Fatal anaphylaxis can occur in all ages, but young patients with food allergies are at a higher risk. Risk taking behavior, including ingestion of the food allergen, unavailability of the epinephrine autoinjector, or delayed treatment with epinephrine are risk factors for death from anaphylaxis.
Diagnosis of anaphylaxis aside from reported or observed symptoms can be difficult as no reliable laboratory testing exists. Histamine can be transiently elevated and while tryptase levels can be elevated they are often normal in food induced anaphylaxis. Therefore, negative testing does not exclude an anaphylactic reaction (Sampson et al. 2006).
Treatment of anaphylaxis is reviewed below and summarized in Table 4 (Sect. 8.2).
7.3 Diagnostic Tests
Diagnostic testing for patients with food allergies is a crucial step in diagnosing or confirming and documenting a sensitization and to estimate the risk for reaction.
The Updated Practice Parameters on Allergy Diagnostic Testing in detail summarizes and describes diagnostic testing for allergies.
7.3.1 Skin Testing
The development of skin testing in the historical context is reviewed and summarized in the Updated Practice Parameters. In brief, skin testing was first described in 1867 by Charles Blackley. He reported placing allergens on abraded skin to test the reactivity. This method of placing an allergen on the skin was further developed by von Pirquet who first established the tuberculosis intradermal testing. Over the following years various clinicians furthered this concept and applied this method to various disease contexts. Schloss rubbed food on a small abraded area on the forearm of children to diagnose food allergy. Later Schick and Cooke developed an intracutaneous method to test allergens on the skin. In the 1950s, the practice of producing a skin abrasion for skin testing was changed because it produced permanent skin changes and since it is custom to prick the skin with a lancet, needle, or plastic prick.
Skin testing is based on the principle that IgE is bound to cutaneous mast cells. Allergen exposure cross-links the IgE molecules and leads to Histamine release (Sampson et al. 2014). The resulting wheal and flare reaction can be measured after approximately 15 min. The wheal and flare is documented in millimeter (wheal mm/flare mm) A positive (histamine) and negative (saline) control are applied and read at the same time as the allergen extracts too assess and account for the reactivity of the skin (Khan et al. 2012).
The size of the wheal and flare reaction depends on the location where the test is applied and is usually larger on the back versus the arm. Specific devices used are known to produce a larger reaction, and it is therefore useful to continue using the same type of prick test device to make testing comparable. Potency of skin testing extract depends on the age of the extract and also the manufacturer. In the case of fruit and vegetables, testing with the fresh fruit is usually more potent as the allergenicity is decreased during the production of the extract.
Larger wheal and flare reaction are predictive of a higher likelihood of reaction to the food tested. The severity of the allergic reaction cannot be extrapolated from the size of the skin test reaction (Sporik et al. 2000).
The positive predictive value of skin testing is variable for different foods used. One study showed an excellent positive predictive value of skin testing in children with peanut, milk, and egg allergy; 100% of children with a skin testing larger than 8 mm (>4 mm for children younger than 2 years old) had a positive food challenge to the food tested (Hill et al. 2004).
Widespread skin conditions can make the application and interpretation of skin testing difficult. In patients who have had a severe or anaphylactic reaction to the food, skin testing should only be performed with caution and assessment of risks and benefits. The same holds true for asthmatic patients, especially for patients with a current asthma flare. The skin of infants and young children might be less responsive to skin testing, on the other hand they might be at a higher risk of developing severe reactions to the testing reagents.
Several additional factors influence the responsiveness of the skin to skin testing. In general there is a variable response to skin testing in the individual patient over time. A recent anaphylactic reaction might leave the skin unresponsive for about 6 weeks after the reaction and skin testing is usually deferred during that time period. Concomitant use of H1-anthistamines, H2- receptor blockers, phenothiazine antiemetics, tricyclic antidepressants, higher doses of methotrexate, topical steroids, and also omalizumab can also render the skin less responsive or unresponsive to allergens and histamine. If patients are unsure about prior antihistamine use or there are other questions about the reactivity of the skin, it is common practice to apply only the positive (histamine) and negative (saline) control to test the responsiveness of the skin before applying all allergens that to be tested.
The practice parameters recommend skin testing to help identify foods that might be provoking an IgE-mediated allergy, but also stress that positive skin testing alone is not diagnostic of an IgE-mediated food allergy.
7.3.2 Serum Testing
Another widely available diagnostic test for food allergy is immunoassay testing. These tests are in vitro assays to identify IgE antibodies directed against allergens. Historically radioallergosorbent testing (RAST) was the most widely used test. Today the method for detection of the allergic antibody does not depend on marking it with radioactivity but rather fluorescent dye. The most common immunoassay testing today is Fluorescent Enzyme Immuno Assay (FEIA) . However, the term RAST is still in use and often also reported by clinical laboratories even though the assay used was FEIA. The result is reported in kU/L. Most large studies in the United States use the ImmunoCAP FEIA from Phadia. Results obtained from other Immunoassays are not fully interchangeable. The laboratories also report a class or percentage value of the test results based on a comparison to a standard curve. However, those values are commonly not taken into consideration by allergists when interpreting these results because of their larger heterogeneity.
Serum testing is significantly more expensive than skin testing for food allergies, but it has several advantages over skin testing. It is also available to physicians who do not practice as allergists. It can also be used in patients who are currently or permanently not candidates for skin testing, for either dermatologic conditions, recent anaphylaxis, or medication use that interferes with skin testing. The wide availability also poses a trap and can lead to frequent and unnecessary testing, which then can lead to unnecessary elimination diets.
Serum testing alone is not diagnostic or exclusive of a food allergy and other factors such as clinical history also have to be taken into account.
About 95% predictive values to predict the positive challenge outcome have been established for milk, egg, peanut, tree nuts, and fish. Similar studies for wheat and soy are not available (Martínez-Aranguren et al. 2014).
Technological advances in protein identification and methods have led to the development of specific IgE testing to individual protein components of the allergenic food. Component testing is available for pollen-related plant-derived foods and for animal derived foods. Two different assays exist for the measurement of component specific IgE.
Fluorescent enzyme immunoassays are available for the detection of IgE directed against individual protein components of the food. Testing can be performed to components of selected foods both plant or animal derived. This testing results quantitative levels to individual components and is used increasingly in daily practice to help distinguish between patients at a high risk for an allergic reaction and patients who are sensitized but clinically tolerant to the food tested. This test is available for protein components of most major food allergens and other food allergens. In the United States component testing for certain allergens is FDA-approved and thus usually covered by third party payers. Insurance coverage often determines if these tests are used in the diagnostic work-up of individual patients.
A broader screening method is the ImmunoCAP ISAC (Immune solid phase allergy chip). This test is a protein microarray where binding to multiple proteins is measured simultaneously (refer to Sect. 7.3.6). This test is a semiquantitative screening testing and not used in daily practice by most allergists (Martínez-Aranguren et al. 2014).
Peanut component testing is the most broadly used component test which is also FDA-approved. Interpretation of IgE binding to specific peanut components helps in the differentiation between patients with pollen allergy-induced symptoms with peanut and patients with primary peanut allergy. Peanut component testing is most helpful in certain scenarios: “Patients who have tolerated peanut earlier in life and subsequently have developed mild to moderate, mainly oral symptoms. Patients with an IgE level of 25 kU/L and below. Patients with a concomitant allergy to tree pollen, mainly birch pollen.” It is less likely to add additional essential information in younger children who have had anaphylactic reactions with peanut exposure (Martínez-Aranguren et al. 2014). Sensitization to the heat stable components Ara h1, Ara h2, and Ara h3 is associated with a more severe, systemic reaction then sensitization to the birch (Bet v1)-related component Ara h8 (Table 1).
Testing for hazelnut components is also commercially available. Testing criteria similar to peanut can be applied to hazelnut component testing. Sensitization to Cor a1, a heat labile Bet v1 analog is usually associated with mild oral symptoms. A study from the Netherlands has shown that sensitization to the components Cor a9 and Cor a14 is associated with systemic reactions (Andrews and Banks 2014). In Mediterranean patients, sensitization to the Lipid Transfer Protein (LTP) Cor a8 is associated with severe reactions (Hansen et al. 2009).
Less data are available on component testing for other allergens like soy and wheat. Component testing for most fruits and vegetables is not commercially available and limited to research settings.
7.3.3 Trial Elimination Diets
Elimination diets are a possible step in the diagnosis of food allergies. At least three different types of elimination diets exist.
Elimination diets are commonly used in conditions that do not cause acute anaphylaxis to the food in question but rather a more subtle, chronic reaction as can be seen in patients with atopic dermatitis or eosinophilic esophagitis.
An elimination of one or more foods from the diet for a limited time can be used to determine if the food is causing or exacerbating a chronic condition. The elimination period should not exceed more than 2–3 weeks and in small children or the elimination of multiple common foods should involve the guidance of a nutritionist. If an improvement of the condition is noted, further testing should be initiated to determine a sensitization to the food in question.
Elimination diets are one pillar in the management of Eosinophilic Esophagitis.
Milk is the most common trigger of symptoms in patients with Eosinophilic Esophagitis and a trial of milk (and wheat) avoidance is a common step in the management. These targeted elimination diets in which less than six foods are eliminated are more successful in children then in adults. Other empiric elimination diets as the elimination of the six major food allergens have been studied and shown success in up to 70% of children and adults (Straumann and Schoepfer 2014).
A second, very different elimination diet is the complete avoidance of all foods that are commonly considered antigenic and only very few “oligoantigenic” foods are allowed in the diet. This approach is used in chronic conditions as atopic dermatitis or chronic hives but very rarely (Sicherer 1999).
Diets low in histamine containing foods or other pseudoallergens are occasionally recommended in patients with chronic idiopathic urticarial. Dietary modification in the management of urticarial is controversial and not generally recommended (Bernstein et al. 2014).
Elemental diets are the third and most extreme form of elimination diets. In this form the patient avoids all proteins in the diet and receives nutrients via an aminoacid-based or extensively hydrolyzed formula. This diet is an accepted step in the management of Eosinophilic Esophagitis and has shown success in >80% of patients (Straumann and Schoepfer 2014). However, extreme caution and the guidance of a nutritionist are recommended especially in young children and infants.
In the diagnosis of food triggers in severe atopic dermatitis, elimination diets are sometimes advised for 2 weeks prior to the food challenge. This approach allows for the clearance of the suspected allergen from the system before it is reintroduced as the continued ingestion might leave the skin irritated and not allow for the evaluation of the effect of the food because of the already irritated skin condition.
7.3.4 Food Diary
Keeping a food diary that records all oral intake but also possible skin contact to foods or cosmetics that may contain food protein over a period of 1–2 weeks can help in the identification of potential allergens that were overlooked by the patient and not elicited during the detailed history.
7.3.5 Food Challenge Testing
Food challenge testing is considered the gold standard test for the diagnosis of IgE-mediated food allergy. It is mainly used in two circumstances, to confirm a diagnosis of a food allergy or to check for persistence or resolution of a known food allergy.
A food challenge is usually the gradual feeding of a food under close supervision. The challenge can be in an open fashion or single or double blinded and placebo controlled as described further below. A challenge is usually preceded by a period of abstinence of the food. The food in question is either avoided for therapeutic reasons as it had or is suspected to have had elicited an acute allergic reaction in the past or for diagnostic reasons as the food is suspected to chronically exacerbate a certain condition as detailed above in “Trial Elimination Diets.” Several clinical factors have to be considered when undertaking an oral food challenge. The physician will consider the risk of a continued allergy. IgE levels or skin test sizes that are associated with a high likelihood of reaction or a recent anaphylactic or other severe reaction to the food are strong indicators of a continued food allergy and likely positive challenge outcome and will usually preclude the challenge.
The decision to perform a food challenge should be made by the physician and the patient and/or patient’s family. Several factors of patient readiness and personal preferences should be considered. A challenge could be considered unnecessary if the patient has no interest including the food into the diet or if the food is considered of low nutritional importance or rare and exotic. Again, personal preference of the patient and family on the other hand might make an indication for a challenge in this case. Foods that are considered staple foods might be challenged even if the risk of failing is higher because avoidance might have nutritional and quality of life implications. A concern that a failed challenge will result in higher IgE levels and increased skin test sizes was not found to be true when examined in a cohort of patients undergoing a food challenge to egg, milk, and peanut (Sicherer et al. 2016).
Three main categories of oral food challenges exist: open, single-blind, and double-blind placebo controlled.
The open food challenge is the most common food challenge performed in daily practice. Both the patient and the observer know which food is being tested and the food is administered in an unmasked fashion. There is little concern for bias in a negative food challenge. Symptoms noted during the challenge can be either subjective or objective and bias can be present in both the patient and the provider.
In a single-blind challenge the taste, texture and color of the food is masked. It can be combined with a placebo challenge to help investigate subjective symptoms. Single-blind challenges help to minimize the subjective patient bias but do not change the observer bias.
Double-blind placebo-controlled food challenges are considered the gold standard of the oral food challenges. It is more labor and time intensive and is usually used in research settings. The DBPCFC aims to minimize both the patient and observer bias.
Certain safety precautions have to be considered before undertaking any kind of food challenge. Challenges should be performed in an office or hospital setting depending on the anticipated risk. Adequate rescue equipment, medication, and trained personnel should be available. Patients are recommended to fast 1–2 h before the challenge and should have stopped all antihistamines, beta agonists and beta adrenergic blockers or other medication that might interfere with challenge outcome or necessary resuscitation.
The physician performing the challenge will examine the patient and consider the current state of health and also all present co-morbidities that will interfere with the challenge or treatment of a reaction or that will increase the likelihood of an acute reaction. There has been one reported fatality with an oral food challenge (http://acaai.org/allergists-respond-death-3-year-old-boy-during-oral-food-challenge).
7.3.6 Future Diagnostic Approaches
The main difference between current diagnostic methods for food allergy and novel diagnostic tools for food allergy is the use of more refined technology. Current methods are mainly based on the use of crude allergen extracts of the food. As described above these extracts have the potential to be cross-contaminated. The most refined and detailed form of allergy testing that is currently on the standard armamentarium of the allergist is component testing as reported above.
Novel diagnostic methods are now focusing on other sources of allergens that are sourced by identification and cloning of the allergens leading to less cross-contamination and contamination by carbohydrate epitopes. These conventional diagnostic tests often leave the patient at an equipoise with an oral food challenge being required to make the final determination of clinical reactivity to a food versus sensitization only noted in diagnostic tests.
Peptide and Protein Microarrays have been developed over the past 25 years. The microarray samples up to 5000 individual datapoints on one single chip. Protein microarrays are used to detect sensitization to multiple allergens simultaneously; peptide microarrays detect allergen epitope recognition patterns. It has been shown that microarray assays correlate well with IgE levels but are less sensitive. Protein microarrays designed for the diagnosis of food allergies exist, but no application is FDA approved yet.
Peptide microarrays have been applied to the definition of IgE binding epitopes in food allergy (Lin et al. 2009; Lin and Sampson 2009). IgE binding epitopes of various foods have been reported (Shreffler et al. 2005; Vereda et al. 2010). However, studies investigating the correlation of clinical reactivity to binding patterns are rare. It has been shown that there is a correlation between reaction severity and epitope diversity with patients exhibiting a more diverse epitope profile also showing more severe allergic reactions to the allergen (Flinterman et al. 2008). Another major disadvantage had been the limitation to sequential linear epitopes. Recently studies have expanded the application of microarray to conformational epitopes, furthering the development of preventative and diagnostic methods based on this platform (Hochwallner et al. 2010).
Basophil activation testing is one of the novel tests that are aiming to address the diagnostic conundrum of true allergy versus sensitization. In basophil activation testing, the change in expression of basophil surface protein after activation with an allergen is measured by flow-cytometry (Knol et al. 1991). Basophil activation testing has been used in other fields of allergy but over the past years has also been applied to food allergy diagnosis and prediction of challenge outcome. Glaumann et al. investigated the basophil allergen sensitivity and antibodies to peanut components compared to challenge outcome of DBPCFC to help in the diagnosis of peanut allergy in allergic children (Glaumann et al. 2012). The authors were able to show that a negative basophil allergen threshold sensitivity excluded peanut allergy. In addition basophil activation tests have been used in the differentiation of patients with a clinical allergy to one food and a noted sensitization to a cross-reactive allergen. The basophils of the patients were activated by the allergen they had shown clinical allergy to but not by the cross-reactive allergen that was noted be positive on component testing but did not elicit a clinical reaction (Wallowitz et al. 2007). Basophil activation testing has been used to identify patients with a more sever phenotype. It has been shown that patients with a current milk allergy showed increased basophil activation compared to patients who had outgrown their milk allergy (Wanich et al. 2009).
8 Treatment/Management
8.1 Treatment of Mild Symptoms
Mild localized symptoms can be managed with a trial of oral antihistamines. Liquid diphenhydramine at a dose of 1–2 mg/kg or cetirizine 5–10 mg PO or other first or second generation antihistamines can be given orally for mild localized hives and oral pruritus. However, treatment with antihistamines should not delay administration of IM epinephrine if clinically indicated (Andreae and Andreae 2009). Steroids are often used as a conjunctive treatment but usually do not have a role in management of mild symptoms.
8.2 Emergency Treatment
The management of anaphylaxis has been well characterized and studied. Both in the outpatient and hospital settings, the foremost treatment for anaphylaxis following elimination of exposure to the responsible allergen is intramuscular epinephrine. IM epinephrine can either come as an auto-injector with weight-range dosing or in a 1:1000 solution that is also delivered according to weight. While epinephrine doses may need to be administered as often as every 5–15 min during an anaphylactic event given signs of shock and vital sign abnormalities, there are other adjunctive therapies that can have a role in the management of anaphylaxis as well. Albuterol, a bronchodilator, can be administered in the event of airway narrowing with or without supplemental oxygen as indicated by blood oxygen saturations. Antihistamines, both first and second generation H1 antagonists as well as H2 antagonists, can have a role in the management of anaphylaxis as well. Similarly, additional fluid volumes can be administered if the anaphylactic patient presents with orthostasis, hypotension, or an incomplete response to IM epinephrine. To ensure adequate blood flow to the vital organs, the patient should also lie supine with legs elevated. In a hospital setting, further pharmacologic measures can be employed as well including corticosteroids, vasopressors other than epinephrine, and glucagon (Table 4).
8.3 Avoidance
Avoidance of allergens has been the core feature of food allergy management to date.
On a society level, food allergy has become a focus of attention and awareness of food allergies has significantly increased over the past one to two decades. In 2004 Congress mandated labeling of the eight major food allergens on all packaged food products by passing the Food Allergen Labeling and Consumer Protection Act (FALCPA). This law applies only to packaged foods and does not regulate labeling of food allergens on restaurant menus, for example. This law also only includes the eight major food allergens and other allergens like garlic or celery might be labeled just under spices. For seafood finned fish and crustaceans are labeled while squid and mollusks like clam, mussels, and oysters are not included. Other countries of the world have different regulations in their labeling laws. In addition the advisory statement “may contain…” has not been regulated and is often used by companies as a low cost measure against law suits following possible food allergen exposures or contaminations of the packaged food (Roses 2011).
This area of uncertainty has become an increasing concern among allergy providers and food allergic patients and their families. Studies investigating foods with advisory labeling have found detectable levels of food proteins in about 5.3% of foods that had the advisory labeling versus 1.9% of foods without advisory labeling (Ford et al. 2010). This rate was even higher (42%) in the case of milk contamination of chocolates with the advisory labeling “May contain milk” (Risks associated with foods having advisory labeling Crotty and Taylor). Until this issue is being addressed and potentially thresholds of reactivity are being defined it is considered safer for allergic patients to avoid those foods. Certain situations require a risk benefit analysis, for example, in the case of the influenza vaccine that might contain residual amounts of egg. The benefit of receiving the influenza vaccine most often outweighs the minimal risk of an allergic reaction. The most recent statement from the committee on infectious diseases states that all children with egg allergy can get the influenza vaccine without further precautions (Committee on Infectious Diseases 2016).
Patients have to be aware that also alcoholic beverages may contain common food allergens and nonfood items such as play dough may contain wheat and other arts and craft items such as finger paint may also contain egg.
The public discussion that followed the labeling laws has also brought increasing awareness of food allergies to the restaurant and hospitality industries as well as to schools.
While some schools opt to have peanut and tree nut free classrooms or cafeterias, most of the other common food allergens cannot be easily excluded from the meal plans. Therefore, education of teachers but also cafeteria personal and school bus drivers on food allergies including safe handling of food, recognition and treatment of food allergy reactions and implementation of avoidance measures.
Education of patients and their families on food allergies, emergency treatment, and avoidance practices is the most important aspect of food allergen avoidance. Young children and toddlers have to be strictly supervised. Kindergarten and school age children will have to be instructed not to share foods and about possible food allergens hidden in arts and craft projects. The older the child the more responsibility they will be able to have regarding their food allergies, starting from communicating with teachers, peers, and also restaurant staff. Teens will also be able to start carrying and administering epinephrine by themselves. An important topic that has to be discussed with school age children and especially teenagers is bullying around the topic of food allergies. Sicherer et al. have conducted extensive research around the topic of food allergy-related quality of life issues and mental wellbeing, including bullying. It was found that children with food allergies who are being bullied in school have significantly lower quality of life compared to children with food allergies who are not being bullied. Parental awareness of the bullying does offset the decreased quality of life significantly (133–136). Teenagers should be encouraged to make their friends and teachers aware of their food allergies and signs and symptoms of possible reactions to ensure prompt recognitions and treatment of possible reactions.
Vigilance around food allergies of all parties involved in the patient’s life is crucial to ensure a favorable outcome as most reactions are reported due to lack of vigilance. Wearing medical identification jewelry is helpful, also in older children who become more independent. In general avoidance and treatment plans should be in place for both home, school, restaurants, and also travel. It was found that during international travel communication of food allergies to restaurants can be difficult due to the language barrier. Carrying “chef cards” that explain the food allergy, possible cross-contamination and also methods to prepare allergen-free food in the local language are recommended. Some families prefer to rent apartments when traveling to be able to prepare their own food safely. Airlines have moved away from completely peanut free flights but might restrict peanut distribution on certain flights if a severely peanut allergic patient is traveling on a flight. For infants and toddlers, parents might want to inspect the seat for food residues in folds and crevices of the seat and wipe down the seat and tray table to clean possible contamination from previous passengers.
The role of the allergist in avoidance is first and foremost in education of the patient and family as well as making sure emergency treatment and emergency treatment plans are well understood and in place. Review of the indications and proper administration of injectable epinephrine is indicated at every return visit.
8.4 Emerging Therapies
While avoidance is crucial and at this time the most important step in avoiding reactions to food allergens, affected individuals are having high hopes for an eventual cure or state of less reactivity to food allergens. While some food allergies are often and others are sometimes outgrown as discussed above, there is a large percentage of food allergic individuals who do not outgrow their food allergies and depend on the combination of avoidance and treatment of accidental ingestions.
Over the past decade significant advances have been made in the investigation of treatments for food allergies. The novel treatment approaches can be broadly classified into two groups, food-allergen-specific treatments and nonfood-allergen-specific treatments. The underlying idea is to achieve a state of sustained unresponsiveness to the food allergen in question. As we will discuss below, safety, tolerability, and also side effects of the therapies have to be investigated. The duration of therapy versus the necessity of continued treatment to maintain tolerance is another topic currently being investigated.
8.4.1 Food-Allergen-Specific Therapies
The main goal of food-allergen-specific therapies is to achieve tolerance to the food allergen in question. This would enable patients to consume the food allergen without a reaction and also would allow for periods of avoidance without development of a reaction upon reintroduction. Not all patients are able to reach this state, and while some patients do not reach the full maintenance dose of the allergen, they are able to tolerate smaller amounts of the food allergen shielding them from reactions with accidental ingestion of small or trace amounts. However, in this case patients often have to continue ingesting the tolerated amount of the food allergen daily because reactivity after a prolonged phase of abstinence cannot be excluded.
Oral Immunotherapy has been shown to be effective in both observational studies and randomized clinical trials. It has also received press coverage over the past years sparking more interest from the food allergy patient community. http://www.nytimes.com/2013/03/10/magazine/can-a-radical-new-treatment-save-children-with-severe-allergies.html.
The underlying concept of oral immunotherapy is that oral exposure to a food protein does elicit an immune system response but usually results in oral tolerance rather than sensitization, as described above. The induction of tolerance is thought to be mediated by upregulation of T regulatory cells in response to small amounts of the protein and T cell anergy or deletion in response to large amounts of the protein (Vickery and Burks 2009). While in the first 12 months a gradual increase in the food-specific IgE levels is seen, those levels subsequently decrease and are accompanied by a gradual increase of IgG4 and IgA (Wright et al. 2016).
Different levels of tolerance can be achieved and exact definition of those states is important for management but also continuing research efforts. While some patients do not tolerate the full maintenance dose, their reaction threshold has increased and they are now able to tolerate larger amounts of the allergen, with the main benefit of protecting them from accidental ingestion of small amounts. Up to 75% of patients receiving oral immunotherapy are reaching this state. However, maintaining desensitization is dependent on continued ingestion of the food. Prolonged intervals of abstinence might result in a return of reactivity to the food.
The main goal of the therapy is induction of tolerance meaning achieving full tolerance even after prolonged intervals of abstinence.
In oral immunotherapy, patients are receiving small increasing amounts of the food, starting with a very small dose, gradual increase until a maintenance dose is achieved. Common protocols have the initial and early step-up doses administered in the clinic with the remainder of the dose increases and the maintenance doses being taken at home. Usually the patient is restricted from ingesting the food included in the OIT during the treatment phase. However, it is unclear what treatment interval is required to maintain sustained unresponsiveness. A general recommendation is to continue ingestion of the food indefinitely about 1–2 times per week. It is not defined what period of abstinence followed by proven maintenance of tolerance defines achievement of complete tolerance. Therefore, the term “sustained unresponsiveness” has been coined to describe permanent tolerance.
Most clinical trials include a period of abstinence and it has been shown that starting OIT at a younger age is highly effective in achieving sustained unresponsiveness (Vickery et al. 2017).
In addition it was noted that lower pretreatment IgE levels and longer treatment times lead to higher rates of sustained unresponsiveness (138, 140).
Side effects from OIT are common and have been reported in all phases of build-up and maintenance. Allergic reactions have been reported in all clinical trials and anaphylactic reactions have also been described. These reactions result in some participants withdrawing from the trials. Development of Eosinophilic Esophagitis has also been reported and the rates and mechanism of that remain to be studied in more detail (141–143).
The underlying mechanism bases on the concept that food extracts applied under the tongue are taken up by dendritic cells and presented to T cells in the draining lymph nodes. Most likely this results in an upregulation and activation of T regulatory cells and a downregulation of mast cells. Sublingual immunotherapy as oral immunotherapy has a much milder side effect profile compared to subcutaneous immunotherapy (Narisety et al. 2015). Protocols are similar to OIT schedules, with SLIT having an even milder side effect profile than OIT, with mainly oropharyngeal pruritus and rare reports of anaphylaxis.
Epicutaneous Immunotherapy follows the same basic mechanism of chronic exposure to the allergen, in this case through application on the skin and subsequent dissemination into the stratum corneum. There is a concern that epicutaneous immunotherapy might lead to sensitization rather than desensitization as based on the concepts of sensitization to food allergens described above (145). Clinical trials have now established the importance of applying the epicutaneous patches to intact skin rather than eczematous skin (Mondoulet et al. 2009). In this form of immunotherapy, no dose escalation is required and the initial dose is also the maintenance dose. The rate of allergic reactions is much lower than in other forms of immunotherapy. Side effects were mainly localized to the skin and the resulting drop-off rate was generally low with good adherence reported in most trials. Outcomes of a recently reported US multicenter clinical trial using EPIT for peanut allergy were a generally good tolerance of treatment, good adherence, and but only a modest response to treatment. Efficacy of treatment was found to be higher in younger participants (Jones et al. 2017).
Subcutaneous immunotherapy as a possible treatment for food allergies has been investigated. Clinical trials have shown efficacy, but they have also documented more severe and frequent side effects and allergic reactions to the treatment compared to oral and epicutaneous immunotherapy (Nelson et al. 1997)
Research mostly on mouse models has been conducted using either short overlapping peptides covering the sequence of the entire protein or a chemically modified peanut extract (Zuidmeer-Jongejan et al. 2015).
A more recent approach has been the development of DNA-LAMP vaccines. The allergen is presented to the immune system using lysosome associated membrane proteins (LAMP). This leads to allergen presentation and triggering not only through the MHC class I but also the MHC class II pathway resulting in a CD-8+ and CD-4+ T-cell response (Su et al. 2016).
Genetically modified proteins have been used for subcutaneous and intramuscular immunotherapy. The underlying concept is that these protein sequences are altered in a way that they are not able to trigger and cross-link IgE molecules and therefore do not stimulate mast cells while still being recognized by T cells. This should lead to more safety and a less severe side effect profile.
8.4.2 Therapies Not Specific for Food Allergens
Nonfood-allergen-specific therapies are mainly aimed at reducing the general reactivity of the immune system.
IgE is the main driver of allergic reactions leading to mast cell activation and subsequent release of histamine and other mediators of allergic reactions.
Anti-IgE treatment using omalizumab a humanized monoclonal antibody directed against IgE has been used as a conjunctive treatment in many clinical trials for food-allergen-specific immunotherapy. It has been shown to reduce side effects of oral immunotherapy. It has not been used as a treatment for food allergies alone.
Similarly an anti-IL-4 fully human monoclonal antibody dupilumab has been used in patients with atopic dermatitis and is also under investigation for patients with eosinophilic esophagitis and food allergies.
Li et al. have extensively investigated the effect of Chinese Herbs and mushrooms on the immune system in patients with asthma and food allergies (Srivastava et al. 2012; López-Expósito et al. 2015). The herbal formula for food allergies that was studied in murine models has been used in clinical trials. It has been shown to be safe and well tolerated with an inhibitory effect on basophil numbers. Further clinical investigation is necessary before herbal formulas can implemented as a nonfood-specific treatment for food allergies.
8.5 Unproven Therapies
As discussed above the rate of patient reported food allergy and physician diagnosed food allergy is discordant in the westernized world. The subjective feeling of food allergy or intolerance and the failure to prove this allergy in standardized and validated testing motivates a subgroup of patients to undergo alternative testing methods to validate their symptoms. In addition skin testing and serum IgE testing can only be used to diagnose IgE-mediated food allergy and do not aid in the diagnosis of other food intolerances or food hypersensitivities. It has been reported that about 1 in 5 patients has pursued alternative testing for food allergies for themselves or their child (Ko et al. 2006).
IgG levels to a particular food have been shown to be present if a food is ingested on a regular basis. IGG to cow’s milk can be found in about 98% of children at the age of 2 years (Siroux et al. 2017). There are no studies showing a role of IgG in food allergies. Nevertheless, there are large panels of IgG levels for food testing available to patients and physicians. While these tests are marketed as not being diagnostic for acute or anaphylactic reactions to foods but are rather advertised as supplying additional information aiding in the diagnosis of chronic fatigue or irritable bowel syndrome, many patients interpret the results as diagnostic for food allergies. Hence, positive IgG levels, as are expected if the food is ingested on a regular basis invariably, lead to unnecessary food avoidances.
Hammond and Lieberman report and summarize other unproven methods that are occasionally used in the diagnosis of food allergies (Hammond and Lieberman 2018).
Pulse testing has been reported by author Dr. Arthur Coca in 1956. The underlying concept is based on the belief that sublingual or intradermal exposure to the investigated food will lead to an increase in the pulse by 16 beats per minute if the food tests positive. It is unnecessary to stress that apart from the missing scientific basis of this test, it can put patients with a true IgE-mediated food allergy at an unnecessary risk for severe allergic reactions. Hammond and Lieberman reported that they were unable to identify any scientific reports studying this test.
Provocation and neutralization tests are also exposing the patient to the food either sublingually or intradermally. Any patient reported symptom within 10 minutes of exposure is considered a positive result. This positive test can be followed by a Neutralization phase where the food is given at a different dosage until the reaction subsides. Usually no placebo is used. Again, also this method poses a significant risk for patients with acute IgE-mediated food allergy.
Cytotoxic testing investigates the morphological change of leukocytes after exposure to an allergen. When this method was first described, changes were detected by microscopy. However, already at that time investigators proved that there was no correlation between test result and clinical presentation as well as no reproducibility of the results (Semizzi et al. 2002).
Applied Kinesiology is another unproven method used in diagnosing food allergy. In this test the patient holds a vial with the allergen that is being testing. The investigator applies light pressure to the opposite arm, if a drop in the strength of that arm is noted the test is considered positive. No studies have provided a scientific basis or validity of this method.
Patch testing is an established diagnostic tool for diagnosing contact dermatitis. At this time there is no standardized patch testing method for IgE-mediated food allergies. Patch testing for mixed IgE-/cell-mediated allergies is discussed elsewhere.
9 Conclusion
Food allergy is an increasingly prevalent disease that has been recognized as a significant public health problem in the past decades. While it is agreed upon that prenatal and early life exposures and interactions play a significant role in the development of food allergies, the exact mechanism and combination of factors that lead to the development of sensitization versus tolerance is not known. Different hypotheses to help understand the context that leads to the development of food allergies have been developed, most recently the dual allergen exposure hypothesis. Based on these concepts, research is being focused on primary prevention strategies to help avoid a further increase in food allergies and also immunologic mechanisms to develop treatment tools to achieve desensitization or tolerance in patients that are already affected by food allergies.
References
Acker WW, Plasek JM, Blumenthal KG, Lai KH, Topaz M, Seger DL, et al. Prevalence of food allergies and intolerances documented in electronic health records. J Allergy Clin Immunol. 2017;1761–1773.
Ahrens B, Quarcoo D, Buhner S, Reese G, Vieths S, Hamelmann E. Development of an animal model to evaluate the allergenicity of food allergens. Int Arch Allergy Immunol [Internet]. 2014 [cited 2017 Nov 9];164(2):89–96. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24903216
Allen KJ, Davidson GP, Day AS, Hill DJ, Kemp AS, Peake JE, et al. Management of cow’s milk protein allergy in infants and young children: an expert panel perspective. J Paediatr Child Health [Internet]. 2009 Sep [cited 2017 Nov 24];45(9):481–6. Available from: http://doi.wiley.com/10.1111/j.1440-1754.2009.01546.x
Andreae DA, Andreae MH. Should antihistamines be used to treat anaphylaxis? BMJ [Internet]. 2009 Jul 10 [cited 2018 Jan 4];339:b2489. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19592404
Andrews T, Banks JR. Sensitization to cor a 9 and cor a 14 is highly specific for a hazelnut allergy with objective symptoms in dutch children and adults. Pediatrics [Internet]. 2014 Nov 1 [cited 2017 Dec 31];134 Suppl(Supplement):S152. Available from: http://pediatrics.aappublications.org/cgi/doi/10.1542/peds.2014-1817HH
Asai Y, Eslami A, van Ginkel CD, Akhabir L, Wan M, Ellis G, et al. Genome-wide association study and meta-analysis in multiple populations identifies new loci for peanut allergy and establishes c11orf30/EMSY as a genetic risk factor for food allergy. J Allergy Clin Immunol [Internet]. 2017 Oct 10 [cited 2017 Nov 6]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/29030101
Audicana MT, Kennedy MW. Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. Clin Microbiol Rev [Internet]. 2008 Apr 1 [cited 2017 Nov 15];21.(2):360–79, table of contents. Available from: http://cmr.asm.org/cgi/doi/10.1128/CMR.00012-07
Bennett AT, Collins KA. An unusual case of anaphylaxis. Mold in pancake mix. Am J Forensic Med Pathol [Internet]. 2001 Sep [cited 2018 Jan 3];22(3):292–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11563743
Bernstein JA, Lang DM, Khan DA, Craig T, Dreyfus D, Hsieh F, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol [Internet]. 2014 May [cited 2017 Dec 31];133(5):1270–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24766875
Bhatia J, Greer F, American academy of pediatrics committee on nutrition. Use of soy protein-based formulas in infant feeding. Pediatrics [Internet]. 2008 May 1 [cited 2017 Nov 24];121(5):1062–8. Available from: http://pediatrics.aappublications.org/cgi/doi/10.1542/peds.2008-0564
Blázquez AB, Berin MC. Gastrointestinal dendritic cells promote Th2 skewing via OX40L. J Immunol [Internet]. 2008;180(7):4441–50. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18354165
Bock SA, Muñoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J Allergy Clin Immunol [Internet]. 2007 Apr [cited 2017 Nov 24];119(4):1016–8. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0091674906038140
Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. Nutr Res [Internet]. 2011 Jan [cited 2017 Nov 9];31(1):61–75. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21310308
Brown SJ, Asai Y, Cordell HJ, Campbell LE, Zhao Y, Liao H, et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol [Internet]. 2011 Mar [cited 2017 Oct 31];127(3):661–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21377035
Carpenter L, Beral V, Strachan D, Ebi-Kryston KL, Inskip H. Respiratory symptoms as predictors of 27 year mortality in a representative sample of British adults. BMJ [Internet]. 1989 Aug 5 [cited 2017 Nov 9];299(6695):357–61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2506967
Caubet J-C, Wang J. Current understanding of egg allergy. Pediatr Clin N Am [Internet]. 2011 Apr [cited 2017 Nov 16];58(2):427–43, xi. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0031395511000162
Chung CH, Mirakhur B, Chan E, Le Q-T, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-α-1,3-galactose. N Engl J Med [Internet]. 2008 Mar 13 [cited 2018 Jan 4];358(11):1109–17. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18337601
Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2016–2017. Pediatrics [Internet]. 2016 Oct 1 [cited 2017 Nov 24];138(4):e20162527–e20162527. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27600320
Des Roches A, Paradis L, Gagnon R, Lemire C, Bégin P, Carr S, et al. Egg-allergic patients can be safely vaccinated against influenza. J Allergy Clin Immunol. 2012;130(5):1213–1216.e1.
Du Toit G, Katz Y, Sasieni P, Mesher D, Maleki SJ, Fisher HR, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008;122(5):984–91.
Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med [Internet]. 2015;372(9):803–13. Available from: http://www.nejm.org/doi/10.1056/NEJMoa1414850
Du Toit G, Sayre PH, Roberts G, Sever ML, Lawson K, Bahnson HT, et al. Effect of avoidance on peanut allergy after early peanut consumption. N Engl J Med [Internet]. 2016;374(15):1435–43. Available from: http://www.nejm.org/doi/10.1056/NEJMoa1514209
Elizur A, Rajuan N, Goldberg MR, Leshno M, Cohen A, Katz Y. Natural course and risk factors for persistence of IgE-mediated cow’s milk allergy. J Pediatr [Internet]. 2012 Sep [cited 2017 Nov 14];161(3):482–487.e1. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22480700
Feng C, Teuber S, Gershwin ME. Histamine (scombroid) fish poisoning: a comprehensive review [Internet]. Clin Rev Allergy Immunol. 2016 [cited 2017 Nov 15]. p. 64–69. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25876709
Fernandes J, Reshef A, Patton L, Ayuso R, Reese G, Lehrer SB. Immunoglobulin E antibody reactivity to the major shrimp allergen, tropomyosin, in unexposed Orthodox Jews. Clin Exp Allergy [Internet]. 2003 Jul [cited 2017 Nov 15];33(7):956–61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12859453
Fleischer DM. Life after LEAP: how to implement advice on introducing peanuts in early infancy. J Paediatr Child Health. 2017;53(S1):3–9.
Fleischer DM, Conover-Walker MK, Matsui EC, Wood RA. The natural history of tree nut allergy. J Allergy Clin Immunol [Internet]. 2005 Nov [cited 2018 Jan 10];116(5):1087–93. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0091674905020452
Fleischer DM, Sicherer S, Greenhawt M, Campbell D, Chan E, Muraro A, et al. Consensus communication on early peanut introduction and prevention of peanut allergy in high-risk infants. Pediatr Dermatol. 2016;33(1):103–6.
Flinterman AE, Knol EF, Lencer DA, Bardina L, den Hartog Jager CF, Lin J, et al. Peanut epitopes for IgE and IgG4 in peanut-sensitized children in relation to severity of peanut allergy. J Allergy Clin Immunol [Internet]. 2008 Mar [cited 2017 Dec 30];121(3):737–743.e10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18234310
Ford LS, Taylor SL, Pacenza R, Niemann LM, Lambrecht DM, Sicherer SH. Food allergen advisory labeling and product contamination with egg, milk, and peanut. J Allergy Clin Immunol [Internet]. 2010 Aug [cited 2017 Nov 24];126(2):384–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20621349
Fukutomi Y, Taniguchi M, Nakamura H, Akiyama K. Epidemiological link between wheat allergy and exposure to hydrolyzed wheat protein in facial soap. Allergy Eur J Allergy Clin Immunol. 2014;69(10):1405–11.
Ganeshan K, Neilsen CV, Hadsaitong A, Schleimer RP, Luo X, Bryce PJ. Impairing oral tolerance promotes allergy and anaphylaxis: a new murine food allergy model. J Allergy Clin Immunol. 2009;123(1):231–238.
Glaumann S, Nopp A, Johansson SGO, Rudengren M, Borres MP, Nilsson C. Basophil allergen threshold sensitivity, CD-sens, IgE-sensitization and DBPCFC in peanut-sensitized children. Allergy. [Internet]. 2012 Feb [cited 2017 Dec 30];67(2):242–7. Available from: http://doi.wiley.com/10.1111/j.1398-9995.2011.02754.x
Gruber P, Becker W-M, Hofmann T. Influence of the maillard reaction on the allergenicity of rAra h 2, a recombinant major allergen from peanut (Arachis hypogaea), its major epitopes, and peanut agglutinin. J Agric Food Chem [Internet]. 2005 Mar 23 [cited 2017 Nov 15];53(6):2289–96. Available from: http://pubs.acs.org/doi/abs/10.1021/jf048398w
Gupta R, Sheikh A, Strachan DP, Anderson HR. Time trends in allergic disorders in the UK. Thorax. 2007;62(1):91–6.
Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128(1):e9–17.
Hammond C, Lieberman JA. Unproven diagnostic tests for food allergy. Immunol Allergy Clin N Am [Internet]. 2018 Feb [cited 2018 Jan 9];38(1):153–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29132671
Han H, Thelen TD, Comeau MR, Ziegler SF. Thymic stromal lymphopoietin-mediated epicutaneous inflammation promotes acute diarrhea and anaphylaxis. J Clin Invest. 2014;124(12):5442–52.
Hansen KS, Ballmer-Weber BK, Sastre J, Lidholm J, Andersson K, Oberhofer H, et al. Component-resolved in vitro diagnosis of hazelnut allergy in Europe. J Allergy Clin Immunol [Internet]. 2009 May [cited 2017 Dec 31];123(5):1134–41, 1141.e1-3. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0091674909002310
Herrera I, Moneo I, Caballero ML, de Paz S, Perez Pimiento A, Rebollo S. Food allergy to spinach and mushroom. Allergy [Internet]. 2002 Mar [cited 2018 Jan 3];57(3):261–2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11906344
Hilger C, Kohnen M, Grigioni F, Lehners C, Hentges F. Allergic cross-reactions between cat and pig serum albumin. Study at the protein and DNA levels. Allergy [Internet]. 1997 Feb [cited 2018 Jan 3];52(2):179–87. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9105522
Hill DJ, Heine RG, Hosking CS. The diagnostic value of skin prick testing in children with food allergy. Pediatr Allergy Immunol [Internet]. 2004 Oct [cited 2017 Dec 31];15(5):435–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15482519
Hochwallner H, Schulmeister U, Swoboda I, Focke-Tejkl M, Civaj V, Balic N, et al. Visualization of clustered IgE epitopes on alpha-lactalbumin. J Allergy Clin Immunol [Internet]. 2010 Jun [cited 2017 Dec 30];125(6):1279–1285.e9. Available from: http://linkinghub.elsevier.com/retrieve/pii/S009167491000504X
Hoffman DR, Day ED, Miller JS. The major heat stable allergen of shrimp. Ann Allergy [Internet]. 1981 Jul [cited 2017 Nov 15];47(1):17–22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7258736
Hong X, Hao K, Ladd-Acosta C, Hansen KD, Tsai H-J, Liu X, et al. Genome-wide association study identifies peanut allergy-specific loci and evidence of epigenetic mediation in US children. Nat Commun [Internet]. 2015 Feb 24 [cited 2017 Nov 6];6:6304. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25710614
Høst A. Cow’s milk protein allergy and intolerance in infancy. Some clinical, epidemiological and immunological aspects. Pediatr Allergy Immunol. 1994;5(5 Suppl):1–36.
Hourihane JO, Dean TP, Warner JO. Peanut allergy in relation to heredity, maternal diet, and other atopic diseases: results of a questionnaire survey, skin prick testing, and food challenges. BMJ. 1996;313(7056):518–21.
Inuo C, Kondo Y, Tanaka K, Nakajima Y, Nomura T, Ando H, et al. Japanese cedar pollen-based subcutaneous immunotherapy decreases tomato fruit-specific basophil activation. Int Arch Allergy Immunol [Internet]. 2015 [cited 2018 Jan 3];167(2):137–45. Available from: https://www.karger.com/Article/FullText/437325
Ito K. Grain and legume allergy. Chem Immunol Allergy [Internet]. 2015 [cited 2018 Jan 8];101:145–151. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26022874
James JM, Crespo JF. Allergic reactions to foods by inhalation. Curr Allergy Asthma Rep [Internet]. 2007 Jun [cited 2017 Nov 15];7(3):167–74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17448326
Jones SM, Sicherer SH, Burks AW, Leung DYM, Lindblad RW, Dawson P, et al. Epicutaneous immunotherapy for the treatment of peanut allergy in children and young adults. J Allergy Clin Immunol [Internet]. 2017 Apr [cited 2017 Nov 30];139(4):1242–1252.e9. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0091674916309666
Joseph CLM, Ownby DR, Havstad SL, Woodcroft KJ, Wegienka G, MacKechnie H, et al. Early complementary feeding and risk of food sensitization in a birth cohort. J Allergy Clin Immunol. 2011;127(5):1203–1210.e5.
Keet CA, Matsui EC, Dhillon G, Lenehan P, Paterakis M, Wood RA. The natural history of wheat allergy. Ann Allergy Asthma Immunol [Internet]. 2009 May [cited 2018 Jan 8];102(5):410–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19492663
Kelso JM. Potential food allergens in medications. J Allergy Clin Immunol [Internet]. 2014 Jun [cited 2018 Jan 4];133(6):1509–18. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24878443
Kelso JM, Greenhawt MJ, Li JT, Joint task force on practice parameters (JTFPP). Update on influenza vaccination of egg allergic patients. Ann Allergy Asthma Immunol. Elsevier; 2013;111(4):301–302.
Khan FM, Ueno-Yamanouchi A, Serushago B, Bowen T, Lyon AW, Lu C, et al. Basophil activation test compared to skin prick test and fluorescence enzyme immunoassay for aeroallergen-specific immunoglobulin-E. Allergy Asthma Clin Immunol [Internet]. 2012 Jan 20 [cited 2017 Dec 30];8(1):1. Available from: http://aacijournal.biomedcentral.com/articles/10.1186/1710-1492-8-1
Knol EF, Mul FP, Jansen H, Calafat J, Roos D. Monitoring human basophil activation via CD63 monoclonal antibody 435. J Allergy Clin Immunol [Internet]. 1991 Sep [cited 2017 Dec 30];88(3 Pt 1):328–38. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1716273
Ko J, Lee JI, Muñoz-Furlong A, Li X, Sicherer SH. Use of complementary and alternative medicine by food-allergic patients. Ann Allergy Asthma Immunol [Internet]. 2006 Sep [cited 2018 Jan 9];97(3):365–9. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1081120610608022
Koppelman SJ, Bruijnzeel-Koomen CA, Hessing M, de Jongh HH. Heat-induced conformational changes of Ara h 1, a major peanut allergen, do not affect its allergenic properties. J Biol Chem [Internet]. 1999 Feb 19 [cited 2017 Nov 9];274(8):4770–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9988715
Kuitunen M. Probiotics and prebiotics in preventing food allergy and eczema. Curr Opin Allergy Clin Immunol. 2013;13(3):280–6.
Kütting B, Brehler R. House dust mite-crustaceans-molluscs syndrome. A rare variant of food allergy in primary sensitization to inhaled allergens. Hautarzt [Internet]. 2001 Aug [cited 2018 Jan 3];52(8):708–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11544942
Lack G. Update on risk factors for food allergy. J Allergy Clin Immunol. 2012;129(5):1187–97.
Lack G, Fox D, Northstone K, Golding J. Factors associated with the development of peanut allergy in childhood. N Engl J Med. 2003;348(11):977–85.
Leduc V, Demeulemester C, Polack B, Guizard C, Le Guern L, Peltre G. Immunochemical detection of egg-white antigens and allergens in meat products. Allergy [Internet]. 1999 May [cited 2017 Nov 16];54(5):464–72. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10380777
Lee JB, Chen CY, Liu B, Mugge L, Angkasekwinai P, Facchinetti V, et al. IL-25 and CD4+ TH2 cells enhance type 2 innate lymphoid cell-derived IL-13 production, which promotes IgE-mediated experimental food allergy. J Allergy Clin Immunol. 2016;137(4):1216–1225.e5.
Leonard SA, Sampson HA, Sicherer SH, Noone S, Moshier EL, Godbold J, et al. Dietary baked egg accelerates resolution of egg allergy in children. J Allergy Clin Immunol [Internet]. 2012 Aug [cited 2017 Nov 14];130(2):473–480.e1. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22846751
Leyva-Castillo JM, Hener P, Michea P, Karasuyama H, Chan S, Soumelis V, et al. Skin thymic stromal lymphopoietin initiates Th2 responses through an orchestrated immune cascade. Nat Commun [Internet]. 2013;4. Available from: http://www.nature.com/doifinder/10.1038/ncomms3847
Li J, Maggadottir SM, Hakonarson H. Are genetic tests informative in predicting food allergy? Curr Opin Allergy Clin Immunol. 2016;16(3):257–64.
Lin J, Sampson HA. The role of immunoglobulin E-binding epitopes in the characterization of food allergy. Curr Opin Allergy Clin Immunol [Internet]. 2009 Aug [cited 2017 Dec 30];9(4):357–63. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00130832-200908000-00014
Lin RY, Cannon AG, Teitel AD. Pattern of hospitalizations for angioedema in New York between 1990 and 2003. Ann Allergy Asthma Immunol. 2005;95(2):159–66.
Lin J, Bardina L, Shreffler WG, Andreae DA, Ge Y, Wang J, et al. Development of a novel peptide microarray for large-scale epitope mapping of food allergens. J Allergy Clin Immunol [Internet]. 2009 Aug [cited 2017 Dec 30];124(2):315–22, 322.e1-3. Available from: http://linkinghub.elsevier.com/retrieve/pii/S009167490900815X
Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2010;126(4):798–806.e14.
Longo G, Berti I, Burks AW, Krauss B, Barbi E. IgE-mediated food allergy in children. Lancet. 2013;382(9905):1656–64.
López-Expósito I, Srivastava KD, Birmingham N, Castillo A, Miller RL, Li X-M. Maternal antiasthma simplified herbal medicine intervention therapy prevents airway inflammation and modulates pulmonary innate immune responses in young offspring mice. Ann Allergy Asthma Immunol [Internet]. 2015 Jan [cited 2017 Nov 30];114(1):43–51.e1. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25465920
Maloney JM, Rudengren M, Ahlstedt S, Bock SA, Sampson HA. The use of serum-specific IgE measurements for the diagnosis of peanut, tree nut, and seed allergy. J Allergy Clin Immunol [Internet]. 2008 Jul [cited 2018 Jan 8];122(1):145–51. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0091674908007331
Maloney JM, Nowak-Węgrzyn A, Wang J. Children in the inner city of New York have high rates of food allergy and IgE sensitization to common foods. J Allergy Clin Immunol. 2011;128(1):214–5.
Martelli A, De Chiara A, Corvo M, Restani P, Fiocchi A. Beef allergy in children with cow’s milk allergy; cow’s milk allergy in children with beef allergy. Ann Allergy Asthma Immunol [Internet]. 2002 Dec [cited 2017 Nov 24];89(6 Suppl 1):38–43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12487203
Martínez-Aranguren R, Lizaso MT, Goikoetxea MJ, García BE, Cabrera-Freitag P, Trellez O, et al. Is the determination of specific IgE against components using ISAC 112 a reproducible technique? Uversky VN, editor. PLoS One [Internet]. 2014 Feb 6 [cited 2017 Dec 31];9(2):e88394. Available from: http://dx.plos.org/10.1371/journal.pone.0088394
Maslova E, Granström C, Hansen S, Petersen SB, Strøm M, Willett WC, et al. Peanut and tree nut consumption during pregnancy and allergic disease in children-should mothers decrease their intake? Longitudinal evidence from the Danish National Birth Cohort. J Allergy Clin Immunol. Elsevier; 2012;130(3):724–732.
McWilliam V, Koplin J, Lodge C, Tang M, Dharmage S, Allen K. The prevalence of tree nut allergy: a systematic review. Curr Allergy Asthma Rep [Internet]. 2015 Sep 2 [cited 2018 Jan 10];15(9):54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26233427
Mondoulet L, Dioszeghy V, Ligouis M, Dhelft V, Dupont C, Benhamou P-H. Epicutaneous immunotherapy on intact skin using a new delivery system in a murine model of allergy. Clin Exp Allergy [Internet]. 2009 Dec 10 [cited 2017 Nov 30];40(4):659–67. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20002446
Morita E, Chinuki Y, Takahashi H. Recent advances of in vitro tests for the diagnosis of food-dependent exercise-induced anaphylaxis. J Dermatol Sci [Internet]. 2013 Sep [cited 2018 Jan 4];71(3):155–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23669019
von Mutius E, Radon K. Living on a farm: impact on asthma induction and clinical course. Immunol Allergy Clin North Am [Internet]. 2008 Aug [cited 2017 Nov 9];28(3):631–47, ix–x. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0889856108000398
Narisety SD, Frischmeyer-Guerrerio PA, Keet CA, Gorelik M, Schroeder J, Hamilton RG, et al. A randomized, double-blind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy. J Allergy Clin Immunol [Internet]. 2015 May [cited 2017 Nov 30];135(5):1275–1282.e6. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0091674914016005
van Neerven RJJ, Knol EF, Heck JML, Savelkoul HFJ. Which factors in raw cow’s milk contribute to protection against allergies? J Allergy Clin Immunol [Internet]. 2012 Oct [cited 2017 Nov 9];130(4):853–8. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0091674912011955
Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol [Internet]. 1997 Jun [cited 2017 Nov 30];99(6 Pt 1):744–51. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9215240
Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010;125(1):191–197.e13.
Noval Rivas M, Burton OT, Oettgen HC, Chatila T. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J Allergy Clin Immunol [Internet]. 2016;138(3):801–811.e9. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0091674916300835%5C, http://www.ncbi.nlm.nih.gov/pubmed/27177780%5C, http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC5014699
Ortolani C, Pastorello EA, Farioli L, Ispano M, Pravettoni V, Berti C, et al. IgE-mediated allergy from vegetable allergens. Ann Allergy [Internet]. 1993 Nov [cited 2018 Jan 3];71(5):470–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8250353
Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127(3):668–676.e2.
Osborne NJ, Ukoumunne OC, Wake M, Allen KJ. Prevalence of eczema and food allergy is associated with latitude in Australia. J Allergy Clin Immunol. 2012;129(3):865–7.
Osterballe M, Hansen TK, Mortz CG, Host A, Bindslev-Jensen C. The prevalence of food hypersensitivity in an unselected population of children and adults. Pediatr Allergy Immunol. 2005;16(7):567–73.
Osterfeld H, Ahrens R, Strait R, Finkelman FD, Renauld JC, Hogan SP. Differential roles for the IL-9/IL-9 receptor??-chain pathway in systemic and oral antigen-induced anaphylaxis. J Allergy Clin Immunol. 2010;125(2):469–476
Oyoshi MK, Larson RP, Ziegler SF, Geha RS. Mechanical injury polarizes skin dendritic cells to elicit a TH2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol. 2010;126(5)
Palmer DJ, Sullivan TR, Gold MS, Prescott SL, Makrides M. Randomized controlled trial of early regular egg intake to prevent egg allergy. J Allergy Clin Immunol. 2017;139(5):1600–1607.e2.
Perez-Gordo M, Lin J, Bardina L, Pastor-Vargas C, Cases B, Vivanco F, et al. Epitope mapping of Atlantic salmon major allergen by peptide microarray immunoassay. Int Arch Allergy Immunol [Internet]. 2012 [cited 2017 Nov 15];157(1):31–40. Available from: https://www.karger.com/Article/FullText/324677
Perkin MR, Logan K, Tseng A, Raji B, Ayis S, Peacock J, et al. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med [Internet]. 2016;374(18):1733–43. Available from: http://www.nejm.org/doi/10.1056/NEJMoa1514210
Pfefferle PI, Prescott SL, Kopp M. Microbial influence on tolerance and opportunities for intervention with prebiotics/probiotics and bacterial lysates. J Allergy Clin Immunol. Elsevier; 2013;131(6):1453–1463.
Popescu F-D. Cross-reactivity between aeroallergens and food allergens. World J Methodol [Internet]. 2015 Jun 26 [cited 2018 Jan 3];5(2):31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26140270
Poulos LM, Waters A-M, Correll PK, Loblay RH, Marks GB. Trends in hospitalizations for anaphylaxis, angioedema, and urticaria in Australia, 1993–1994 to 2004–2005. J Allergy Clin Immunol. 2007;120(4):878–84.
Prescott S, Allen KJ. Food allergy: riding the second wave of the allergy epidemic. Pediatr Allergy Immunol. 2011;22(2):155–60.
Restani P, Beretta B, Fiocchi A, Ballabio C, Galli CL. Cross-reactivity between mammalian proteins. Ann Allergy Asthma Immunol [Internet]. 2002 Dec [cited 2017 Nov 24];89(6 Suppl 1):11–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12487198
Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol [Internet]. 2007 Sep [cited 2017 Nov 16];120(3):638–46. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17628647
Roses JB. Food allergen law and the food allergen Labeling and consumer protection act of 2004: falling short of true protection for food allergy sufferers. Food Drug Law J [Internet]. 2011 [cited 2017 Nov 24];66(2):225–242, ii. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24505841
Rupa P, Mine Y. Immunological comparison of native and recombinant egg allergen, ovalbumin, expressed in Escherichia coli. Biotechnol Lett [Internet]. 2003 Nov [cited 2017 Nov 16];25(22):1917–24. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14719827
Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol [Internet]. 2001 May [cited 2018 Jan 8];107(5):891–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11344358
Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report – second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med [Internet]. 2006 Apr [cited 2018 Jan 4];47(4):373–80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16546624
Sampson HA, Aceves S, Bock SA, James J, Jones S, Lang D, et al. Food allergy: a practice parameter update-2014. J Allergy Clin Immunol [Internet]. 2014 Nov [cited 2017 Dec 30];134(5):1016–25.e43. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0091674914006721
Sánchez-Borges M, Suárez-Chacon R, Capriles-Hulett A, Caballero-Fonseca F, Iraola V, Fernández-Caldas E. Pancake syndrome (oral mite anaphylaxis). World Allergy Organ J [Internet]. 2009 May [cited 2018 Jan 3];2(5):91–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23283016
Savage JH, Matsui EC, Skripak JM, Wood RA. The natural history of egg allergy. J Allergy Clin Immunol. 2007;120(6):1413–7.
Savage JH, Kaeding AJ, Matsui EC, Wood RA. The natural history of soy allergy. J Allergy Clin Immunol. 2010;125(3):683–6.
Savage JH, Lee-Sarwar KA, Sordillo J, Bunyavanich S, Zhou Y, O’Connor G, et al. A prospective microbiome-wide association study of food sensitization and food allergy in early childhood. Allergy [Internet]. 2017 Aug 2 [cited 2017 Nov 9]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/28632934
Semizzi M, Senna G, Crivellaro M, Rapacioli G, Passalacqua G, Canonica WG, et al. A double-blind, placebo-controlled study on the diagnostic accuracy of an electrodermal test in allergic subjects. Clin Exp Allergy [Internet]. 2002 Jun [cited 2018 Jan 9];32(6):928–32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12047441
Sheehan WJ, Graham D, Ma L, Baxi S, Phipatanakul W. Higher incidence of pediatric anaphylaxis in northern areas of the United States. J Allergy Clin Immunol. NIH Public Access; ;2009;124(4):850–852.e2.
Shreffler WG, Lencer DA, Bardina L, Sampson HA. IgE and IgG4 epitope mapping by microarray immunoassay reveals the diversity of immune response to the peanut allergen, Ara h 2. J Allergy Clin Immunol [Internet]. 2005 Oct [cited 2017 Dec 30];116(4):893–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16210066
Sicherer SH. Food allergy: when and how to perform oral food challenges. Pediatr Allergy Immunol [Internet]. 1999 Nov [cited 2017 Dec 31];10(4):226–34. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10678717
Sicherer SH. Clinical implications of cross-reactive food allergens. J Allergy Clin Immunol [Internet]. 2001 Dec [cited 2018 Jan 3];108(6):881–90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11742262
Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133(2):291–307.e5.
Sicherer SH, Furlong TJ, Maes HH, Desnick RJ, Sampson HA, Gelb BD. Genetics of peanut allergy: a twin study. J Allergy Clin Immunol. 2000;106(1):53–6.
Sicherer SH, Muñoz-Furlong A, Sampson HA. Prevalence of seafood allergy in the United States determined by a random telephone survey. J Allergy Clin Immunol. 2004;114(1):159–65.
Sicherer SH, Wood RA, Vickery BP, Perry TT, Jones SM, Leung DYM, et al. Impact of allergic reactions on food-specific IgE concentrations and skin test results. J Allergy Clin Immunol Pract [Internet]. 2016 Mar [cited 2018 Jan 2];4(2):239–45.e4. Available from: http://linkinghub.elsevier.com/retrieve/pii/S2213219815006583
Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature [Internet]. 2011;477(7363):229–33. Available from: http://www.nature.com/doifinder/10.1038/nature10329
Siroux V, Lupinek C, Resch Y, Curin M, Just J, Keil T, et al. Specific IgE and IgG measured by the MeDALL allergen-chip depend on allergen and route of exposure: the EGEA study. J Allergy Clin Immunol [Internet]. 2017 Feb [cited 2018 Jan 9];139(2):643–654.e6. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0091674916305218
Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow’s milk allergy. J Allergy Clin Immunol. 2007;120(5):1172–7.
Sporik R, Hill DJ, Hosking CS. Specificity of allergen skin testing in predicting positive open food challenges to milk, egg and peanut in children. Clin Exp Allergy [Internet]. 2000 Nov [cited 2017 Dec 31];30(11):1540–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11069561
Srivastava KD, Bardina L, Sampson HA, Li X-M. Efficacy and immunological actions of FAHF-2 in a murine model of multiple food allergies. Ann Allergy Asthma Immunol [Internet]. 2012 May [cited 2017 Nov 30];108(5):351–358.e1. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22541407
Straumann A, Schoepfer A. Update on basic and clinical aspects of eosinophilic oesophagitis. Gut [Internet]. 2014 Aug [cited 2017 Dec 31];63(8):1355–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24700438
Su Y, Connolly M, Marketon A, Heiland T. CryJ-LAMP DNA vaccines for Japanese red cedar allergy induce robust Th1-type immune responses in murine model. J Immunol Res [Internet]. 2016 [cited .2017 Nov 30];2016:4857869. Available from: http://www.hindawi.com/journals/jir/2016/4857869/
Togias A, Cooper SF, Acebal ML, Assa’ad A, Baker JR, Beck LA, et al. Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases–sponsored expert panel. J Allergy Clin Immunol. 2017;139(1):29–44.
Tordesillas L, Goswami R, Benedé S, Grishina G, Dunkin D, Järvinen KM, et al. Skin exposure promotes a Th2-dependent sensitization to peanut allergens. J Clin Invest. 2014;124(11):4965–75.
Tordesillas L, Berin MC, Sampson HA. Immunology of Food Allergy. Immunity 2017;47(1):32–50
Umasunthar T, Leonardi-Bee J, Turner PJ, Hodes M, Gore C, Warner JO, et al. Incidence of food anaphylaxis in people with food allergy: a systematic review and meta-analysis. Clin Exp Allergy [Internet]. 2015 Nov [cited 2018 Jan 4];45(11):1621–36. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25495886
Untersmayr E, Szalai K, Riemer AB, Hemmer W, Swoboda I, Hantusch B, et al. Mimotopes identify conformational epitopes on parvalbumin, the major fish allergen. Mol Immunol [Internet]. 2006 Mar [cited 2017 Nov 15];43(9):1454–61. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0161589005003020
Vereda A, Andreae DA, Lin J, Shreffler WG, Ibañez MD, Cuesta-Herranz J, et al. Identification of IgE sequential epitopes of lentil (Len c 1) by means of peptide microarray immunoassay. J Allergy Clin Immunol [Internet]. 2010 Sep [cited 2017 Dec 30];126(3):596–601.e1. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0091674910010250
Vicente-Serrano J, Caballero ML, Rodríguez-Pérez R, Carretero P, Pérez R, Blanco JG, et al. Sensitization to serum albumins in children allergic to cow’s milk and epithelia. Pediatr Allergy Immunol [Internet]. 2007 Sep [cited 2018 Jan 4];18(6):503–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17680908
Vickery BP, Burks AW. Immunotherapy in the treatment of food allergy: focus on oral tolerance. Curr Opin Allergy Clin Immunol [Internet]. 2009 Aug [cited 2017 Nov 24];9(4):364–70. Available from: http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00130832-200908000-00015
Vickery BP, Berglund JP, Burk CM, Fine JP, Kim EH, Kim JI, et al. Early oral immunotherapy in peanut-allergic preschool children is safe and highly effective. J Allergy Clin Immunol [Internet]. 2017 Jan [cited 2017 Nov 30];139(1):173–181.e8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27522159
Vissers YM, Blanc F, Skov PS, Johnson PE, Rigby NM, Przybylski-Nicaise L, et al. Effect of heating and glycation on the allergenicity of 2S albumins (Ara h 2/6) from peanut. Niess J-H, editor. PLoS One [Internet]. 2011 Aug 25 [cited 2017 Nov 15];6(8):e23998. Available from: http://dx.plos.org/10.1371/journal.pone.0023998
Wal J-M. Bovine milk allergenicity. Ann Allergy Asthma Immunol [Internet]. 2004 Nov [cited 2017 Nov 24];93(5 Suppl 3):S2–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15562868
Wallowitz ML, Chen RJY, Tzen JTC, Teuber SS. Ses i 6, the sesame 11S globulin, can activate basophils and shows cross-reactivity with walnut in vitro. Clin Exp Allergy. [Internet]. 2007 Jun [cited 2017 Dec 30];37(6):929–38. Available from: http://doi.wiley.com/10.1111/j.1365-2222.2007.02725.x
Wang J, Calatroni A, Visness CM, Sampson HA. Correlation of specific IgE to shrimp with cockroach and dust mite exposure and sensitization in an inner-city population. J Allergy Clin Immunol. 2011;128(4):834–7.
Wanich N, Nowak-Wegrzyn A, Sampson HA, Shreffler WG. Allergen-specific basophil suppression associated with clinical tolerance in patients with milk allergy. J Allergy Clin Immunol [Internet]. 2009 Apr [cited 2017 Dec 30];123(4):789–94.e20. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0091674909001195
Wood RA. Food allergen immunotherapy: current status and prospects for the future. J Allergy Clin Immunol. 2016;137(4):973–82.
Wright BL, Kulis M, Orgel KA, Burks AW, Dawson P, Henning AK, et al. Component-resolved analysis of IgA, IgE, and IgG4 during egg OIT identifies markers associated with sustained unresponsiveness. Allergy. [Internet]. 2016 Nov [cited 2017 Nov 24];71(11):1552–1560. Available from: http://doi.wiley.com/10.1111/all.12895
Zuidmeer L, Goldhahn K, Rona RJ, Gislason D, Madsen C, Summers C, et al. The prevalence of plant food allergies: a systematic review. J Allergy Clin Immunol [Internet]. 2008 May [cited 2018 Jan 8];121(5):1210–1218.e4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18378288
Zuidmeer-Jongejan L, Huber H, Swoboda I, Rigby N, Versteeg SA, Jensen BM, et al. Development of a hypoallergenic recombinant parvalbumin for first-in-man subcutaneous immunotherapy of fish allergy. Int Arch Allergy Immunol [Internet]. 2015 [cited 2017 Nov 30];166(1):41–51. Available from: https://www.karger.com/Article/FullText/371657
Author information
Authors and Affiliations
Corresponding author
Section Editor information
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this entry
Cite this entry
Sylvestre, S., Andreae, D.A. (2019). IgE Food Allergy. In: Allergy and Asthma. Springer, Cham. https://doi.org/10.1007/978-3-030-05147-1_26
Download citation
DOI: https://doi.org/10.1007/978-3-030-05147-1_26
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-05146-4
Online ISBN: 978-3-030-05147-1
eBook Packages: MedicineReference Module Medicine