Abstract
While a melanoma may be suspected clinically, a definitive diagnosis usually requires pathologic assessment of a tissue biopsy. Pathologic diagnosis of melanoma requires evaluation of changes in the architectural and cytologic features and must be interpreted in the clinical context of the biopsy including the age of the patient and site of the lesion. The pathology report should document pathologic features important for guiding patient management, including those characteristics upon which the diagnosis was based and also prognostic factors. The traditional Clark-McGovern classification of melanoma has been validated to have molecular underpinning, and in the 2018 World Health Organization Classification of Skin Tumors, clinical, epidemiologic, pathologic, and molecular features have been integrated to define nine pathways of melanoma pathogenesis. Recent molecular studies have also opened new avenues for the treatment of patients with metastatic melanoma, and molecular pathology is likely to play an important role in the expanding field of personalized melanoma therapy.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
Introduction
For accurate diagnosis and appropriate management, melanoma patients are reliant on the knowledge, skills, and experience of both their treating clinician and their pathologist. The prognosis and further management of patients with primary cutaneous melanoma are determined to a major extent by the histopathological diagnosis and a number of crucial, pathologically assessed/measured parameters. These tumor parameters include thickness, ulceration, tumor mitotic rate, and whether or not the tumor involves the resection margins. For patients with metastatic melanoma, the presence of an oncogenic BRAF mutation and other factors such as the distribution and density of tumor-infiltrating lymphocytes and tumor mutation burden may also influence patient management. Pathologists should possess a thorough knowledge of the factors that are important in determining a melanoma patient’s prognosis and management and provide both clinician and patient with the information required to make the most appropriate decisions. In turn, knowledge of the pathology and diagnostic features of melanocytic tumors will assist clinicians to avoid potential diagnostic pitfalls, enable them to better understand diagnostic problems, and allow them to manage difficult cases more effectively.
The Role and Challenges of Pathologic Assessment of Melanocytic Tumors
For any atypical pigmented lesion, the primary goal of pathologic assessment is to determine whether it is benign (i.e., a nevus or other benign lesion) or malignant (i.e., a melanoma). If it is a melanoma, the secondary goal is to determine its metastatic potential (i.e., the risk of it spreading elsewhere) and the risk it will cause death of the patient. For those who have not attempted to do so, these may appear simple tasks. However, in reality the pathologic diagnosis of melanocytic tumors can be one of the most difficult areas of diagnostic histopathology (van Dijk et al. 2008; Veenhuizen et al. 1997). Microscopic diagnosis of melanocytic tumors requires a sound knowledge of diagnostic criteria, an awareness of potential pitfalls and an ability to make a logical and reasoned judgment on the basis of all information available (McCarthy and Scolyer 2004). A range of architectural and cytologic characteristics and features of the host response must be assessed and correlated with clinical data including the age of the patient and site of the lesion (Elder 2006). Part of the difficulty arises because each individual pathologic feature can occur both in nevi and in melanoma, and no single feature can be considered definitively diagnostic of a particular disease entity. For example, both Spitz nevi and melanoma often include large epithelioid cells and dermal mitotic figures and may show pagetoid epidermal invasion (Fig. 1) (Crotty et al. 2002; Scolyer et al. 2002; Dahlstrom et al. 2004; Petronic-Rosic et al. 2004). Hence, in this example, none of these features can be used alone to determine whether a particular tumor is benign or malignant. Furthermore, while Spitz nevi usually occur in children and melanomas in adults, either tumor can occur in patients at any age. Because of such difficulties, the pathologic diagnosis of melanocytic tumors also requires experience and considerable judgment. Only by integrating all available clinical and pathologic data can a correct pathologic diagnosis be made.
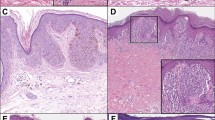
(a and b). Spitz nevus. Seven-year-old girl, back lesion. Spitz nevi share many histopathologic features with melanoma and in some cases may be difficult to distinguish from melanoma. This Spitz nevus shows symmetry, lateral circumscription, epidermal thickening, hypergranulosis, a “rain down” pattern in the superficial dermis, uniform cells with eosinophilic cytoplasm, paucity of mitotic figures, and maturation with depth
A pathologist can render an unequivocal pathologic diagnosis of nevus or melanoma accurately, reproducibly, and rapidly in most cases. However, there is a small subset of melanocytic tumors for which it is difficult, even for experts, to accurately predict their biologic behavior. Such difficulties were highlighted in the disturbing results of one study of atypical melanocytic tumors with spitzoid features which were reviewed by 10 international pathologist experts in the diagnosis of melanocytic (Barnhill et al. 1999). In some melanocytic lesions that proved lethal, more than half of the experts considered the lesion benign, and in more than one half of the cases, there was no unanimous view among the experts as to whether the lesion was a nevus or melanoma. Others have reported similar problem (Cerroni and Kerl 2001; Gerami et al. 2014). It is important for clinicians to be aware of such difficulties and for pathologists to clearly communicate in their report when there is uncertainty of the malignant potential of a melanocytic tumor and, in such instances, to consider the need for further expert opinions. While clearly the safest course of action would be to manage histologically ambiguous melanocytic tumors as melanoma, this is not always appropriate (Thompson and Scolyer 2004; Elder and Xu 2004). For example, the patient may have significant medical comorbidities making the risk of surgery and/or sentinel node biopsy unacceptably high or the lesion may involve a cosmetically or functionally sensitive anatomic site where wide local excision may result in disfigurement that is unacceptable to the patient. Molecular techniques are already assisting in the classification of melanocytic tumors (see chapter “Molecular Pathology and Genomics of Melanoma”). Nevertheless, until the diagnostic and prognostic value of such methods is established, pathologic diagnosis will remain the “gold standard” for the diagnosis of melanocytic tumors.
Biopsying Clinically Suspicious Pigmented Tumors
The type of biopsy performed may affect the accuracy of pathology evaluation of a melanocytic tumor. Although clinical considerations are important in determining the most appropriate biopsy technique, an excision biopsy with 2 mm margins is preferred as this offers the best opportunity for correct pathologic diagnosis (Thompson et al. 2005; Swetter et al. 2019). Incomplete biopsies of melanocytic tumors (punch, incision, curette and some superficial shave biopsies) may contribute to pathologic misdiagnosis, either by providing unrepresentative sampling of a heterogeneous tumor (i.e., a partial biopsy may sample only the benign part of a lesion and miss a coexisting melanoma) or by providing insufficient tissue for adequate assessment of the pathologic criteria necessary to permit correct diagnosis. Furthermore, superficial biopsies that do not show the entire lesion will not allow an accurate measurement of the Breslow tumor thickness (Fig. 2) (Scolyer et al. 2006). However, a deep “scoop” shave biopsy can provide complete information about a relatively small, superficial lesion such as a dysplastic nevus where the purpose of the biopsy is to rule out melanoma, and a very broad superficial shave biopsy can be successful in demonstrating the essential features of a very broad in situ lesion such as a lentigo maligna, where invasion is suspected to be absent or minimal (Swetter et al. 2019). Another potential pitfall that may follow incomplete biopsy of a nevus is that it may regrow from residual nevocytes after incomplete removal. Regenerating nevi often display many histologic features that commonly occur in melanomas (pagetoid epidermal invasion, cytologic atypia, occasional dermal mitotic figures, and HMB45 positivity). For these reasons such lesions have sometimes been termed “pseudomelanomas” (an unfortunate and misleading term) and are prone to overdiagnosis as melanomas (Dymock and Menz 1986; Kornberg and Ackerman 1975; Suster 1986). Because accurate assessment of pathologic features of a primary melanoma allows a reliable estimate of prognosis and guides selection of appropriate management (width of excision margins, appropriateness of sentinel node biopsy), inaccuracy of pathologic assessment can have serious clinical repercussions.
Punch biopsy of a melanocytic tumor. Sixty-four-year-old male, shoulder lesion. This punch biopsy shows part of a subtle melanocytic junctional tumor. Partial biopsies of melanocytic tumors may provide unrepresentative sampling and therefore may result in misdiagnosis and inappropriate or delayed patient management. If there are no clinical reasons to do otherwise, an excision biopsy with 2 mm margins is recommended for the diagnosis of any atypical pigmented lesion for which there is clinical concern about the possibility of melanoma
Pathologic Assessment of Primary Melanomas
Biopsy specimens of primary cutaneous melanoma should be placed in a suitable fixative such as 10% buffered formalin from 6 to 48 h prior to dissection. The specimen is carefully examined macroscopically. The dimensions of the specimen are measured including a detailed description of the lesion (size, shape, color, border, and contour) and its proximity to the resection margins. The presence of marking sutures or clips should be indicated on the pathology request form and noted in the pathology report. For smaller specimens the entire specimen should be processed. The surgical margins should be inked. In general, excision biopsies should be sliced transversely in 2–3 mm slices, sequentially including through the center or thickest part of the lesion. For lesions less than 3 cm in diameter, the entire lesion should be embedded for microscopic examination. The tissue blocks should be selected to facilitate microscopic assessment of the thickest portion of the tumor and determination of the relationship of the tumor to the surgical margins. In many cases, it is prudent to examine microscopic sections cut at intervals (levels) through each tissue block. The skin surface and cut surfaces of wide excision specimens should be examined carefully for macroscopic evidence of residual tumor and then serially sectioned into 2–3 mm slices. If the melanoma was completely excised and had no unusual features (such as desmoplasia or neurotropism) in the original biopsy and there is no suspicion of residual tumor on visual inspection then it may be sufficient to submit only one or two slices from the center of the scar for microscopic examination (Martin et al. 1998; Johnson and Sviland 1998; Kirkham 1998). Frozen section examination is not recommended for the assessment of primary cutaneous melanocytic tumors because the significant artifacts caused by freezing the tissue may compromise subsequent analysis of paraffin-embedded sections.
Accuracy of Pathologic Assessment Is Enhanced by Clinical Correlation
Although correlating clinical and pathologic features is critical for optimal patient care, it is a source of great frustration that little useful clinical information is provided on many pathology request forms submitted with pigmented skin tumor samples. In a recent study from the Victorian Melanoma Service in Australia, no useful clinical information whatsoever was provided in almost one half of 1,200 primary cutaneous melanomas diagnosed over a 10-year period (Scolyer et al. 2019). Although in most instances a final pathologic diagnosis can be made with minimal clinical information, some difficult problems require close and detailed communication between the clinician and the pathologist (Scolyer et al. 2004a, 2005). For example, trauma, topical treatments, pregnancy, or recent sun burn in benign melanocytic lesions may cause histologic changes that are usually seen in melanoma (Scolyer et al. 2004a). Lack of awareness of such clinical details may therefore lead to misdiagnosis. It is also important that clinicians inform pathologists of involvement of the margins in any previous biopsy and the histologic subtype and microstage of a previously biopsied melanoma because these factors may influence how the specimen is examined pathologically and therefore the accuracy of the pathology report. For example, desmoplastic melanoma may be extremely difficult to distinguish from scar tissue on histology (McCarthy et al. 2006; Chorny and Barr 2002; Robson et al. 2001). In this situation, very careful microscopy and the use of immunohistochemical analysis for markers such as S100 and SOX10 may be necessary for accurate diagnosis (McCarthy et al. 2004). On the other hand, immunostaining is unlikely to be used routinely in the examination of wide excision specimens if the pathologist is unaware that a previous biopsy disclosed desmoplastic melanoma. These examples indicate clearly that lack of clinicopathologic correlation may contribute to diagnostic errors.
The Role of Specimen Orientation
Orienting specimens with marking sutures (or other techniques) at the time of surgery is often helpful to pathologists if assessment of surgical margins is critical in determining the need for or extent of further surgery (Fig. 3). The pathologist should, in turn, assist the clinician by providing a specimen diagram or photograph that accurately illustrates the extent of the tumor and its proximity to the resection margins. Photography can also be helpful when assessing clinically heterogeneous lesions. By reviewing clinical photographs of the lesion with the pathologist, the clinician can direct the pathologist to areas of particular clinical concern, such as possible regression or recent change in color, and therefore improve clinicopathologic correlation. This is especially helpful when new techniques or clinical skills, such as dermoscopy or confocal microscopy, are being refined (Crotty and Menzies 2004). When there is a focal area of change within a preexisting lesion, it is critical that this area is examined microscopically by the pathologist to determine whether it represents a melanoma developing within a nevus or other benign lesion. It has been shown that scoring the area of concern with a superficial punch technique extending through the epidermis into the superficial dermis can improve the accuracy of melanoma diagnosis in this scenario (Grogan et al. 2018).
Seventy-five-year-old female, melanoma left temple. A. There is an old skin graft site related to a previous non-melanoma skin cancer, and this was included in the excision because the graft site was causing the patient some discomfort. The vertical primary melanoma scar is present in the inferior part of the specimen. Melanoma involved the margins of the original excision site. B. The re-excision specimen is orientated with a marking suture inferiorly. The lines and letters indicate where the tissue blocks were taken. The asterixes indicate where residual tumor involved the margins. C. Microscopic photograph showing tumor extending to the inferior margin as marked on the excision specimen
Melanoma Tumor Progression: The Concept of Radial and Vertical Growth Phases
The majority of melanomas are characterized by an initial, relatively indolent, phase of growth which clinically appear as irregular, variably pigmented and flat lesions with irregular peripheral margins (Clark et al. 1984, 1986, 1989; Elder et al. 1980, 1984; Herlyn et al. 1985). This phase is termed the radial growth phase (RGP) and is characterized by progressive radial or horizontal spread in the skin. In the absence of regression, it has been proposed that melanomas in the radial growth phase have no capacity to metastasize and are therefore cured by complete excision (Clark et al. 1984). Such proliferations are usually confined to the epidermis (in situ melanoma) but may also show microinvasion into the superficial dermis (Fig. 4). Microinvasive melanoma or invasive melanoma without vertical growth phase is defined as a melanoma invasive to the superficial, papillary dermis in which there are no dermal nests of melanoma cells larger than the largest epidermal nest and no dermal mitotic figures (Elder 2006). In contrast, melanomas in the vertical growth phase (VGP) have been defined as those showing dermal mitotic figures or dermal nests larger than any epidermal nest; such lesions are reportedly capable of metastasizing (Lefevre et al. 2003; Elder et al. 1984).
Microinvasive radial growth phase melanoma. Seventy-six-year-old female, lesion of cheek. By definition, for microinvasive radial growth phase melanoma the largest dermal nest of melanoma cells must be no greater in size than the largest epidermal nest and no dermal mitotic figures should be identified
The identification of the pathologic features that define the distinction of the RGP and VGP in superficial dermally invasive melanoma may be dependent on how the specimen is prosected macroscopically and on the number of sections examined microscopically. For example, the probability of identifying rare dermal mitotic figures in a thin, invasive melanoma will increase as more microscopic sections are examined. For this reason, some experts are loath to state categorically that any dermal invasive melanoma is incapable of metastasizing even when it appears to be in the RGP. Nevertheless, in one major study, all of 161 patients whose pure RGP-confined melanomas showed no histologic evidence of regression in the primary excision specimen had metastasis-free survival at a median follow-up of 13.7 years (Guerry et al. 1993). However, exceptional cases are reported and the number of metastasizing RGP lesions, though small, continues to increase (Guerry et al. 1993; Taran and Heenan 2001; Guitart et al. 2002; Berman 2006; Cook et al. 2002). The risk of metastasis in RGP melanoma is increased when there is regression and high lymphatic vessel density (Yun et al. 2011).
Patients with RGP-confined melanomas tend to be younger than those with VGP melanoma (Guerry et al. 1993). Later in its evolution, VGP melanomas may form an expansile nodule and aggressively invade the reticular dermis and/or subcutaneous fat. For patients with VGP melanoma, their prognosis is directly related to the measured Breslow thickness (Breslow 1970) and to other histologic parameters as discussed below (Clark et al. 1969, 1986; Busam and Barnhill 1995; Crowson et al. 2001b; Day et al. 1981, 1982c, a, b; Balch et al. 1978, 1980, 1982, 2001b; Harrist et al. 1984; Leon et al. 1991).
Pathways of Melanoma Pathogenesis and Clinicopathologic Classification of Melanoma
Traditionally, melanoma has been classified into “histogenetic” subtypes based on proposals by Clark (Clark 1967; Clark et al. 1969; Busam and Barnhill 1995; Crowson et al. 2001b), McGovern (McGovern 1970, 1972) and others (Crowson et al. 2001a; Barnhill 1995; Scolyer et al. 2011). A formal classification of melanoma was developed at a consensus conference held during the joint meeting of the International Union Against Cancer and International Pigment Cell Society in Sydney in 1972 (McGovern et al. 1973) and was updated in 1982 (McGovern et al. 1986). This classification included four major subtypes of melanoma: superficial spreading melanoma (SSM), lentigo maligna melanoma (LMM), acral lentiginous melanoma (ALM), and nodular melanoma (NM). The distinction between these major subtypes is based on a lesion’s histologic features, anatomic site and the degree of solar damage in the adjacent skin. The distribution of intraepidermal melanocytes during the early phase of melanoma growth (i.e., the RGP) is most important. SSM, LMM, and ALM are all distinguished based on the pattern of their RGP. NM was proposed as a fourth category for lesions lacking a RGP extending significantly beyond the VGP component. There are also clinical differences between the histogenetic subtypes of melanoma such as their peak age incidence, commonest anatomic site and degree of solar exposure, and in some instances the approach to management may vary with subtype (Swetter et al. 2019). Interestingly this traditional histogenetic classification has now essentially been validated by genetic analysis (see chapter “Molecular Pathology and Genomics of Melanoma”) (Curtin et al. 2005; Saldanha et al. 2006). The frequency of each of the major subtypes of melanomas varies considerably in different patient populations. For example, there is a higher incidence of LMMs in Caucasians living in subtropical and tropical areas and of ALM in Japanese and other Asian populations, while the absolute incidence of this (and likely also other “non-solar” melanomas) remains more or less constant in different ethnicities (Swetter et al. 2005; Elder 1995).
Other less common but clinicopathologically distinctive forms of melanoma have also been described (Jelfs et al. 1994). These include desmoplastic melanoma (Conley et al. 1971), melanoma arising within (or resembling) a blue nevus (so-called malignant cellular blue nevus) (Merkow et al. 1969; Granter et al. 2001) nevoid melanoma (Schmoeckel et al. 1985), mucosal melanoma, and a form of melanoma with prominent melanin synthesis termed pigment-synthesizing , equine-type (animal-type) melanoma (Crowson et al. 1999). The latter lesion (or a subset) has been described as pigmented epithelioid melanocytoma (Zembowicz et al. 2004).
Expanding upon the work of Bastian (2014), the fourth edition of the World Health Organization (WHO) Classification of Skin Tumors, published in 2018, classifies melanoma on the basis of clinical, pathologic, and molecular features (Table 1). Nine pathways to melanoma formation are described based upon the role of ultraviolet light in the disease pathogenesis, the cell (or tissue) of origin of the tumor, and the presence of specific recurrent genetic alterations (Table 1). The recent WHO classification also recognizes benign precursor lesions in each pathway and highlights the formal recognition of lesions that are intermediate in the disease progression from benign to fully malignant tumors (these intermediate lesions are now generally referred to under the rubric “melanocytomas”).
In populations composed predominantly of Caucasians, most melanomas occur in sun-exposed skin with either a low or high degree of cumulative sun damage (CSD). However, other types of melanomas may also occur in skin or mucosal surfaces with no or little UV exposure and in these melanomas ultraviolet light is thought to play no role in their pathogenesis (Hayward et al. 2017). The latter types of melanomas predominate in non-Caucasian populations.
The biologic behavior of the various subtypes of melanoma when matched for other important clinical and pathologic prognostic parameters is not significantly different (i.e., the estimate of prognosis for a patient with a clinically localized melanoma is principally related to the Breslow thickness of the tumor rather than its histogenetic subtype) for most melanoma subtypes with a few notable exceptions (as detailed below) (Ackerman and David 1986). It has even been suggested that subclassification of melanoma is irrelevant. Despite such extreme opinions, the subclassification of melanoma highlights many important clinical, pathologic, and molecular features of melanomas (including melanomas occurring at certain anatomic sites) a number of which are of great practical importance in establishing the correct diagnosis clinically and microscopically.
Low Cumulative Sun Damage Melanoma/Superficial Spreading Melanoma
Also known as pagetoid melanoma (reflecting its frequent histologic finding of prominent pagetoid epidermal invasion), SSM is the commonest form of RGP and comprises 70–75% of all melanomas in most European-derived populations (Busam and Barnhill 1995; Crowson et al. 2001b; Guerry et al. 1993; Taran and Heenan 2001; Clark et al. 1984). Clinically and microscopically SSM may arise at any anatomic location but occurs most often on the trunk and sun-exposed areas of upper arms and lower legs (Holman et al. 1983; Holman and Armstrong 1984). It occurs in relatively younger patients (compared with high CSD melanomas) and is usually associated with a low or moderate degree of CSD in the adjacent skin. Melanoma arising in a preexisting dysplastic nevus (atypical mole) is usually of superficial spreading type (Clark et al. 1978). Clinical features of SSM and other melanoma subtypes are discussed in more detail in chapter “Clinical Presentations of Melanoma” (or Part VI, “Uncommon Presentations of Melanoma”). Genetically, low CSD melanomas are usually characterized by the presence of a BRAFV600E mutation. They are also associated with a high mutation burden, a strong UV mutation signature, and multiple DNA copy number changes (Hayward et al. 2017).
By definition, SSM must have a RGP (Busam and Barnhill 1995; Crowson et al. 2001b). The RGP is initially confined to the epidermis (Clark 1967; Clark et al. 1969, 1984, 1986; Guerry et al. 1993; Taran and Heenan 2001) and usually is formed by cells with an epithelioid cytology and with pagetoid disposition of variably sized nests and single cells percolating through the entire epidermis up to the corneal layer (Fig. 5). The melanoma cells usually have round or oval nuclei with thick rims of chromatin, macronucleoli, and abundant eosinophilic or clear cytoplasm that may contain melanin granules. The latter are of variable size and shape but are mostly small as they represent single melanosomes. Scattered mitotic figures are usually present. The epidermis associated with the melanoma is often thickened compared to adjacent skin. The peripheral edge of the tumor is usually sharply demarcated.
Superficial spreading melanoma. Forty-four-year-old male, lesion of back. Numerous melanoma cells show upward pagetoid scatter in the thickened epidermis. The melanoma cells have large pleomorphic nuclei with vesicular chromatin and prominent nucleoli and a moderate amount of pale staining cytoplasm
The presence of a lymphocytic infiltrate in the papillary dermis underlying an SSM may herald the onset of microinvasion. Although delicate fibroplasia is common in the superficial dermis below the SSM, well-organized lamellar fibrosis around rete ridges, as seen in dysplastic nevi, is uncommon in RGP melanoma, except in the areas where there is a contiguous, associated dysplastic nevus.
The next phase in the progression of RGP of SSM is extension of microinvasive melanoma into the papillary dermis (Fig. 4). This extension comprises nested and singly dispersed cells cytologically similar to the intraepidermal cells, albeit often with more abundant cytoplasm. The nests should not be larger than those along the dermoepidermal junction and dermal mitotic figures should not be identified. The presence of either of these features indicates progression to VGP (Fig. 5).
In contrast to NM, there is extension of the radial growth phase intraepidermal component past the confines of the invasive, dermal component (Fig. 6) (Clark et al. 1969). In the absence of regression, RGP melanoma is very infrequently associated with metastasis (Guerry et al. 1993; Taran and Heenan 2001; Ronan et al. 1987; Paradela et al. 2010), although there are rare exceptions to this rule, with late-developing metastases (Guitart et al. 2002; Berman 2006; Cook et al. 2002; Yun et al. 2011). As discussed in more detail below, regression is characterized microscopically by fibroplasia forming a band of fibers parallel to the epidermal surface, vertically oriented ectatic vessels within the collagenized zone, and admixed melanophages (Fig. 22). An infiltrate of lymphocytes and plasma cells and stromal edema may also be present. Melanoma cells are absent in the zone of regression although residual tumor may be present on either or both sides. Distinguishing a zone of regression from scarring (such as that related to trauma or previous shave biopsy) can be very difficult and may sometimes be impossible without a corroborative history.
Superficial spreading melanoma. Thirty-year-old female, lesion of thigh. Extension of the radial growth phase component of a melanoma (three rete ridges) beyond the invasive vertical growth phase component is integral to the definition of superficial spreading melanoma and is best appreciated at low power magnification
Pitfalls
Melanoma in situ may be mimicked by other malignant intraepidermal lesions exhibiting a pagetoid pattern including Paget disease (an intraepidermal proliferation of malignant epithelial cells, usually with apocrine or eccrine differentiation), Pagetoid Bowen’s disease (squamous cell carcinoma in situ), intraepidermal sebaceous carcinoma, epidermotropic Merkel cell carcinoma, and other malignant skin adnexal neoplasms (Haupt and Stern 1995; Petronic-Rosic et al. 2004). Careful attention to morphologic features and the judicious use of appropriate histochemical and immunohistochemical stains should facilitate their distinction in problematic cases.
High-Cumulative Sun Damage Melanoma/Lentigo Maligna Melanoma
Lentigo maligna (Hutchinson’s melanotic freckle, precancerous melanosis of Dubreuilh) usually occurs on sun-exposed skin of elderly persons (though younger individuals may be affected in regions of high sunlight exposure). It most often occurs on the face and the head and neck region, and less commonly on extrafacial locations (Hutchinson 1890; Dubreuilh 1894; Clark and Mihm 1969; Cox et al. 1996, 1998; Finan and Perry 1982). Lentigo maligna has sometimes been designated as melanoma in situ (Finan and Perry 1982), although some authors have suggested that it constitutes a premalignant phase in melanoma progression (Tannous et al. 2000) The term LMM is sometimes applied to invasive melanoma on chronically sun-damaged skin, as a contradistinction between in situ (lentigo maligna) and invasive (LMM). In our opinion, it is preferable to indicate if a lesion of LMM is either in situ or invasive. The lifetime risk of invasive melanoma arising in lentigo maligna is estimated to be 5% and 30% (Weinstock and Sober 1987; Menzies et al. 2019). Long-term exposure to ultraviolet irradiation is the main risk factor for lentigo maligna, and the diagnosis should not be made if the lesion occurs on skin that histologically shows no evidence of solar elastosis (Little et al. 1980). Predominant mutations include NF1, NRAS, BRAFnon-V600E, and perhaps KIT. High CSD melanomas typically show a very high mutation burden, a strong UV radiation mutation signature, and multiple DNA copy number changes.
Lentigo maligna is usually characterized histologically by polygonal-shaped melanoma cells with variably sized, hyperchromatic, angulated nuclei dispersed singly and, initially, confined to the basal layer of epidermis in a discontinuous lentiginous pattern with extension down into eccrine ducts and hair follicles (Figs. 7 and 8). A characteristic cytologic feature is the presence of multinucleated giant melanoma cells disposed along the basal layer of the epidermis. These are termed “star-burst giant cells,” and are identified in up to 85% of cases of lentigo maligna (Cohen 1995, 1996; Cohen et al. 1994), although similar cells can be seen in benign melanocytic lesions. The epidermis in lentigo maligna is characteristically atrophic, manifesting thinning and loss of rete ridges, and there is underlying elastotic dermal collagen often associated with telangiectasia and melanophages (Tannous et al. 2000). As the lesion progresses, there is a confluent (back-to-back/side-by-side/cheek-by-jowl) pattern of growth of atypical melanocytes along the basal layer of the epidermis. This is followed by the development of a nested growth pattern comprising variably sized discohesive junctional nests along the dermoepidermal junction that often assume a disposition parallel to the long axis of the epidermis to form distinctive oblong “swallow’s nests.” Compared to SSM, LMM generally exhibits less pagetoid scatter, less nesting, a more poorly circumscribed border, less epidermal thickening and less pigment, and more elastosis (Viros et al. 2008). With progression, there is more prominent suprabasilar pagetoid infiltration, usually of epithelioid cells similar to those observed in SSM, perhaps representing a “final common pathway.” These findings are harbingers of the next phase of lesional evolution, dermal invasion.
Early phase lentigo maligna. Sixty-seven-year-old female, lesion of cheek. Lentigo maligna is characterized pathologically by an increase in atypical junctional melanocytes occurring in severely sun-damaged skin. The latter is manifest by epidermal atrophy with loss of rete ridges and superficial dermal solar elastosis (the pale blue zone in the superficial dermis)
The presence of a lichenoid infiltrate with admixed melanophages in a sclerotic papillary dermis may provide a clue to evolution from in situ to microinvasive melanoma. At first, the inflamed papillary dermis is penetrated by single melanoma cells. The cytology of the microinvasive cells is usually identical to the epidermal component, i.e., with an epithelioid or nevoid morphology and variably pigmented cytoplasm and are usually readily distinguishable from pigment-laden macrophages by their morphologic characteristics. Nevertheless, immunohistochemistry for melanoma markers such as SOX10, S100, HMB45, or MelanA and the macrophage marker CD68 may on occasion assist in the latter distinction. Metastases from such microinvasive foci are rare (in the absence of regression) (Guerry et al. 1993; Taran and Heenan 2001; Yun et al. 2011). In the absence of unequivocal invasive melanoma, regressive stromal changes including neovascularization and an inflammatory host response raise the possibility that prior microinvasion may have occurred. At our institutions, we usually indicate the latter in the pathology report with information along the lines of: “Melanoma in situ, lentigo maligna type, with histologic features consistent with dermal regression.”
As with SSM, the progression of LMM from a RGP to a VGP is characterized by the formation of tumor within the dermis that exceeds the size of any nest within the epidermis or by the presence of any dermal mitotic figure. The VGP cells in LMM are often fusiform (spindled) or nevoid in appearance. As discussed in more detail below, the VGP of LMM may take the form of desmoplastic melanoma. This is characterized by spindled melanoma cells associated with and separated by variable stromal desmoplasia often also involving dermal or subcutaneous nerves (perineural invasion/neurotropism).
Pitfalls
Not all cases of lentigo maligna have the prototypic appearance. In some cases, the epidermis is hyperplastic with rete elongation and some dermal fibrosis along the rete ridges resembling a dysplastic nevus (Farrahi et al. 2005). The distinction is made by identifying foci more typical of lentigo maligna. Incomplete biopsies (such as punch, small ellipses, and some shave biopsies) may therefore provide unrepresentative sampling and represent a diagnostic pitfall. Clinicopathologic correlation may help prevent misdiagnosis.
It is often truly difficult to ascertain whether a low- to moderate-density proliferation of singly disposed atypical melanocytes in the basal epidermis of sun-damaged skin represents true early lentigo maligna or just melanocytic hyperplasia related to photoactivation (also termed solar melanocytosis) in chronically sun-damaged skin (Gilchrest et al. 1979; Montagna et al. 1989; Gross et al. 1999; Acker et al. 2001; Bahwan 1997). This is not surprising since both processes form a morphologic continuum. Sun-induced atypical melanocytic proliferations simulate LMM microscopically, but have less pronounced atypia and lack the density, nesting, or upward migration by melanocytes of LM.
Assessment of level of invasion in LMM may be difficult as the dermis is often thin with abundant elastotic material obscuring the demarcation between papillary and reticular dermis. Invasion of the perifollicular stroma in hair follicles extending into the reticular dermis may be misinterpreted as true level IV melanoma, especially if the section is tangential and does not include the follicular epithelium. Thickness measurements should not be based on perifollicular extension except if that is the only invasion discernible (Dodds et al. 2018). In that circumstance, depth is most appropriately measured from the limit of infiltration of the perifollicular dermis to the inner layer of the outer root sheath epithelium or to the center of the follicle.
Assessment of Excision Margins in Lentigo Maligna
There is often a tendency for the lesional cells in lentigo maligna to “trail off” at the periphery such that there is a gradual diminution in both the number of melanocytes and their degree of atypia, and these changes merge with those of sun-induced melanocytic proliferations (Scolyer et al. 2004a). For these reasons, assessment of the distance between the tumor and the margins of excision can be subjective and imprecise. In our experience, overestimation by pathologists of the significance of melanocytic hyperplasia and atypia due to photoactivation is a common event, particularly when en face sections are used to examine margins. Examination of a “mirror-image” biopsy taken from the opposite site of the face may assist interpretation (Gross et al. 1999).
Acral Melanoma
Acral melanoma (AM) occurs mainly on the palms, soles, subungual regions, and digits. Such melanomas probably occur at a similar incidence among racial groups but are proportionately more common in those of African and Asian origin because of the lesser frequency of solar-induced melanomas at other sites in such populations. AM is similar microscopically to melanoma in mucosal areas (which is therefore usually designated as mucosal lentiginous melanoma) (Kato et al. 1996, 1999; Harmelin et al. 1998; Cho et al. 1998; Yasuoka et al. 1999; Kuchelmeister et al. 2000; Levit et al. 2000; Krige et al. 1995; Jimbow et al. 1984; Cascinelli et al. 1994; Ridgeway et al. 1995; Chang et al. 1998; Saida 2000). Although usually pigmented, a rare AM is amelanotic (Yasuoka et al. 1999). Not all acral melanomas are of acral lentiginous type; in one series of 62 plantar melanomas, 14.5% were classified as NM and 3.2% as SSM (Kato et al. 1999). SSMs in acral sites in Caucasians are most frequent on the dorsal aspect of the hands and feet (Kuchelmeister et al. 2000). AM is characterized by a relatively low mutation burden, absent UV radiation mutation signatures, and multiple chromosomal complex structural rearrangements. AM often has multiple amplifications of genes such as CCND1, KIT, and TERT, although these do not as yet have therapeutic importance since they are not readily targeted with currently available molecular therapies. BRAF and NRAS mutations occur in a proportion of AM (about 10–15%) but are much less frequent than in CSD melanomas. KIT mutations occur in about 10% of AM, and some patients with advanced AM respond to KIT inhibitor targeted therapy.
The early stages of AM in situ are characterized by scattered single, atypical, often angulated melanocytes with scant cytoplasm, along the junctional zone and may be associated with a junctional/subjunctional infiltrate of tumor-infiltrating lymphocytes. Subsequently, the atypical melanocytes form a confluent array along the basal epidermis and are often associated with epidermal hyperplasia (Fig. 9). Transepithelial pigment elimination with haphazard melanization of the dense acral keratin layer is distinctly different from the organized pigment columns seen with benign acral nevi (these are seen only when sections are cut perpendicular to the ridge and furrow lines). This results in the parallel ridge pattern of pigmentation (parallel band-like pigmentation in ridges of dermatoglyphic lines of the palms and sole) of AM on dermoscopy (Ishihara et al. 2006). As the RGP progresses, it often becomes associated with pagetoid epidermal invasion, often of epithelioid melanocytes showing an increasing degree of cytologic atypia. Mitotic figures are often seen in the intraepidermal component.
Invasive RGP AM shows intradermal cells in a single-cell or nested pattern with a cytology similar to the tumor cells in the epidermis but often with more abundant cytoplasm and fine pigment granules. As with SSM and LMM, nests larger than those in the epidermis or dermal mitotic figures signify progression to VGP melanoma (see below).
It has been identified that histologically normal melanocytes in apparently uninvolved skin adjacent to acral melanomas harbor genetic changes that can be detected by array-based comparative genomic hybridization and fluorescence in situ hybridization (Bastian et al. 2000; North et al. 2008). These “field cells” have a mean extension of 6.1 mm beyond the histopathologic margin of in situ melanomas and of 4.5 mm beyond the margin of invasive melanomas (North et al. 2008). It has been suggested that such cells represent an early phase of melanoma that precedes melanoma in situ, and it has been hypothesized that they provide a plausible explanation for the tendency of acral melanomas to recur locally despite apparently complete excision.
Nodular Melanoma
NMs are typically characterized by a bulky dermal component with an epidermal component that does not extend more than three rete ridges beyond the dermal VGP component (Fig. 10). In some instances of NM, no epidermal component may be apparent. They account for approximately 10–15% of melanomas in most predominantly Caucasian Western populations. They may occur in any anatomic site but are most common on the trunk, head and neck, and legs. NM typically presents as a rapidly growing pale or pigmented papule or nodule and may be superficially ulcerated (Kelly et al. 2003; Liu et al. 2006).
It is hypothesized that NM is not a single entity but represents a group of melanomas in which, because of rapid growth, the intraepidermal component is either short-lived or no longer recognizable. The possibility of origination from skin adnexa has also been considered. It is therefore likely that NM can occur in melanomas of each of the other subtypes of melanoma that are associated with a preceding RGP. The etiology is therefore likely heterogeneous, paralleling that of RGP melanomas. Although data are currently very limited, similarly the mutation profile of NM likely overlaps with other melanoma subtypes including SSM, LMM, AM, and mucosal melanomas.
Histologic Features of the Vertical Growth Phase of Melanoma
The presence of the VGP signifies the capacity of a melanoma to metastasize. It usually appears clinically as a pigmented or amelanotic nodule supervening within a preexisting macule or plaque (RGP). The notable exception is NM (Fig. 9-4, A) which by definition lacks a RGP component extending more than three rete ridges beyond the VGP component (Busam and Barnhill 1995; Crowson et al. 2001b; Clark et al. 1984, 1986, 1989; Kato et al. 1995). The histologic descriptions that follow for VGP melanoma arising in a RGP lesion of LMM, AM, or SSM apply equally to NM.
As described above, the histologic features that define the early VGP are the presence of at least a dominant nest within the papillary dermis that is larger than any nest within the epidermis or the presence of any dermal mitotic figures. Typically, the VGP is composed of cells that show prominent nucleoli, coarse chromatin, and irregularly thickened and/or notched nuclear membranes. Apoptotic cells may be observed but are not a prerequisite. The dominant cytomorphology of the cells within the epidermis is often epithelioid; however, those cells of the VGP nodule may be epithelioid, ovoid, spindled, or small, and often there is a mixture of cell types. The cells of the VGP may show greater cytoplasmic pigmentation than the intraepidermal component. This may represent a form of clonal evolution. The presence of superficial dermal regression in a thin melanoma indicates a higher risk of metastasis than would otherwise be the case if regression was absent (Yun et al. 2011). The presence of early VGP portends a risk for metastasis of approximately 10% after a follow-up of 8 years (Elder et al. 1984).
In the discussion of the histologic features of VGP melanoma below, it is classified by predominant cell type to highlight its characteristics and potential pitfalls.
Predominantly Epithelioid Cell Vertical Growth Phase
The VGP is usually predominantly composed of epithelioid melanocytes with a round or ovoid cytoplasmic profile, round or oval nuclei with coarse chromatin, prominent nucleoli, and chromatin rims that are irregular in thickness and often notched (Fig. 11). Cytoplasmic intranuclear pseudoinclusions are typically seen. The cytoplasm is often abundant with an eosinophilic or amphophilic staining quality and a variable amount of melanin. Cell cohesion is variable. Mitotic figures are usually present (often deep in the dermis) and may be atypical. The latter manifest as multipolar mitotic figures that reflect an aneuploid DNA content (Crowson et al. 2001b). A variable inflammatory response is seen, the nature of which may have prognostic significance (see below).
Predominantly Spindle Cell Vertical Growth Phase
A VGP that is composed predominantly of spindle cells may occur in any type of melanoma but is most common in LMM, AM, and mucosal melanomas. The spindle cells may form sheets, fascicles, or even a storiform architectural pattern (Fig. 12). There is usually no diminution in cell size from the superficial to the deep aspect of the tumor (i.e., maturation is absent).
Desmoplastic melanoma is a variant of the spindle cell VGP in which the spindled melanocytes are separated by and associated with variable, dense fibroplasia (see below). The spindled melanoma cells are often fusiform and attenuated with low-grade nuclear atypia but may contain large, hyperchromatic nuclei with irregularly distributed chromatin and multiple and/or prominent nucleoli. Neurotropism is a common accompaniment (30–50% of cases) and is usually manifest as perineural invasion but occasionally may take the form of intraneural invasion. Neural transformation, a rare phenomenon where the tumor cells themselves form nerve-like structures, is regarded as a form of neurotropism by some authorities. Desmoplastic melanoma cells almost invariably express S-100 protein and SOX10; however, they are usually negative for HMB45 antigen, Melan-A, and MITF in contrast to other melanoma subtypes (Skelton et al. 1997; Busam et al. 2001). Desmoplastic melanoma, when defined as having the above features in more than 90% of the invasive component, has a low rate of nodal metastasis but rather tends to recur locally, especially when margins are positive because the subtle proliferation is not recognized histologically, or when there is neurotropism.
Pitfalls
In the absence of an in situ component or intracytoplasmic melanin pigmentation, it is important to distinguish spindle cell melanoma from other spindle cell lesions such as atypical fibroxanthoma, spindle cell squamous cell carcinoma, scar, or sarcomas (McCarthy et al. 2004). Careful attention to morphologic features and the judicious use of appropriate immunochemistr y should enable distinction.
Mixed Spindle Cell and Epithelioid Cell Vertical Growth Phase
The VGP is often formed by a mixture of spindled and epithelioid cells but usually also includes cells with a variably elongated appearance (representing the spectrum of morphologic shapes in between). The cytologic appearances are similar to those described above.
Nevoid Vertical Growth Phase (“Nevoid Melanoma”)
A VGP composed of nevoid cells is often very subtle and because of its histologic similarity to a nevus is often termed nevoid melanoma (Fig. 9-4, D and E). For this reason, it may be difficult to diagnose and as such is a frequent source of false negative diagnosis of melanoma leading to medicolegal action (particularly when the correct diagnosis of melanoma is not made until after the lesion has metastasized) (Troxel 2003). By definition, the lesions resemble a nevus at low power, but have distinguishing features including increased cellularity with sheet-like rather than nested growth that provide a clue to diagnosis even at first glance (see below for more detailed description). Histologic features that allow recognition of nevoid melanoma include asymmetry at low power, long thin rete ridges, expansile dermal growth, an infiltrative deep margin, incomplete maturation, a mixture of cell types, subtle nuclear atypia (visible nucleoli and irregular chromatin), and the presence of dermal mitotic figures (McCarthy and Scolyer 2004). The latter are invariably present, often frequent and sometimes deep and atypical. Often the cells have nuclear outlines with irregular folds, longitudinal grooves, and conspicuous nucleoli. Cytoplasm is usually sparse. Proliferation markers such as Ki67 may be increased (most studies report that more than 2% of nuclei deeply located are positive) (Li et al. 2000). In most nevoid melanomas, absent deep maturation is a helpful clue to distinguish it from a banal dermal nevus. However, in some cases, the melanocytes manifest an apparent diminution in both nest and cell size with depth, which is a particularly treacherous pitfall (Ruhoy et al. 2000). There may be a variable inflammatory host response. Because most nevoid melanomas lack a discernible RGP, they are best categorized as an unusual subtype of NM. Although some studies have reported that nevoid melanoma may behave in a more indolent fashion than the other VGP variants (Wong et al. 1993), despite a deceptively banal morphology, aggressive behavior is possible.
Other Melanoma Subtypes and Variants
Desmoplastic Melanoma
Desmoplastic melanoma (DM) is a fibrosing variant of melanoma (Conley et al. 1971). It is uncommon constituting approximately 2–4% of melanomas referred to major melanoma centers. Although it may be suspected clinically when a firm nodule is noted within an area of lentigo maligna, in a background of normal-appearing skin, it is often mistaken for a scar/keloid, fibroma, or basal cell carcinoma. DM usually affects sun-damaged skin of the head and neck area, but can occur at any site, including acral skin and mucosae. It has a slight predilection for men and typically occurs in the sixth to eighth decades (Quinn et al. 1998; Busam et al. 2004; Jain and Allen 1989; Smithers et al. 1990; Carlson et al. 1995). DM differs in several respects from the majority of “conventional” melanomas. It tends to have a higher local recurrence rate and a lower incidence of regional lymph node involvement (Hawkins et al. 2005). However, with wide local excision by experienced surgeons and careful margin assessment by pathologists, the local recurrence rate is approximately 4%, significantly lower than reported in the older literature (Arora et al. 2005; Hawkins et al. 2005). Most sentinel lymph node biopsies from patients with a primary cutaneous DM are histologically negative (Gyorki et al. 2003; Pawlik et al. 2006). Recently, it has been suggested that in patients with thick and deeply invasive melanomas, the presence of desmoplastic features may be associated with longer survival (Shaw et al. 2006).
DM has distinctive genomic profile with mutation of NF1 being characteristic. They also have a high tumor mutation burden (with a strong UV signature), making them more likely to be responsive to check point inhibitor immunotherapy with anti-PD1 (Eroglu et al. 2018). Morphologically similar DM that occurs in acral and mucosal sites does not share this feature.
DM is histologically characterized by fusiform melanocytes dispersed in a variable fibrous matrix (Fig. 13a) (Busam 2005). Cell density is typically low. Individual tumor cells have a fibroblast-like or Schwann cell-like shape albeit with variable nuclear atypia (nuclear enlargement, hyperchromatism) (Fig. 13b). The vast majority of DM are completely amelanotic. Focal lymphocytic aggregates are not infrequent within the tumor and are diagnostically useful. If the classic pauci-cellular desmoplastic appearance is present throughout the entire tumor (at least 90% of the dermal component), it is classified as pure (Fig. 13a) (Busam et al. 2004). If there is a significant (10% or more) non-desmoplastic component (typically densely cellular compact fascicles of spindle cells or clusters of epithelioid tumor cells), the tumor is considered mixed (or combined) DM (Fig. 13c). Spindle cell melanomas without desmoplasia should not be classified as DM.
Desmoplastic melanoma. (a) Pure-type. Note the presence of a lentigo maligna in the overlying epidermis. (b) Pure-type. The spindled tumor cells are separated by collagenous stroma. (c) Mixed-type. A non-desmoplastic spindle cell melanoma (right side of picture) is juxtaposed to a desmoplastic melanoma
Approximately two thirds of DM are associated with an intraepidermal precursor/in situ lesion, typically of lentigo maligna type (Fig. 13a). However, some DMs (usually mixed variants) arise in association with superficial spreading or acral lentiginous melanoma. Because DMs without a clinically pigmented background are often diagnosed at an advanced clinical stage, such tumors tend to be thick and deeply infiltrative at surgical excision and perineural invasion/neurotropism is common. However, some DMs are not neurotropic and neurotropism may occur in melanomas that are not desmoplastic.
Differential Diagnosis
Histologically, DM can be confused with a sclerosing melanocytic nevus, a scar, a fibroma, or a non-melanocytic malignant spindle cell neoplasm (McCarthy et al. 2004, 2006).
Desmoplastic Melanoma Versus Sclerosing Nevus (ScN)
This distinction is usually straightforward, but can be very difficult (Harris et al. 1999), if only a small, partial biopsy is available for examination. Most DMs tend to occur on sun-damaged skin, in elderly individuals. They tend to be broad and deeply infiltrative, and tend to show associated intraepidermal (in situ) melanoma. In contrast, most ScN occur on the trunk and extremities in young individuals. The majority of ScN (except for some large sclerosing blue nevi) are small, superficial, and wedge-shaped lesions with evidence of maturation (zonal change ranging from large epithelioid cells in sclerosing Spitz nevi, to small attenuated fusiform nevocytes in nevic components located in the reticular dermis). If a junctional component is present in a ScN, it typically demonstrates a predominant nested pattern, typical of more conventional nevi. Mitotic figures and lymphoid aggregates are more common in DM, but rare mitotic figures can be seen in nevi as well as occasional scattered lymphocytic clusters. Thus, these features need to be interpreted in context with other evidence for or against the diagnosis of melanoma. Immunostains can help distinguish DM from sclerosing nevus. Most ScN express Mart-1/Melan-A, while most DMs do not. HMB45 shows decreased labelling from top to bottom (maturation) in ScN but is patchy or absent in DM; also, as expected, sclerosing blue nevi show diffuse labelling with HMB45.
Desmoplastic Melanoma Versus Scar or Fibroma
Confusion of a DM with a scar or fibroma is a serious and potentially tragic misdiagnosis, because such an error may lead to significant delay of definitive treatment. DM may have a scar-like appearance and its tumor cells may resemble fibroblasts, but typically some DM cells have larger and more hyperchromatic nuclei than typical fibroblasts. Furthermore, scattered lymphocytic aggregates or an associated atypical intraepidermal or intrafollicular melanocytic proliferation may provide a clue to the presence of DM. Once suspected, DM can usually be confirmed or excluded by immunohistochemistry for S-100 protein or SOX10. However, the diagnosis of DM should not be based on immunostains alone, but rather must take into account atypical architectural and cytologic features, since isolated S-100 protein or SOX10-positive cells may occur in an inflamed scar, likely corresponding to proliferating Schwann cells (Jackett et al. 2016).
Desmoplastic Melanoma Versus Non-melanocytic Desmoplastic Malignant Spindle Cell Neoplasms
DM may also be confused with sclerosing variants of cutaneous sarcomas or spindle cell carcinomas. Immunohistochemical studies usually allow definitive distinction, since the majority of sarcomas that need to be considered in the differential diagnosis of DM are negative for S-100 protein or SOX10. An important exception is malignant peripheral nerve sheath tumors that express SOX10 but only focal S100; also, in contrast to most melanomas, they may lose expression of H3K27me3 histone (Prieto-Granada et al. 2016b; Schaefer et al. 2016). Spindle cell/sarcomatoid carcinomas differ from desmoplastic melanoma not only by their lack of staining for S-100 protein, but also by their expression of cytokeratins. In differentiating DM from leiomyosarcomas, it is important to use a panel of markers, since DM usually express SMA.
Nevoid Melanoma
Nevoid melanoma is a subtype of melanoma that resembles a nevus in general architecture, and to some extent also in cytology and, as a consequence, are prone to misdiagnosis (Troxel 2003, 2006; Massi 2007). Misdiagnosis may have disastrous consequences for the patient and is a frequent basis for medicolegal actions. The term nevoid melanoma was first coined by Schmoeckel et al. (Schmoeckel et al. 1985) for melanomas that somewhat resemble a benign nevus architecturally and cytologically by virtue of their symmetry, dome-shaped or verrucous silhouette, and composition by cells that resemble nevocytes (Wong et al. 1993, 1995; Zembowicz et al. 2001; Schmoeckel et al. 1985; Kim and Murphy 2000; Barnhill 1998a; Levene 1980; Suster et al. 1987; Blessing et al. 2000; Kossard and Wilkinson 1997; McNutt et al. 1995; McNutt 1998). Most nevoid are protuberant or verrucous tan to flesh-colored nodules that usually occur on the trunk or proximal limbs of young adults. It is controversial whether NM is associated with a worse or similar prognosis than other types of melanomas. In Schmoeckel et al.’s original series of 33 patients published in 1985, 15 developed metastases and eight died of disseminated melanoma (Schmoeckel et al. 1985). However, more recent studies have suggested that the nevoid melanoma has a similar prognosis to non-nevoid melanoma subtypes.
Microscopically nevoid melanomas are characteristically dome-shaped or verrucous, well circumscribed laterally and often associated with epidermal acanthosis (Fig. 14) (McNutt 1998; Wong et al. 1993). Prominent pagetoid epidermal invasion is usually absent. The dermal component comprises superficial nests of atypical nevoid tumor cells. Although the cells at the deep aspect of the lesion are often smaller than the more superficial cells, they usually exhibit conspicuous nucleoli, nuclear membrane irregularity, and hyperchromasia with high nuclear to cytoplasmic ratios and mitotic figures. The apparent diminution in the size of the cells and nests has been termed “pseudomaturation” and contributes to misdiagnosis of the lesions as benign. Clues to the diagnosis include an infiltrative deep margin, expansile dermal nodules, poor true maturation, cell crowding (“puffy shirt” sign, Diwan AH and Busam KJ, personal communication), a mixture of cell sizes, nucleoli (particularly in the deeper dermal cells), elongated long thin rete ridges, and at least occasional dermal mitotic figures (often including deep and abnormal forms) (Fig. 14). Some nevoid melanomas are HMB45 negative but most have a few, scattered HMB45-positive dermal cells. Proliferation markers (e.g., Ki67) may be increased (most show more than 2% of nuclei are positive). Preliminary data suggests that nevoid melanomas commonly harbor NRAS mutations.
Nevoid melanoma. (a) Thirty-two-year-old female, melanoma of leg. At low power, this superficial shave biopsy shows part of a nevocellular melanocytic tumor associated with some elongated rete ridges and keratin horn cysts. At this magnification the features are reminiscent of a congenital nevus. (b) Thirty-two-year-old female, melanoma of leg. At high power magnification (×400) the small nevoid cells show significant hyperchromasia, pleomorphism, and mitotic activity which all represent subtle clues to the diagnosis. This patient subsequently developed widespread metastatic disease. Nevoid melanomas are an important cause of melanoma misdiagnosis and may not be correctly identified until after they have metastasized. (c and d) Thirty-nine-year-old female, melanoma shoulder. At low power magnification, expansile growth is seen in the dermis in the left portion of the lesion. A residual dermal nevus is present in the right-hand portion of the lesion. (d, e, and f) Thirty-nine-year-old female, melanoma shoulder. At higher power magnification, the tumor cells show intermediate-sized hyperchromatic nuclei with sheet-like growth and scattered mitoses
Some examples of nevoid melanoma have been termed minimal deviation melanoma or small cell melanoma. Because these terms have often been poorly defined and their application confusing, it would appear appropriate to refrain from using them without specific clarification.
Pigmented Epithelioid Melanocytoma
“Pigmented epithelioid melanocytoma” (PEM) is a term proposed by Zembowicz et al. for a group of heavily pigmented melanocytic tumors that were originally diagnosed as epithelioid blue nevus or animal-type melanoma (also termed equine-type , pigment-synthesizing, or melanophagic-type melanoma) (Zembowicz et al. 2004). Epithelioid blue nevus was originally described as a distinctive form of blue nevus associated with Carney’s myxoma syndrome (Carney and Ferreiro 1996). The terms animal or equine-type melanoma reflect the fact that similar tumors occur around the perianal skin of old gray horses (Crowson et al. 1999; Darier 1925; Levene 1979). Histologically, PEM is usually situated in the dermis and composed of heavily pigmented epithelioid and spindle cells. There is often associated epidermal hyperplasia or ulceration, and numerous admixed pigment-laden macrophages. Although most cases appeared to behave in an indolent manner in short follow-up period of the original report, 46% of cases showed metastatic deposits in regional lymph nodes. It was not possible to segregate the metastasizing and non-metastasizing tumors on the basis of their histopathologic features. A review of similar tumors from the Sydney Melanoma Unit also identified overlapping features with epithelioid blue nevus and animal-type melanoma, corroborating the observations of Zembowicz et al., although the frequency of lymph node metastases appeared to be lower (Scolyer et al. 2004b). Further support for the unique nature of PEMs was provided by the demonstration of a loss of expression of a protein kinase A regulatory subunit, type one alpha (R1alpha) (coded by the PRKAR1A gene on chromosome 17q22–24) in 28 of 34 PEMs (82%) but not in human and equine melanomas or a range of nevi (Zembowicz et al. 2007). Mutations of the PRKAR1A gene are found in more than 50% of patients with Carney’s syndrome. More recent studies with longer follow-up have supported initial reports that PEM was associated with indolent clinical behavior (Mandal et al. 2009). PEM may occur as a component of a combined nevus. In the latter setting, PEM usually harbors BRAFV600E and PRKAR1A mutations as well as showing loss of PRKAR1A expression. A subset of PEMs has also been shown to demonstrate gene fusions involving the PRKCA gene (Cohen et al. 2017).
Melanoma Arising From a Blue Nevus
Melanoma associated with a blue nevus (usually a cellular blue nevus) is very rare. It is most common on the scalp but may occur at any location (Ozgur et al. 1997; Granter et al. 2001). Malignant transformation is usually suspected clinically on the basis of rapid enlargement, ulceration, and change in color. As such lesions are often large (average size 3–13 cm) and extend deeply; they tend to have a poor prognosis. However, when matched with other malignant melanomas for known prognostic parameters, clinical outcome may not be significantly different.
In most examples, a clearly malignant tumor clone is present within or adjacent to a cellular blue nevus (Fig. 15). The melanoma usually displays expansile, sheet-like growth of large, hyperchromatic, pleomorphic, and mitotically active cells. Areas of necrosis are occasionally present. The mitotic rate is usually high (averaging 8–10 mitotic figures/mm2) and includes atypical forms. There is often a moderately dense inflammatory infiltrate at the tumor base. It can be difficult to determine the biologic potential of cellular blue nevus-like tumors with less severely atypical features than those that characterize diagnosable melanomas arising in association with a blue nevus. Such lesions have been termed atypical cellular blue nevi. Most reported cases of atypical cellular blue nevus have not metastasized in the reported follow up period; however, a recent study reported poor interobserver agreement for the diagnosis of atypical cellular blue nevi (Barnhill et al. 2008). Highlighting similarities with uveal melanoma, it has recently been described that loss of BAP1 is sometimes associated with a diagnosis of melanoma arising from a blue nevus (Costa et al. 2016).
Melanoma arising in a cellular blue nevus. Forty-seven-year-old male, melanoma of scalp. This patient presented with a rapidly growing nodule in a longstanding pigmented lesion that had previously been clinically unchanged for many years. (a) At lower power magnification, a cellular nodule is present near the deep edge of the specimen which represents the focus of melanoma. (b) The superficial part of the tumor shows features of a cellular blue nevus. (c) The melanoma is characterized by high cellularity, nuclear enlargement, pleomorphism, and multiple mitotic figures. A focus of necrosis is present (top left side of picture)
Mucosal Melanoma
Melanoma involving mucosal sites is uncommon but may arise (in descending order of frequency) in the oral cavity, the vagina, anorectal region, nasal cavity, penis, and esophagus (see chapter “Mucosal Melanoma”) (Pandey et al. 1998; Larsson et al. 1999). Mucosal melanoma usually presents clinically as an irregular brown patch or mass (Gorsky and Epstein 1998); however, some are amelanotic . Esophageal and nasal mucosal melanomas usually present with nasal obstruction or bleeding (Crawford et al. 1995).
The RGP of mucosal melanomas is typically lentiginous and often resembles AM. The invasive component is often spindle celled and may have an associated desmoplastic stromal response. Osteoid formation has been reported in nasal and anorectal melanomas (Friedmann 1998; Hoorweg et al. 1997; Dodds et al. 2019).
Vulvar and Penile Melanoma
Approximately 3–8% of all primary vulvar malignancies are melanomas, accounting for 3–7% of all melanomas in women (Wechter et al. 2004; Gonzalez-Bosquet et al. 1997; Dunton and Berd 1999; Ragnarsson-Olding et al. 1993, 1999a, b; DeMatos et al. 1998; Raber et al. 1996; Egan et al. 1997; Carlson and Mihm 1997; Carlson et al. 2002; Piura et al. 1999; Tasseron et al. 1992; Raspagliesi et al. 2000). They often present as a polypoid lesion that may be amelanotic (Ragnarsson-Olding et al. 1999a). The mean age of patients with vulvar melanoma is 61.4 years (DeMatos et al. 1998), although it can occur at all ages, including childhood (Raber et al. 1996; Seifried et al. 2015). Penile melanomas are rare.
The majority of vulval and penile melanomas have a radial growth phase. The junctional component is generally poorly circumscribed and usually shows diffuse pagetoid epidermal invasion by large atypical melanoma cells. There is often a dense dermal lymphoid infiltrate that may involve the junctional zone. Surface ulceration is common. The dermal component shows histological features typical of melanoma: sheet-like growth, poor maturation, cytologic atypia, and dermal mitotic figures.
Because a subset of genital nevi may show atypical features (as being one of the nevi of “special site”), particularly of the epidermal component, they have been termed atypical melanocytic nevus of genital type (Clark et al. 1998; Gleason et al. 2008). The junctional component of such tumors may show some architectural and cytologic atypia and potentially may be misdiagnosed as melanoma. Vulvar melanomas are recognized by greater cytologic atypia, prominent and diffuse pagetoid epidermal invasion, and features of the dermal component including mitotic activity. Nevertheless, interpretation of small, crushed, or partial biopsies can be extremely challenging. Another finding that may raise the possibility of melanoma is the association with features of lichen sclerosus (Carlson et al. 2002).
Conjunctival Melanoma
The group of acquired melanocytic lesions in the conjunctiva is designated by ocular pathologists as “primary acquired melanosis” (PAM), with or without atypia. Melanoma of the conjunctiva is associated with melanoma in situ (PAM with atypia) in 75% of cases (Folberg et al. 1992; Guillen et al. 1985). A small number of conjunctival melanomas arise in association with junctional or compound nevi. Most melanomas of the conjunctiva arise in the limbal or bulbar conjunctiva. Pigmented lesions of the palpebral conjunctiva are very rare, and most are either PAM with atypia or frank melanoma. Conjunctival melanomas exhibit the irregularities in border, color, and size that are associated with their more common cutaneous counterparts. One remarkable clinical difference between conjunctival nevi and melanomas is that a nevus has one feeder artery and a vein whereas melanomas have numerous vessels surrounding the lesion. Any thickening of the pigmented lesion should raise suspicion of malignant transformation. The equivalent of nodular melanoma is often a blue-black rapidly growing nodule, but rare melanomas are amelanotic. PAM with atypia and radial growth phase of conjunctival melanomas can resemble lentigo maligna (with cells disposed in the basilar region) or superficial spreading melanoma (with epithelioid cells either in basilar and/or pagetoid array) (Sugiura et al. 2007). Microinvasion of the substantia propria may occur in the radial growth phase of conjunctival melanoma. The vertical growth phase can exhibit epithelioid cells, spindle cells, small polyhedral nevoid cells, or balloon cells (Grin et al. 1998; Magro et al. 2006). A helpful histologic feature is the presence of dilated glandular structures (cysts) within conjunctival nevi, a finding rarely observed in conjunctival melanoma. As with their cutaneous counterpart, immunohistochemistry may be helpful to diagnose conjunctival melanoma. HMB45 highlights the presence of pagetoid migration and intraepithelial single-cell pattern of growth, and labels with a patchy pattern the component in the lamina propria. Anti-Ki67 labels scattered cells in the component in the lamina propria.
Prognosis
As in the skin, the prognosis of conjunctival melanoma is related in part to the depth of invasion. In one study, 29% mortality was seen in patients with lesions 0.8–1.5 mm in thickness (Folberg et al. 1985). Other prognostic features have been cited including greater than five mitotic figures per high power field and intralymphatic spread (Jakobiec et al. 1989). SLN examination has been employed in a manner similar to cutaneous melanoma (Savar et al. 2009) (see also below).
Management
Melanotic lesions of the conjunctiva should always be examined and possibly biopsied to determine if there is atypia. The first line of treatment for atypical PAM/melanoma in situ is surgical excision. If the lesion is widespread, adjunctive cryotherapy and even topical chemotherapy with agents such as mitomycin can be used. In some elderly patients, extensive PAM and even melanoma with microinvasion will respond to fractionated radiotherapy. Surgery is the preferred treatment for melanoma, followed by cryotherapy or radiotherapy. Enucleation is reserved for lesions where there is invasion into the globe involving, for example, the sclera.
Atypical Spitz Nevi/Atypical Spitzoid Tumors and Spitz Melanoma (Malignant Spitz Tumor)
Spitz nevi (SN) are benign melanocytic lesions which share histopathologic features with melanomas. In fact, they were first described by Sophie Spitz herself in 1948 as “juvenile melanomas” (Spitz 1948). Subsequently, Allen and Spitz recognized that, despite their concerning histopathologic appearance, SN had a paradoxically benign behavior (Allen and Spitz 1954), although one of the lesions resulted in metastasis and death this involved the deep fascia of the foot and may have been a clear cell sarcoma. Since the original description, the clinical and pathologic features of SN have been well described in the literature. While SN typically occur in children and melanomas typically occur in adults, both tumors can occur in patients of any age. In cases displaying all or most of the classical histologic features, particularly when occurring in a young patient, a confident diagnosis of Spitz nevus can be made. However, for those tumors with atypical histological features, it may be difficult to correctly predict biologic behavior just from histopathology. Such tumors have been referred to by a variety of names including atypical Spitz nevus, atypical Spitz tumor, and spitzoid tumors of uncertain malignant potential. However, even expert dermatopathologists have a relatively low concordance in distinguishing Spitz nevus with atypical features from melanoma.
To understand this group of disorders one must be aware of the histologic evolution of the compound Spitz nevus and the variant termed pigmented spindle cell nevus of Reed. Morphologically, when fully developed, a compound SN is a reticular dermal lesion that presents clinically as a nodule that may be flesh-colored, pink, red, reddish-brown, or black. Histologically, it is wedge-shaped, as an inverted triangle with the apex in the deep dermis and the base along the long axis of the epidermis. SN are well-circumscribed, symmetrical lesions. Two types of maturation occur. The first has reference to the large nests superficially that become smaller and break up into fascicles and single cells in the deeper portion of the lesion. This is the architectural aspect of maturation. The other, cytologic maturation, refers to the cells of SN becoming smaller as they descend into the dermis but always maintaining the same nuclear and cytoplasmic characteristics. Rare mitotic figures can be observed, but they are usually present in the epidermal or superficial dermal components and usually number less than 2 per mm2 (or < 6 in children under the age of 10). They seldom appear in the deep component or near the edges of the lesion.
On the other hand, the pigmented spindle cell nevus of Reed is seen as a plaque-like lesion in which most of the cells are present in the epidermis in discrete nests that are sharply circumscribed at the edges of the lesion. These cells are spindled and may be admixed with epithelioid cells and show varying degrees of pigmentation. There are often numerous suprabasilar epidermal melanocytes. In the nevus of Reed, a dermal component may be present and is usually a nested proliferation of cells cytologically identical to those of the epidermal nests, usually confined to the papillary dermis in contrast to the cells of Spitz nevi. There are virtually no mitotic figures in the dermal aspect, whereas intraepidermal mitotic figures can be noted.
Atypical Spitz tumors represent variations in these described patterns. These lesions often present as large expansile nodules that may measure several millimeters in thickness and be greater than 1 cm in width. Those well-demarcated, large nodules usually exhibit mitotic activity. In one study of atypical Spitz tumors, the factors that were associated with recurrence and/or metastasis were high depth of the lesion, extension into subcutaneous fat, and the number of mitotic figures (usually deemed significant if >5/mm2 in children or > 2 in adults) (Crotty 1997). In another study, mitotic figures were the most significant predictor of metastatic behavior (Walsh et al. 1998). For these lesions we recommend complete excision and that consideration be given to managing them as if they were a melanoma of similar thickness. In some cases, particularly lesions with many mitotic figures, a sentinel lymph node biopsy should be considered, especially if the patient is adult.
Melanocytic Tumors of Uncertain Malignant Potential (MELTUMP), Intermediate Melanocytic Proliferations and Melanocytomas
It is possible to accurately, rapidly, and reproducibly diagnose and predict the clinical behavior of most nevi and melanomas from their morphologic features. However, occasional melanocytic tumors, including those resembling Spitz nevi (including those with loss of BAP1 expression), blue nevi, and deep penetrating nevi, share many features common to nevi and melanomas, and the difficulty of predicting their biologic behavior is well documented (McCarthy and Scolyer 2004). Such tumors have been termed by some authorities as atypical melanocytic tumors of uncertain/unknown malignant potential (MELTUMPs) to highlight the uncertainty as to whether they are nevi or melanomas (Elder and Xu 2004). If this term is used, it should be spelled out fully and accompanied with a differential diagnosis and an outline of the reasons for the uncertainty.
Clinical, pathologic, molecular, and follow-up data have accumulated over recent years that indicate that the traditional dichotomous categorization of tumors as benign or malignant is likely too simplistic and there is a truly intermediate class of melanocytic tumors. In the fourth edition of the WHO Classification of Skin Tumours, the term “melanocytoma ” was proposed to encompass tumors fitting into this intermediate group. The most commonly encountered and diagnostically challenging entities in this group of tumors are atypical spitzoid tumors, since they may cause considerable discordance among pathologists (Barnhill et al. 1999; Cerroni and Kerl 2001). Melanocytomas generally show atypical features such as nuclear atypia, macronucleoli, (atypical) mitotic activity, necrosis, or ulceration (Elder and Xu 2004). However, the morphology of the cells lacks the degree of pleomorphism and severe atypia and other features that would allow a definitive diagnosis of melanoma. The lesions often have some resemblance to a Spitz nevus, an atypical banal nevus, a deep penetrating nevus, or a blue nevus. In rare instances, melanocytomas have arisen in association with a longstanding nevus that had been present for many years, causing a clinically detectable change. Such lesions often display two distinct cellular components, one with features of a banal nevus and the other showing differing morphology often with features of a deep penetrating nevus or BAP1 inactivated spitzoid nevus (Wiesner et al. 2011). Recent molecular data indicate that the second cell component usually arises when a BRAFV600E mutant nevus cell acquires a second genetic “hit” such as BAP inactivation or a mutation affecting signally in the Wnt signaling pathway (in the case of deep penetrating nevus) (Yeh et al. 2017). The vast majority of melanocytomas will have a benign course; however, there is evidence to suggest that their transformation rate may be higher than that of banal nevi.
Management
When there is genuine uncertainty as to whether a melanocytic tumor is benign or malignant, this uncertainty should be very clearly conveyed to the treating clinician. The clinician is then able to make a management decision on a case-by-case basis. While the safest course of action is to manage the tumor as if it were a melanoma, this may not always be appropriate. For tumors occurring on cosmetically sensitive areas such as the face, wide local excision of the tumor site may be particularly difficult, and a sentinel node biopsy procedure may be a complex undertaking. In many cases, the surgeon will convey the pathologist’s uncertainty to the patient, and present them with management options so that they can decide whether they wish to err on the side of caution but accept that there will be cosmetic sequelae from the wide excision and possible surgical morbidity from the sentinel node biopsy procedure. Furthermore, it must also be emphasized that the clinical, biological, and prognostic significance of sentinel node metastases from MELTUMPS remains uncertain. Studies reporting longer follow-up in larger numbers of patients are required to address these issues. It cannot necessarily be inferred that sentinel node metastases from MELTUMPs have the same significance as those from unambiguous melanomas.
Spitz Melanoma/Malignant Spitz Tumor
Spitz melanoma refers to a limited group of melanocytic lesions, comprised typically of large spindle and/or epithelioid cells, and often associated with epidermal thickening and sometimes pseudoepitheliomatous hyperplasia (Spitz features), that demonstrate severe cellular pleomorphism and high mitotic activity, usually greater than 6/mm2 that by definition are genetically characterized by the presence of either HRAS mutations or kinase gene fusions such as those involving ALK, ROS1, MET, RET, or NTRK. The mitotic figures may be atypical and may be identified throughout the lesion including deeply located cells. Often a high percentage of cells (>5%) stain for Ki-67, even in the deeper component. Ulceration is common. The tumors are composed of spindled and epithelioid cells. At any given level, there is severe cellular pleomorphism. There is no evidence of maturation, and the tumor cells become more atypical in the deeper layers and may form asymmetrical expansile nodules in the deep dermis and subcutaneous fat. In the absence of metastasis, molecular tests such as fluorescent in situ hybridization or comparative genomic hybridization to identify the presence of multiple chromosomal copy number gains and losses are usually required for diagnosis. These lesions should always be treated as melanoma, although they may have a better prognosis than usual melanomas of similar microstage. Some lesions that may appear spitzoid but lack the characteristic genomic features of Spitz tumors (e.g., BRAFV600E or NRAS mutated tumors) are best regarded as non-Spitz melanomas.
Histopathologic Features of Prognostic Importance
As described in chapter “Melanoma Prognosis and Staging,” the prognosis for a patient with clinically localized VGP primary cutaneous melanoma is principally correlated with its vertical depth of tumor growth (Breslow thickness). However, other factors are also important, particularly dermal mitotic rate, the presence of ulceration, perineural invasion, vascular invasion, and satellitosis, as well as patient characteristics, such as sex and age. It is important that all relevant histologic features are described in the pathology report to allow the most accurate estimate of prognosis to be made and an appropriate management plan prepared. As discussed below, a synoptic reporting format can assist (CAP template).
Breslow Thickness
Numerous studies performed over the past 30 years have shown that the prognosis for a patient with clinically localized primary cutaneous melanoma is principally related to its Breslow thickness (Balch et al. 1978, 1982, 2001b; Vollmer and Seigler 2000; Azzola et al. 2003). Breslow thickness is measured from the top of the granular layer of the epidermis or, if the surface is ulcerated, from the base of the ulcer, to the deepest dermal invasive cell (Fig. 16). Determination of Breslow thickness can be problematic in some circumstances. For example, in the case of periadnexal extension of melanoma, thickness measurements should not be based on those areas of periadnexal extension except if it is the only focus of invasion (Dodds et al. 2018). In that circumstance, depth is measured from the center of adjacent sweat glands or hair follicle to the furthest extent of infiltration into the periadnexal dermis. The Breslow thickness cannot be determined if a superficial shave biopsy transects a melanoma and includes only its superficial portion. In such instances, the pathologists can only report the melanoma to be “at least” a certain thickness. Correlation with the re-excision specimen is necessary. Similarly, tangentially cut sections are not suitable to provide an accurate Breslow thickness. In such cases, Clark level may be the only estimate of tumor thickness (see below). Other problems may arise from differing interpretations of the nature of dermal cells (i.e., whether they represent melanoma or a preexisting nevus).
Clark Level of Invasion
Tumor invasion may also be qualitatively expressed as Clark level, according to the anatomic compartment of invasion (i.e., papillary or reticular dermis or subcutis) (Fig. 17; Clark 1967). Clark levels are defined as follows (Clark et al. 1969):
-
Level I: Melanoma cells confined to the epidermis (melanoma in situ).
-
Level II: Melanoma cells invade into but do not fill or expand the papillary (superficial) dermis.
-
Level III: Melanoma cells fill and expand the papillary dermis with extension of tumor to the papillary-reticular dermal interface.
The boundary between the papillary and reticular dermis may be hard to identify, particularly if there is severe solar elastosis or in some sites such as the scalp, acral skin, and mucosal or anogenital regions. The papillary dermal collagen fibers are fine and oriented vertically, whereas the reticular dermal collagen bundles are coarse and have a more horizontal orientation, and therefore recognition of their distinction can be assisted using polarization microscopy because dermal collagen is birefringent. Another useful landmark in separating the papillary and reticular dermis is the presence of a capillary plexus at the interface. Polypoid tumors that expand but do not fill the papillary dermis should be classified as level III.
-
Level IV: Melanoma cells infiltrate into the reticular dermis.
-
Level V: Melanoma cells infiltrate into the subcutaneous fat.
Melanoma involving periadnexal adipose tissue (which represents extensions of and is continuous with the subcutis) should not be interpreted as level V invasion.
Most evidence suggests that the Breslow thickness measurement of the depth of invasion of a melanoma is a much more accurate prognostic indicator than its Clark level. Furthermore, evidence also suggests that pathologist’s assessment of Clark level of invasion has poorer interobserver reproducibility than Breslow thickness, mitotic rate, or ulceration (Scolyer et al. 2003).
Ulceration
The presence of non-traumatic ulceration (Fig. 18) is an important prognostic factor (Balch et al. 1978, 1980, 1982, 2001b; Buzaid et al. 1997; Kim et al. 1999). In an analysis of 17,600 melanoma patients, ulceration ranked second to tumor thickness as the most powerful predictor of survival in patients with clinically localized primary melanoma (Balch et al. 2001b). It is also likely that the size of the area of ulceration is prognostically relevant, and therefore the diameter of ulceration should be measured, using an ocular micrometer (Grande Sarpa et al. 2006). Difficulties may occur in assessing the presence of ulceration if there is only a focal loss of the epidermis and whether the epidermal deficiency is due to ulceration or sectioning artifact. The absence of fibrin or granulation tissue from putative areas of ulceration are clues that the loss of epidermis is actually due to sectioning artifact providing only part of the epidermis. Distinguishing non-traumatic and traumatic (procedural) ulceration is important as the latter is of no prognostic significance. While this differentiation is relatively easy when a clinical history of a previous biopsy is provided or a well-demarcated dermal scar is present, at other times it can be extremely difficult or impossible to distinguish between the two (Ruiter et al. 2002). Traumatic ulceration is often related to anatomic site or architectural configuration of the tumor (i.e., whether it is polypoid or pedunculated). Clues that ulceration is traumatic in origin include sharp demarcation with “squared off” edges or the presence of a “v” or wedge-shaped area of underlying granulation tissue.
Mitotic Rate
Mitotic rate is a very important prognostic factor (Fig. 19) (Gimotty et al. 2005, 2007; Barnhill et al. 2005; Ostmeier et al. 1999; Retsas et al. 2002; Azzola et al. 2003; Nagore et al. 2005; Francken et al. 2004), ranking second only to Breslow thickness in significance in an analysis of 3661 patients from the Sydney Melanoma Unit (Azzola et al. 2003). In that study, highly statistically significant differences in patient survival were found between each mitotic rate group (p values <0.0001) irrespective of whether the mitotic rate was grouped according to method A (0, 1–4, 5–10, and > = 11 mitotic figures/mm2) or method B (0–1, 2–4, and > =5 mitotic figures/mm2). Similar findings have been reported by others (Fig. 20). (Thompson et al. 2011; Gershenwald et al. 2017).
Dermal mitotic rate. Dermal mitotic rate is a very important prognostic indicator in primary cutaneous melanoma. (a) Fifty-two-year-old male, melanoma of back. Numerous mitotic figures are present (arrows) which were computed as up to 18/mm2. (b) Forty-eight-year-old male, melanoma of back. This melanoma included few mitotic figures (none are present in the illustrated field)
Kaplan-Meier melanoma-specific survival curves according to mitotic rate in patients with AJCC stage I and II melanoma from the AJCC eighth edition International Melanoma Database. (Figure 2 in Gerhsenwald et al. CA Cancer J Clin 2017; 177(6): 472–92. Reproduced with permission.) (Gershenwald et al. 2017)
Melanoma mitotic rate should be expressed as number of mitotic figures per square millimeter rather than mitotic figures per high power microscopic field because the field diameter may vary significantly between different microscopes, and this may potentially impart significant measurement error when using different microscopes (Ellis and Whitehead 1981). The recommendations of the 1982 International Pathology Workshop which revised the 1972 Sydney Classification of Melanoma should be followed (McGovern et al. 1986). In the 1972 Sydney classification, the number of mitotic figures in at least 10 HPFs over the entire lesion was determined and then expressed as the average number of mitotic figures/5 HPFs (McGovern et al. 1973). In contrast, the 1982 Workshop highlighted the appropriateness of expressing the mitotic rate per millimeter squared because it would be independent of the magnification and microscope used and results obtained in different studies would therefore be more comparable. The mitotic rate is determined by beginning the mitotic count in the high power field (×400 magnification) with most mitotic figures and then counting in successive high power microscopic fields (i.e., the “hotspot method”). Unless a standardized method is used to determine the mitotic rate, there is likely to be significant measurement error between observers because the number of mitotic figures often varies greatly between different parts of a tumor. A recent study showed excellent interobserver reproducibility for mitotic rate determination using this method even though the pathologists who performed the measurements had widely differing experience in the assessment of melanocytic tumors (Scolyer et al. 2003). We recommend a formal calibration of the field diameter of a microscope using a stage micrometer to determine the number of high power fields that equates to a square millimeter (Crowson et al. 2001b; Mihm and Googe 1990).
Inflammatory Host Response (Including TILs)
A lymphocytic response to the VGP component of a melanoma has been shown to influence prognosis in some studies (Clark et al. 1989).(Mihm et al. 1996; Clemente et al. 1996) The most commonly applied grading scheme for quantitating the presence of TILs is as follows. A non-brisk infiltrate refers to only focal areas of lymphocytic infiltration in the tumor. They may be isolated, multifocal, or even segmental. Absent is diagnosed when there are no lymphocytes present or, if present, they do not interact with tumor cells. For example a cuff of lymphocytes around the periphery of the tumor with no infiltration is considered absent. Furthermore, lymphocytes within the tumor nodule, but in perivenular array or in fibrous nodules in the tumor substance, without infiltration of the tumor itself, are considered absent. Infiltration either of the entire base of the tumor or diffuse permeation of the VGP is designated “present, brisk” (Fig. 21). To be significant, lymphocytes must infiltrate and disrupt tumor nests and/or show apposition to tumor cells (Crowson et al. 2001b; Mihm and Googe 1990). Other numerical methods have been suggested to quantitate TILs to provide prognostic information (Saldanha et al. 2017; Azimi et al. 2012),
Polyclonal VGP refer to tumors with separate islands or expansile nodules of melanoma cells of different morphologies usually separated from one another by fibrous bands. If one of these nodules has no infiltration, and the other(s) do have an infiltrate, the host response is designated absent because patients with these tumors have the same survival as those that fulfill the “absent” criteria defined above.
Regression
Regression is caused by a host immunologic response directed against a melanoma resulting in loss of part or all of the melanoma. It is a temporal phenomenon that may be arbitrarily categorized into three stages: early (tumor infiltrating lymphocytes – see above), intermediate, and late. Complete or late regression is characterized by an area of absent melanoma in the epidermis and dermis (Fig. 22), often flanked on one or both sides by residual melanoma. Often the epidermis is attenuated with loss of the rete ridges. The underlying dermis shows angiofibroplasia. In intermediate stage regression, there are often a few associated lymphocytes, variable numbers of melanophages, some edema, and telangiectasia. The prognostic significance of regression is controversial. Some studies have suggested it portends a worse prognosis, while others have suggested it is either not an important prognostic parameter or is associated with a more favorable outcome (Aung et al. 2017). Difficulties in interpreting such studies include lack of a standardized definition or criteria for its diagnosis and poor interobserver reproducibility. The reason for regression being an adverse prognostic factor in some studies is not entirely clear. One possible reason is that when regression is present in the deep dermal portion of a tumor, it may cause an underestimation of the original Breslow thickness of the lesion and therefore its metastatic risk is underestimated. It has also been proposed that a strong immune response to the tumor may cause tumor cells to undergo progressive genetic aberrations, leading to the ultimate generation of more aggressive tumor clones (Prehn 1996). Conversely, studies associating a more favorable prognosis to late regression have been interpreted as suggesting that regression indicates a more effective host immunologic response to the tumor. Further analysis are needed to determine if quantification of the amount of regression correlates with prognosis (Aung et al. 2017).
Regression. Seventy-three-year-old male, melanoma of chest. The zone of regression is characterized by an area of angiofibroplasia in the superficial dermis with numerous associated pigment-laden macrophages. No residual melanoma cells are present. The overlying epidermis is thin and shows loss of rete ridges (common features associated with late regression)
Microscopic Satellites
In the eighth edition of the AJCC melanoma staging system (Amin and Edge 2017), the definition of a microscopic satellite was refined. It is now defined as a nest of tumor metastatic melanoma cells adjacent or deep to a primary melanoma identified on pathological examination of the primary tumor site (usually in a wide excision specimen). The microsatellite must be discontinuous from the primary tumor and not separated from the primary tumor by only fibrous scarring and/or inflammation as this may represent regression of the intervening tumor. There is no minimal size threshold or distance from the primary tumor for defining microsatellites. It is important to emphasize that satellites should not be included in the measurement of Breslow thickness.
A number of studies have shown a statistically significant decrease in survival in patients who have microscopic satellites associated with their primary tumor (Day et al. 1981; Harrist et al. 1984; Leon et al. 1991; Gershenwald et al. 2017). Furthermore, the presence of microsatellites is associated with decreased overall survival and relapse free survival by univariate analysis; however, in multivariate analysis, vascular invasion, tumor vascularity, ulceration, as well as thickness better predicted both regional and distant metastasis (Shaikh et al. 2005; Kashani-Sabet et al. 2001, 2002). These studies support work done at Harvard Medical School that directly correlated the presence of tumor lymphangiogenesis with positive sentinel lymph nodes and survival (Dadras et al. 2003, 2005).
Blood Vessel and Lymphatic Invasion
Some studies have shown that frequency of angiolymphatic invasion increases with increasing depth and level of tumor invasion (Schmoeckel et al. 1983), while others have shown vascular invasion to be a significant predictor of metastasis (Kashani-Sabet et al. 2001; Mraz-Gernhard et al. 1998) or of reduced survival (Schmoeckel et al. 1983; Gilchrist et al. 1977; Kapelanski et al. 1979; Larsen and Grude 1979; Johnson et al. 1985; Barnhill et al. 1996). The presence or absence of melanoma cells within blood and lymphatic vessels should be documented in the pathology report; however, the actual observation of melanoma cells within blood vessels in histologic tissue sections is rare. Pathologic misinterpretation may arise in the assessment of lymphovascular invasion if a space around a tumor thrombus is regarded as retraction artifact or if a tumor thrombus completely occludes a vessel and the presence of a vessel is not recognized. The application of endothelial markers, such as CD31 and CD34, and new specific lymphatic endothelial markers, such as lymphatic endothelial hyaluronan receptor-1 (LYVE-1) (Banerji et al. 1999) and podoplanin (Breiteneder-Geleff et al. 1997) (Petersson), may be of assistance when there is uncertainty. It is perhaps the low frequency of the identification of lymphatic or vascular invasion that has precluded its emergence as an independent prognostic variable in many series (Clark et al. 1989).
Angiotropism
It has been proposed that the migration of tumor cells along the external surfaces of vessels (angiotropism) may be an important mechanism by which some melanoma cells spread to nearby and distant sites (Barnhill 2001). This phenomenon has been termed “extravascular migratory metastasis” (Barnhill and Lugassy 2004) and is akin to the known propensity of melanoma to spread along nerves and skin adnexal structures. Although further larger studies are required, initial results suggested that the presence of angiotropism may be an important factor predicting local, in-transit, regional, and distant recurrence in melanoma patients (Barnhill et al. 2002; Barnhill and Lugassy 2004; Van Es et al. 2008)
Neurotropism
The propensity of some melanomas to spread along the perineurium or occasionally within the endoneurium is termed neurotropism (Fig. 23). While it is a common feature of desmoplastic melanoma (about 30% of cases), it may also occur in non-desmoplastic melanomas. The presence of neurotropism is associated with an increased risk of local recurrence of melanoma and may require wider excision margins and or adjuvant radiotherapy in some cases (Baer et al. 1995; Varey et al. 2017).
Desmoplasia
Melanomas with a spindle cell morphology that are associated with and separated by variable stromal desmoplasia are termed desmoplastic melanomas (see also below). As discussed in more detail below, desmoplasia may represent almost all (>90%) of the dermal component of a melanoma (pure desmoplastic melanoma) or be associated (usually more superficially) with a non-desmoplastic dermal invasive melanoma (termed mixed or combined desmoplastic melanoma) (Busam et al. 2004; Hawkins et al. 2005). Recent evidence suggests that although pure desmoplastic melanoma is associated with higher rates of local recurrence, survival times are longer than for patients with non-desmoplastic melanoma of similar stage (Busam et al. 2004; Hawkins et al. 2005). For combined or mixed desmoplastic melanoma, rates of local recurrence and survival times are intermediate between those of pure desmoplastic melanoma and non-desmoplastic melanoma. Furthermore, there is a lower frequency of sentinel node positivity in desmoplastic melanomas. Thus, it has even been suggested that for patients with pure desmoplastic melanoma, sentinel node biopsy is unnecessary because the frequency of involvement is low (Gyorki et al. 2003; Pawlik et al. 2006).
Anatomic Site
Melanomas in the head and neck area, upper back, axial skeleton, subungual region, and/or on the palms or soles have a worse prognosis than extremity-based lesions when matched for other known prognostic factors (Balch et al. 1978, 1982, 2001b; Azzola et al. 2003).
Sex
Women have a better prognosis than men in some but not all studies (Balch et al. 1978, 1982, 2001b; Massi et al. 1999). The statistical significance of gender as a prognostic factor is confounded by anatomic site. Furthermore, obesity in males patients is associated with improved survival in male patients treated with targeted or immune therapy, although the mechanism for this is unclear (McQuade et al. 2018).
Age
Older patient age at diagnosis is an independent predictor of worse outcome in melanoma (Balch et al. 1978, 1982, 2001b). Interestingly, the frequency of sentinel node melanoma metastasis is inversely correlated with patient age. Possible explanations for this clinical observation include differences in tumor biology and/or host immunity in patients of differing ages and are the subject of ongoing investigations.
Lymph Node Metastasis
The presence of melanoma metastases within regional lymph nodes is an adverse prognostic feature and is an important parameter in the AJCC/UICC melanoma staging system (Balch et al. 2001a; Balch 2002; Gershenwald et al. 2017). As discussed in more detail below, the use of the sentinel lymph node biopsy technique in melanoma patients has revolutionized nodal staging. The number of positive regional lymph nodes is also an important prognostic factor with prognosis declining as the number of positive lymph nodes increases. Quantification of tumor burden using methods such as measurement of the size of the intranodal metastasis or distance from the border of the lymph node correlates with prognosis and as such it is recommended to be included in melanoma reporting (see below).
The Melanoma Pathology Report Including a Synoptic Format
Pathological features of localized primary cutaneous melanoma are the principal indicators of prognosis and guide initial definitive treatment including width of excision margins and appropriateness of sentinel node biopsy. For accurate prognostication and optimal patient care, thorough assessment and documentation of pathologic features of the melanoma is essential, and the pathologist has a responsibility to provide this information in the pathology report (Cochran et al. 1998). This aids the clinician in predicting the likely outcome and in planning subsequent treatment, and communicating this information to the patient (Scolyer et al. 2005). The presence of certain adverse prognostic factors may also qualify the patient for entry into clinical trials. The pathology report should include important pathologic features (such as those listed above) that affect patient prognosis and management.
The Synoptic Pathology Report
A synoptic pathology report refers to the presentation of information in a tabular rather than descriptive form. In our view, both the pathologist and clinician benefit from the discipline of respectively reporting and reading melanoma pathology reports in a synoptic format because such reports ensure that all information required to make management decisions and determine prognosis are addressed and included in the report and can be considered in management decisions (Scolyer et al. 2005). Synoptic reports also act as a checklist that not only helps ensure completeness in the reporting of pathology but also consistency, because information is presented in a predictable and easy-to-read format. Use of the synoptic format can save time for the persons completing/typing the report because they allow the use of words or phrases instead of sentences. Furthermore, the synoptic format facilitates efficient extraction of information for registries, data collection, and research purposes. An example of a melanoma synoptic report is provided in Table 2.
The value of synoptic reporting in melanoma was highlighted in a study which showed that the most important factor predicting the completeness of pathology reports (including for parameters critical to staging and guiding management such as Breslow thickness, Clark level, ulceration, mitotic rate, and distance to specified margins) was not whether they were generated within or outside a specialist melanoma treatment center, but whether or not they were in a synoptic format (Karim et al. 2008). This highlights the fact that synoptic melanoma pathology reports are of value not only in specialist units/tertiary institutions but also in non-specialist pathology practices.
While synoptic pathology reports provide a framework for the minimum set of features to be assessed, they should not restrict the pathologist to documenting only those features. The facility for free text to allow the pathologist to document other features and to explain any points of uncertainty and the reasons for coming to a particular opinion must be available in all synoptic reports. The use of a synoptic report may not always be appropriate for melanocytic tumors which are difficult to classify or for those where there is genuine uncertainty as to whether a lesion is benign or malignant. Under such circumstances, we recommend that the pathologist should document the evidence in favor/against a particular diagnosis, give a preferred diagnosis, but express the degree of uncertainty. If melanoma is favored, the synoptic template may also be completed, but there is always the concern that the clinician, by seeing the synoptic report, may not comprehend the diagnostic difficulty and assuming a diagnosis of melanoma may manage the patient in a suboptimal manner (Table 3).
Melanoma in Children and Adolescents
Patients less than 20 years of age harbor between 1.3% and 2% of all melanomas. In prepubertal children, melanomas are truly rare, representing 0.3–0.4% of melanomas occurring in the young (see chapter “Melanoma in Children and Teenagers”) (Ceballos et al. 1995; Reintgen et al. 1989; Trozak et al. 1975; Rao et al. 1990). Melanoma in childhood occurs in three main settings: congenital melanoma, melanoma developing de novo, and melanoma arising in a preexisting nevus (see chapter “Acquired Precursor Lesions and Phenotypic Markers of Increased Risk for Cutaneous Melanoma”) (Scalzo et al. 1997; Berg and Lindelof 1997; Temple et al. 1991; Ceballos et al. 1995; Whiteman et al. 1997; Lu et al. 2015; Prieto-Granada et al. 2016a; Verzi et al. 2017; Wilmott et al. 2019). Since most melanocytic lesions in children are benign, there may be a bias of some pathologists and dermatopathologists to underdiagnose melanoma in such clinical scenario.
Histopathology
Most studies suggest that childhood melanoma is diagnosed when the tumor is at an advanced clinical stage with many greater than 1.5 mm and invasive to levels IV and V. In a review of childhood melanomas in patients younger than 13 years who subsequently died of melanoma, all but one were bulky tumors greater than 8 mm in thickness with surface ulceration and a high mitotic rate (Prieto-Granada et al. 2016a).
Most melanomas arising de novo are superficial spreading in type, followed by nodular and unclassifiable melanomas (Scalzo et al. 1997; Melnik et al. 1986; Rao et al. 1990).
The histologic findings in lesions that clinically resemble adult melanoma are similar to the adult lesions. As discussed above, melanomas that resemble Spitz nevi are more problematic. Several parameters help in assessing these lesions. The mean thickness of Spitz nevi is approximately 1 mm, whereas lesions that are thicker or that penetrate the subcutis raise for the possibility of malignancy (Spatz et al. 1999). Lesions greater than 10 mm in diameter or ulcerated tumors are especially worrisome (Spatz et al. 1999). The relationship of nests of cells and individual cells is a critical feature to assess. In childhood melanoma, there is great variability in nest sizes, and dermal nests are larger than intraepidermal nests. In contrast to Spitz nevi, childhood melanomas show a striking “side-by-side” nuclear pleomorphism that is visible at any given level of the lesion. In Spitz nevi, the cells are of similar type (i.e., spindle shaped or epithelioid) and are similar to one another at the same level. Absence of maturation is another key factor as is the presence of a deep expansile nodule, especially if it contains mitotically active cells. Mitotic figures are a highly significant key diagnostic criterion: when there are more than six dermal mitotic figures per mm2 or mitotic figures are present within 0.25 mm of the lesional edge (Spatz et al. 1999; Crotty et al. 1992). Atypical mitotic figures are of special concern. Some investigators have found that the presence of fine dusty cytoplasmic melanin pigment in deep cellular nests of lesions in adolescents were associated with risk of metastatic behavior (McCarthy et al. 1994). In aggregate, the features that favor benignancy are age less than 10 years, sharp demarcation, symmetry, diffuse maturation, spindle cells and nuclei, few or absent mitotic figures, epithelial hyperplasia, and Kamino bodies (Crowson et al. 2001b; Crotty et al. 1992; Spatz et al. 1999; McCarthy et al. 1994). Nevertheless, 6–8% of spitzoid melanocytic proliferations are unclassifiable (Rapini 1999). A large study with 137 patients showed that nodular histologic type, fusiform or spitzoid cytology, high Breslow thickness, vertical growth phase, high dermal mitotic activity, ulceration, and vascular invasion were associated with impaired prognosis, while adjacent nevus and radial growth phase were associated with a better prognosis. Multivariate analysis showed that age ≥ 11 years and higher Breslow thickness predicted an increased risk of metastasis (Paradela et al. 2010). Barnhill et al. (1999) noted that significant discrepancies occur even among expert observers because of a lack of objective criteria for predicting the biologic behavior of these tumors. Even when Spitz-like tumors metastasize to regional node fields in childhood, they are not often fatal (Barnhill 1998b; Hung et al. 2013; Sepehr et al. 2011; Scolyer et al. 2010; Berk et al. 2010; Ludgate et al. 2009; Murali et al. 2008).
Melanoma in congenital nevi usually exhibit epithelioid melanocytes and show mitotic activity, inflammation, necrosis, sharp demarcation from the adjacent nevus, and absent maturation.
A diagnosis of melanoma in childhood is often very difficult and cannot be made on the basis of any single criterion in isolation. Rather, it is a construction based upon assessment of multiple features of cytology and architecture in concert with the clinical history (Barnhill 1998b; Barnhill et al. 1995, 1999; Crotty et al. 1992; McCarthy et al. 1994; Rapini 1999; Spatz et al. 1999; Spitz 1948; Skov-Jensen et al. 1966; Weedon and Little 1977; Reintgen et al. 1989; Tate et al. 1993; Chun et al. 1993; Sander et al. 1999). Immunohistochemical features may be helpful, particularly high proliferation rates with anti-Ki67 (in patients older than 1 year of age) and irregular labeling with HMB45. Notwithstanding the combined experience of the authors, we regularly encounter lesions that defy precise classification and do not permit confident prediction of biological behavior. It is appropriate to express uncertainty in such cases in order to allow for a fully informed approach to management. For these difficult lesions, the performance of a sentinel lymph node biopsy may be considered, a technique that allows one to obtain modest additional information concerning the possibility of metastatic behavior. However, many studies have indicated that detection of metastasis in SLN in childhood lesions may not represent the same prognostic value as in adults (Reed et al. 2013; Paradela et al. 2010). Further studies with even longer follow-up will be required to establish the clinical significance of SLN examination in children with melanocytic lesions.
Cutaneous Metastases of Melanoma
If the diagnosis is made in the context of an appropriate clinical history, it is usually not difficult to establish a correct pathologic diagnosis of primary cutaneous melanoma for those familiar with the spectrum of features of melanocytic tumors (Plaza et al. 2010). However, it can be extremely difficult or even impossible to definitively determine whether a melanoma is a primary tumor or a metastasis on the basis of histology/cytology alone (Guerriere-Kovach et al. 2004). This is particularly the case for a melanoma of the dermis in the absence of an in situ component in the overlying epidermis. In some instances, such tumors represent a primary melanoma with regression of the superficial dermal and epidermal components. The pathologist should recognize this phenomenon by the presence of subtle clues such as the presence of rare single atypical epidermal melanocytes, epidermal thinning with loss of rete ridges, fibrosis and vascular proliferation in the dermis overlying the lesion, a defect in the band of superficial solar elastosis, and a band-like lymphohistiocytic inflammatory cell infiltrate (which usually includes numerous melanophages). In cases where difficulty remains, it is prudent to examine microscopically additional tissue from the lesion, including further sections cut from the original and additional tissue blocks. In some instances it is impossible to be certain from the pathologic features alone whether a melanoma is primary or metastatic. In such cases, correlation with clinical information is essential, as this may provide further and critical clues. Furthermore, some primary melanomas may arise in the dermis without origin from the epidermis, including some cases of desmoplastic melanoma, melanomas arising in congenital nevi, and blue nevus-like melanoma (so-called malignant blue nevi). Such melanomas may incorrectly be reported as metastatic melanoma on pathological criteria alone. The observation of an associated, benign component indicates some such cases that they are primary lesions and not metastatic melanomas. Because some apparently primary melanomas (so-called primary dermal melanomas) may be pathologically indistinguishable from dermal melanoma metastases (Cassarino et al. 2008; Swetter et al. 2004), we recommend that pathologists be extremely cautious in diagnosing metastatic melanoma when there is no clinical history of a prior primary melanoma. If there is no clinical evidence to suggest a prior primary, we recommend such cases be managed as for a primary melanoma of a similar thickness because their prognosis appears to reflect this. If the lesion is indeed metastatic, it is likely that additional metastases will appear within a relatively short interval.
Conversely, some metastatic melanomas can show prominent epidermotropism, mimicking a primary tumor (White and Hitchcock 1998; Abernethy et al. 1994; Bahrami et al. 2007; Plaza et al. 2010). In most epidermotropic melanomas the overlying junctional change does not extend beyond the dermal component. However, in some instances, the clinical features may be the only clues to the recognition that the tumor is, in fact, a metastasis. Awareness of this phenomenon, together with precise communication between clinicians and pathologists, is vital to minimize the risk of misdiagnosis. It has been recognized in recent years that occasionally the pathologic features of some metastatic melanomas may closely resemble blue nevi (Busam 1999). Although the presence of subtle nuclear pleomorphism, occasional mitotic figures, and an associated lymphoid infiltrate may provide pathologic clues to the correct diagnosis, correlation with clinical information is, as always, essential in reaching a final diagnosis.
Regional Lymph Node Metastases of Melanoma
Approximately 15–20% of patients with a primary cutaneous melanoma >1 mm thick develop ipsilateral regional lymph node metastases, usually as the first evidence of metastatic spread. Such metastases are more likely in patients with thicker, deeper, ulcerated primaries that show frequent dermal mitotic figures. The presence of true endolymphatic tumor in the region of the primary tumor is associated with an increased risk of nodal metastases, but the correlation is not 100%. The time that elapses between removal of the primary and development of nodal metastases varies according to tumor depth and thickness. Most nodal metastases develop within 4 years, but a significant minority of metastases develops later, up to and even beyond 10 years after the primary surgery.
The prophylactic removal of all lymph nodes within a nodal basin on the grounds that primary melanomas of similar pathology have, in the past, generated nodal metastases (elective lymphadenectomy) is little practiced now, having been largely replaced by lymphatic mapping and sentinel node (SN) biopsy (see below). Removal of clinically positive regional nodes (palpable nodal enlargement or abnormal features on ultrasound, with or without guided fine needle aspiration) (therapeutic lymphadenectomy) is still widely practiced (in contrast to the abandonment of complete lymph nodal dissection for patients with a positive sentinel lymph node) (Faries et al. 2017). These approaches generate specimens containing multiple lymph nodes that (with their adjacent non-lymphoid tissue) all require pathologic assessment, and tissues from both approaches are managed similarly.
Laboratory Assessment of Regional Lymphadenectomy Specimens
Specimens received fresh or in fixative should be measured, and the presence of orienting stitches or clips recorded. The specimen is fixed for 18–24 h in a generous volume of buffered formalin (or alternative). The nodes are bisected and the total number recorded. After examination of HE-stained sections the number of tumor-positive nodes is recorded. Whether the nodes are totally or partially replaced by tumor and the presence of extra-capsular extension should be recorded (Cochran et al. 1992, 2000).
It is not customary (because of workload and fiscal concerns) to use immunohistochemistry in evaluating all or even most lymphadenectomy specimens, but this approach should be used to evaluate suspicious or anomalous findings or to confirm that tumor in a node is melanoma. It should be remembered that benign melanocytes (showing S100+, Mart-1+, Ki67−, and P16+) are found in nodal capsules and trabeculae in up to 24% of cases (Carson et al. 1996; Biddle et al. 2003). These clusters of benign cells are designated as nodal nevi (a term preferable to the sometimes used “subcapsular” nevi since they are overwhelmingly located in the capsule surrounding the lymph node), and sometimes in the fibrous trabeculae. The number of lymph nodes that are the site of metastases is a critical indicator of likely clinical outcome, with prognosis declining as the number of positive nodes increases (Callery et al. 1982; Balch et al. 2001b). Where numerous nodes are matted together by tumor, it may be impossible to determine the number of nodes affected. Matting and fusion of nodes by tumor is by itself a highly unfavorable sign.
Lymphatic Mapping and Sentinel Lymph Node Biopsy
The advent of lymphatic mapping and SLN biopsy (Morton et al. 1992), staging techniques designed to identify patients with early spread of melanoma to the lymph nodes, has dramatically altered the work of pathologists evaluating lymph nodes from melanoma patients, since it allows pathologists to thoroughly evaluate a limited number of lymph nodes, those most likely to be positive (Morton et al. 2014).
The critical challenge for pathologists evaluating SLN biopsies is to accurately determine the presence or absence of metastatic melanoma to facilitate prediction of likely clinical outcome for the individual patient and determination as to whether an immediate completion lymph node dissection should be considered (Morton et al. 2006).
Laboratory Assessment of Sentinel Lymph Nodes
These comments are based on recommendations from the International Sentinel Node Society and the American Joint Committee on Cancer Melanoma Expert Panel (Gershenwald et al. 2017; Chakera et al. 2009).
SLN specimens should be gently handled in the operating room to avoid crush or cautery artifacts. If specimen transfer to the laboratory is likely to exceed 2 h, the tissues should be placed in fixative in the operating room area immediately after surgical removal. Surgeons may mark the SLN with clips or stitches of different lengths and colors to highlight blue-colored areas of the SLN observed during surgery and areas suspicious for tumor. Any such special markers should be described in the pathology requisition form, preferably with a simple explanatory diagram.
Dissected SLNs are examined for blue coloration, measured (at least maximum diameter in mm), and a note taken of the reported radioactivity recorded during intraoperative gamma counter evaluation. The node is then either bisected or breadloafed , depending on the protocol employed, often influenced by the size of the node. When using bisection, since melanomas metastasize first to the subcapsular sinus in the plane of entry of afferent lymphatics, it has been recommended that the SLN be bisected through its longest meridian. This critical cut should provide two lymph node segments as nearly equal in volume as possible. Alternatively, breadloafing produces a larger surface to be examined in a single histologic section. The cut surfaces are closely examined with the naked eye and perhaps a hand lens (or dissecting microscope) for the presence of visible metastases or collections of melanin or carbon pigmentation. At this stage imprints may be made for cytology (see below); however, this is not standard in most institutions. The slices are placed in cassettes and fixed in formalin for 12–24 h prior to standard processing through alcohols to paraffin. Samples provided for research should not interfere with the determination of nodal tumor status (Cochran et al. 2003).
SLNs encased in fat are usually dissected free (but this may destroy evidence of tumor in afferent lymphatics) and each node handled as noted above. Since the reported radioactivity cannot be assigned in this case to an individual node, all nodes in the specimen are to be regarded as SLN and subjected to appropriately detailed evaluation. Most patients have a single SLN, but two and three SLNs may be encountered. If more lymph nodes are present, the specimen is likely a partial lymph node dissection, rather than an SLN biopsy and surgeons using this approach must be made aware of work load and fiscal issues that arise from special processing of multiple lymph nodes.
Laboratory Confirmation That a Submitted Node Is Truly Sentinel
Accurate staging of clinically localized primary melanoma requires that node(s) claimed as SLN are truly sentinel lymph nodes. Because nuclear medicine and surgical approaches that identify SLN are fallible, it is possible that a node claimed as sentinel may not be the true SLN. This can occur when the true SLN is partially or entirely replaced by tumor, deviating lymph flow to other nodes. There is a need for objective measures that pathologists can use to confirm the SLN status of submitted lymph nodes. The blue dye injected by the surgeon usually dissipates by the time the node arrives in the laboratory or is at most faintly visible. Similarly, radioactive isotope decays rapidly from peak emission values measured in the operating room and laboratory measurements would be suboptimal. Few pathology laboratories are equipped to measure radioactivity.
A stable, inert marker such as particulate carbon that selectively accumulates in SLN and is readily visible on standard microscopy would be ideal for confirmation of SLN status. Carbon particles injected intradermally with blue dye at the start of surgery accumulate preferentially and usually exclusively in the SLN. Particles settle in the subcapsular sinus and in lymphoid tissues around the point of entry of afferent lymphatics. Carbon accumulations also indicate the area of the node most likely to be the site of metastatic tumor cells delivered from the primary tumor via the same lymphatics as carbon particles and blue dye. Intensive evaluation of nodal tissues can thus be focused on this area of the node. This approach cannot be applied if the patient has a permanent black tattoo, because carbon-based particles from tattoos often migrate to the regional nodes. Drug regulations vary and this use of carbon particles is not currently permitted in some countries (Haigh et al. 2001; Morton et al. 2003), and is not generally standard of care.
Intraoperative Evaluation of Sentinel Nodes
Although LM/SLNB were initially developed using intraoperative frozen sections (Morton et al. 1992), experience has shown that frozen sections are unreliable in this role (Cochran et al. 2003). Although intraoperative frozen sections allow the possibility of immediate completion lymph node dissection if the SLN contains melanoma cells, the preparation of a full-face frozen section often requires the sacrifice of relatively substantial amounts of nodal tissue. And since SLN metastases are often small and narrowly located beside the nodal meridian, diagnostic tissue may be entirely destroyed during preparation of frozen sections. Additionally, identification of melanoma cells is more difficult in frozen tissue sections relative to slides prepared from well-fixed tissues. It is therefore strongly recommended that SLNs from melanoma patients are evaluated using sections from properly fixed paraffin-embedded SLN. If there is a need for intraoperative assessment, visual assessment of the cut face of the node is recommended and cell smears may be obtained by scraping the nodal cut surfaces, or tumor imprints prepared by pressing the cut surfaces of nodes onto glass slides for cytological evaluation.
The Need to Evaluate Multiple Levels of the Sentinel Lymph Node
There is little disagreement that there is a need to examine multiple H and E stained levels of both halves of SLN. What is not settled and continues to cause considerable debate is how many sections should be examined and how extensively separated these sections should be.
In early studies of micrometastases in lymph nodes (Cochran et al. 1988), data showed that (in contrast to other types of tumors, such as breast cancer) early metastases of melanoma are selectively present in the tissues that lie adjacent to the longest meridian through the node. On this basis it has been recommended (Morton et al. 1992; Cochran et al. 2000; Cochran 2000) that ten full face serial sections entirely representative of the node are cut from both cut faces of the node (Fig. 1) and stained by H&E (sections 1, 3, 5 and 10), S100 (section 2), HMB45 (section 4), and MART-1 (section 6). If tumor cells are not found in a clinical context where the thickness of the primary melanoma suggested that there was a high probability that the SLN would be positive, the above protocol can be repeated to evaluate deeper areas of the SLN. Antibodies to tyrosinase and cocktails to multiple melanoma epitopes may also be used according to local practice. According to the particular mix of thickness of the melanomas in a given study, this approach yields 16–20% of tumor-positive SLN, a figure similar to the rate of ipsilateral regional nodal failure seen in patients treated by wide excision alone. This level of sampling thus appears to detect all nodal metastases that progress to clinically detectable size by 10 years after excision of a primary melanoma. Subsequent studies have shown that simpler protocols employing fewer number of sections provide equivalent results, with similar “false-negative” rates of nodal relapse (Prieto and Sandra 2002).
Other studies have shown that evaluation of sections cut from deeper in the SLN will, in some patients, identify (usually) small numbers of melanoma cells, increasing the number of positive SLN (Cook et al. 2003; Spanknebel et al. 2005; Riber-Hansen et al. 2009). The clinical significance of detection of these additional melanoma cells remains undetermined and elucidation will require prolonged follow-up studies.
The entire slide is scanned at low power and the different nodal compartments assessed, with particular attention to the subcapsular sinus. Tumor cells may replace substantial areas of the node or be relatively few in number and dispersed singly or as microcolonies in subcapsular sinuses, lymphoid parenchyma, deeper sinuses, and afferent lymphatics (with the same clinical implication as intranodal tumor) (Cochran et al. 2003). High-power (×400) fields are examined for single or clustered tumor cells. The MART-1 preparation can be examined first followed by the S100 and HMB45 stained sections, and the H&E sections. Other protocols use other markers (SOX10, MITF) or even cocktails with different combinations of MART1/HMB45/Tyr/SOX10. The H&E sections are most likely to demonstrate microcolonies, but single cells may be more difficult to identify, even if they are heavily melanized since they may resemble melanophages. Melanoma metastases extend into the lymphoid parenchyma or central nodal sinus when expansile colonies have formed. Extracapsular invasion is infrequent, but should be recorded (Cochran et al. 2004a). Measurement of the amount of tumor present and its distribution are discussed below.
The Role of Immunohistochemistry in Evaluation of Sentinel Lymph Nodes
Single tumor cells or small micrometastases can be extremely difficult to identify in H&E-stained sections and even experienced pathologists may miss up to 12% of patients with SN metastases (Cochran 2000). It is therefore essential to supplement conventional stains with immunohistochemistry. The choice of immunohistochemical reagents is important (Ohsie et al. 2008). Antibodies to S100 protein stain essentially 100% of melanomas but are not specific, staining paracortical dendritic leukocytes, some sinus histiocytes, fat cells, Schwann cells of nerves, and capsular/trabecular nevocytes (Carson et al. 1996). Despite the challenge of interpreting S-100-stained sections, some authors consider that S100 (expressed by all melanomas) is still helpful but should always be used in parallel with other more specific (though less sensitive) antibodies. The other commonly used antibodies, MART-1/MelanA, HMB45, and tyrosinase, are more specific for cells of melanocytic lineage but are not expressed by up to 25% of melanomas (Ohsie et al. 2008). Further studies with the application of SOX10 may determine the effectiveness of that marker. True false-positive assessment of SLN tissue would be due to misidentification of non-melanoma cells. For a cell to be called “positive” for metastasis, it must be morphologically consistent with melanoma. Confusion between melanin-containing macrophages and immunopositive melanoma cells may be reduced by the use of red aminoethylcarbazole rather than brown diaminobenzidine to allow antibody visualization. Other potential sources of false-positive interpretation (as noted above) are S100-reactive dendritic cells, capsular and trabecular nevi, histiocytes, intranodal and perinodal nerves, and ganglion cells. HMB45 reactivity in the absence of melanoma cells may be seen in the focally calcified trabeculae of inguinal or pelvic lymph nodes, an alteration most marked in older patients. Occasionally, mast cells show apparent reactivity with MART-1 and/or HMB45. Furthermore, nodal nevus with features of blue nevus is strongly positive with HMB45.
Identification of Nodal Nevi and Their Separation from Metastatic Melanoma
Discrimination of benign nevocytes from metastatic melanoma requires meticulous consideration of nodal architecture and the cytological and immunophenotypical features of the cells under consideration (Cochran et al. 2003; Prieto 2017). Melanoma cells are usually (but not always) larger than nevocytes and are located individually and in small aggregates within the subcapsular sinus and lymphoid tissues of the node. They are seldom seen in the nodal capsule other than within afferent lymphatics entering the node. Features that assist separation of melanoma cells from nevocytes include large size, high nuclear to cytoplasmic ratio, prominent “bird’s eye” nucleoli, mitotic figures (especially atypical forms), and the presence of the fine punctate melanin granules (single melanized melanosomes) that indicate a cell that synthesizes melanin. Coarse melanin granules usually indicate the presence of the melanosomal aggregates that are characteristic of macrophages (melanophages). Up to 25% of melanoma patients have nevocytes in the connective tissue capsule or trabeculae of one or more regional lymph node(s) draining skin surfaces (Carson et al. 1996). Extension of nevocytes into perivascular stroma or ultrafine reticulations of the trabeculae can make interpretation difficult; the cells seeming to lie in the lymph node parenchyma, a location more characteristic of melanoma cells (Cochran et al. 2003; Wen et al. 2004). It may be helpful to evaluate the nodal tissue using connective tissue stains such as Masson trichrome and reticulin that will disclose the arborizations pattern of the nodal architecture. Nevocytes stain positively for S100 (nucleus and cytoplasm), MART-1 (cytoplasm) has weak or absent reactivity for HMB45 and is negative for Ki67. Consideration of high-quality immunohistochemical preparations is usually helpful in determining whether suspicious cells are derived from a nevus or a melanoma.
Sentinel Lymph Node Evaluation in Assessment of the Malignant Potential of Ambiguous (Nevoid) Melanocytic Lesions
Lymphatic mapping and SLN biopsy have been used in the management of patients with melanocytic skin lesions for which the pathologist cannot definitively diagnose melanoma or a nevus (Kelley and Cockerell 2000; Zuckerman et al. 2001; Lohmann et al. 2002; Su et al. 2003). If the pathologist, after due consultation, remains less than totally confident of the diagnosis, the patient may be managed by wider excision, lymphatic mapping, and SLN biopsy. Additional regional surgery is not required if the SN is tumor-free or contains only capsular or trabecular nevus cells. If the node contains true intraparenchymal metastases, completion lymph node dissection may be offered and adjuvant therapy discussed. This is not a truly diagnostic procedure, but the pattern of extension of the lesion within the regional nodes (non-threatening nevus-type spread within the capsule or trabeculae or the more ominous involvement of the nodal parenchyma that is characteristic of melanoma) may assist determination of the most appropriate management strategy. A similar immunohistochemical approach to that outlined above can be applied to evaluation of SLN removed during the management of ambiguous nevoid lesions such as atypical spitzoid neoplasms and atypical cellular blue nevi. However, in the case of Spitz tumors, studies have demonstrated that these patients do not (with only rare if any exceptions) go on to experience progressive disease.
Molecular Biology as a Supplement to Histological Evaluation of Sentinel Nodes
Conventional microscopy may fail to detect limited occult melanoma in the SN of some melanoma patients. This may be used as an argument for more extensive nodal sampling (Cook et al. 2003) and/or the use of approaches such as real-time polymerase chain reaction (RT-PCR) (Morton et al. 2003). The possibility that RT-PCR could identify melanoma cells by detecting mRNA for melanoma-associated markers in SLN with no histological or immunohistological evidence of tumor cells attracted considerable interest. However, there is currently no compelling reason to abandon microscopy and analyze SN exclusively by RT-PCR. The techniques used to extract mRNA for evaluation by RT-PCR destroy the tissue, and the cell from which any enhanced signal derives can never be identified. In addition to deriving from melanoma cells, a molecular signal could originate from capsular and trabecular nevocytes, Schwann cells of intranodal nerves, macrophages that have ingested melanosomes, or melanoma-macrophage hybrids. There is concern that overinterpretation of the results of RT-PCR studies could lead to patient overtreatment (Starz et al. 2003). The efficacy and clinical relevance of molecular analysis of the SLN was analyzed in the second Multicenter Selective Lymphadenectomy Trial (MSLTII) which compared immediate completion lymphadenectomy with observation in the management of patients with a tumor-positive SLN (including for this study patients with a histologically negative but RT-PCR positive SLN) (Faries et al. 2017). The data from this trial suggests that patients with a histologically negative but RT-PCR positive SLN have similar outcomes to those with both histologically negative and RT-PCR negative SLNs.
Measurement of the Amount of Tumor Present and Its Distribution in Sentinel Lymph Nodes
In the past, patients who had a melanoma-positive SLN were usually offered immediate completion dissection of the affected node basin (completion lymphadenectomy). When dissected non-SLNs are evaluated microscopically (HE and immunohistochemistry), only one-third of completion lymphadenectomy specimens contain nodes that harbor melanoma cells (Wen et al. 2004). Thus, in two-thirds of patients who have a melanoma-positive SLN, regionally metastatic tumor is present only in the SLN and it has been demonstrated that such patients may not require potentially morbid completion lymphadenectomy, but rather are offered careful follow-up with ultrasound of the regional node basin, which should be done by appropriately qualified and experienced personnel. Tumor volume (Wagner et al. 1999), tumor burden (Ranieri et al. 2002), the micrometer-measured depth of invasion of melanoma from the lymph node capsule (Starz et al. 2001), SLN tumor burden (% area of the node occupied by melanoma) (Cochran et al. 2004b), and/or disposition of tumor within the different nodal compartments (Dewar et al. 2004) of a positive SLN may predict the likelihood that melanoma is present in non-SLNs and patient outcomes (Fig. 24). Since no survival benefit was demonstrated in clinical trials of patients who received a completion lymphadenectomy after a positive sentinel node, this procedure is no longer offered routinely in patients with a positive sentinel node (Leiter et al. 2016; Faries et al. 2017).
Melanoma Metastatic to Visceral Organs and Other Sites
Some melanomas have a predilection for gastrointestinal (in particular the submucosa of the small intestine), brain, and lung . Melanoma metastases to other sites including bone, breast, heart, spleen, and pancreas usually occur in patients with disseminated metastases. Recent evidence suggests that specific chemokines and their respective receptors may facilitate metastasis to specific organs. A recent study suggests that the site-specific metastasis by melanoma to the small intestine is related to expression of the chemokine receptor CCR9 in melanoma cells and response to CCL25, the ligand for CCR9, in the small bowel (Amersi et al. 2008).
Fine Needle Biopsy in Melanoma Patients
Fine needle biopsy (FNB) is a rapid, minimally invasive technique that is widely used for the investigation and diagnosis of palpable and non-palpable masses in melanoma patients. FNB has a number of advantages over core needle biopsy or open surgical biopsy. It is less invasive, cheaper, does not require expensive operating theater, and is associated with fewer complications and less morbidity. Because FNB specimens can be procured and prepared for assessment in a few minutes, it can provide a diagnosis quicker than more technically demanding formal biopsies. An on-demand FNB service is provided at some melanoma treatment centers, immediate results being communicated directly to treating clinicians, saving time, allowing expeditious planning of management, and reducing the stressful period of uncertainty for the patient (Doubrovsky et al. 2008). It is a particularly useful technique for evaluation of clinically suspicious visible or palpable masses in melanoma patients. The approach is also useful in the assessment of small tumor deposits detected by computerized tomographic or ultrasound scanning. A recent large study with a sensitivity of 92.1% and a specificity of 99.2% (Murali et al. 2007) demonstrated the high diagnostic accuracy of FNB used to evaluate metastatic melanoma. While FNB has no current role in the initial diagnosis of primary melanoma, it should be the first-line diagnostic modality for confirmation of clinically and/or radiologically suspected metastases in melanoma patients.
Melanoma exhibits a wide variety of morphologic appearances, and the smears prepared from FNB specimens of metastatic melanoma are similarly heterogeneous. Nevertheless, the smears are usually highly cellular with a discohesive population of large pleomorphic epithelioid cells in disorganized three-dimensional aggregates and associated singly dispersed cells (Fig. 25). Prominent nucleoli, intranuclear pseudoinclusions, eccentric nuclei, hyperchromasia, and a moderate amount of cytoplasm are typical. Focal intracytoplasmic melanin pigment is identified in up to 50% of cases. Diagnostic pitfalls include the overinterpretation of paucicellular smears and the misdiagnosis of tumors such as metastatic carcinoma or lymphoma as metastatic melanoma. However, when performed by appropriately trained and experienced cytopathologists, instances of misdiagnosis are rare (0.6% in one large series) (Murali et al. 2007). Collection of additional material for ancillary investigations, such as the preparation of a cell block for immunochemistry, also assists in reducing diagnostic errors, and this material can also potentially be used for genomic studies.
Fine needle biopsy: cytologic appearance of melanoma. Sixty-year-old male, metastatic melanoma in axilla. (a and b) A Romanovsky stained smear showing a dissociating population of large epithelioid melanoma cells with eccentrically placed nuclei. E. Prominent nucleoli and occasional intranuclear pseudoinclusions are present in this Papanicolau stained cytology preparation
Clear Cell Sarcoma (Melanoma of Soft Parts)
Melanoma arising in deep soft tissue was described by Enzinger (1965) in 1965 under the designation “clear cell sarcoma of tendons and aponeuroses.” The malignant cells of this sarcoma typically have clear cytoplasm, thus the designation as clear cell sarcoma. As these tumors demonstrate evidence of melanocytic differentiation on ultrastructural and immunohistochemical analysis, they have also been termed “melanoma of soft parts” as a synonym. Nevertheless, there are clear clinical, morphologic, cytogenetic, and molecular differences between cutaneous melanoma and clear cell sarcoma, indicating that they are distinct and separate neoplasms. Clear cell sarcoma usually occurs in young adults and has a slight female predominance but may occur in patients of any age. Tumors present typically as a tender nodule in the deep soft tissues of the extremities, although they can occur at any location (Chung and Enzinger 1983). More than one half of patients with clear cell sarcoma ultimately develop metastases, typically to the lymph nodes, lung, or bone (Fletcher 2000). At time of surgical excision, tumors are usually circumscribed and lack a connection to the overlying skin. They may include foci of hemorrhage, necrosis, or pigmentation due to either old hemorrhage or melanin production.
Seventy-five percent of cases manifest a unique t(12;22)(q13;q12) translocation that is reportedly absent in cutaneous melanoma. This translocation fuses the Ewing Sarcoma (EWS) gene of chromosome 22 to the activating transcription factor-1 gene (ATF-1) on chromosome12 and can be detected in paraffin-embedded tissue with the use of appropriate probes by fluorescent in situ hybridization (FISH) (Bridge et al. 1991; Antonescu et al. 2002; Reeves et al. 1992; Zucman et al. 1993; Speleman et al. 1997; Mrozek et al. 1993). Other dermal tumors with articular fusion genes have been recently described, and these have appeared to be less aggressive than clear cell sarcomas or usual melanomas (Cellier et al. 2018).
The tumor is usually composed of clusters of cells separated by fibrocollagenous stroma. The cells have eosinophilic or clear cytoplasm, and multinucleated giant cells with peripheral wreath-like nuclei are usually identifiable. The presence of multinucleated giant cells and relative paucity of mitotic figures are morphologic features favoring clear cell sarcoma over metastatic melanoma. Like melanoma, clear cell sarcoma expresses S-100 protein in the majority of cells, and most are also positive for Melan-A and HMB45 antigen (Chang and Folpe 2001). When there is diagnostic uncertainty as to whether a deep-seated tumor is a clear cell sarcoma or a melanocytic tumor such as a melanoma metastasis or (atypical) cellular blue nevus, careful clinicopathologic correlation and cytogenetic or FISH analysis may be helpful in establishing the correct diagnosis.
Molecular Pathology of Melanoma
The molecular pathology of melanoma is a broad field and encompasses the entire spectrum of melanocyte biology, host response, angiogenesis, and mutations, as well as a host of different techniques to study these various molecular events. In this brief review, we discuss diagnostic molecular markers, melanoma stem cells, and therapeutic targets.
The commonest molecular technique used in melanoma pathology is immunohistochemistry. Immunohistochemistry is widely available and an integral part of modern histopathologic practice. The most commonly used immunohistochemical markers related to melanoma diagnosis are antibodies for S-100 protein (a calcium binding protein), Mart-1/Melan-A (a melanocyte differentiation antigen expressed as a transmembrane protein), and HMB45 (a part of the gp100 pre-melanosome complex). The key enzyme, tyrosinase, and the transcriptional regulator of the tyrosinase gene, the so-called microphtalmia transcription factor (MITF), are also used, as well as SOX10. Arguably the major role of immunohistochemistry in melanocytic pathology is to assist in the differential diagnosis of melanocytic and non-melanocytic lesions, separation between benign and malignant melanocytic lesions, and especially detection of micrometastatic disease in sentinel nodes by highlighting the presence of small numbers of metastatic melanoma cells (see above). The routine use of immunohistochemistry improves the detection of occult melanoma metastases in SNs, resulting in an increase in the detection rate from 15% to 20%.
Ki-67 has proven very important in identifying cells actively participating in the cell cycle. Recent studies have shown the importance of detecting cell cycle activity in thin melanomas (<1 mm) (Gimotty et al. 2005). The tumor suppressor genes, p16 and p53, are other important markers. The molecular abnormalities of these genes affect cell growth, DNA repair, and cellular susceptibility to apoptosis.
Vascular markers (CD34, CD31, LYVE, D2-40) have been employed to help detect vascular invasion.
Therapeutic Targets
Molecular pathology studies have led to a greater understanding of the molecular pathogenesis of many cancers including melanoma. Melanoma develops as a result of accumulated abnormalities in genetic pathways that promote cell proliferation and prevent normal pathways of apoptosis in response to DNA damage. Knowledge of the mechanisms that underpin these pathways has provided new opportunities for the development of more specific targeted cancer therapies. In recent years, multiple new therapeutic strategies have radically transformed the care of melanoma patients, particularly those with advanced stage disease. Examples of new treatment approaches include molecularly targeted therapy using BRAF inhibitors (usually administered in combination with MEK inhibitors for patients with BRAF V600 mutant metastatic melanoma) and immunotherapy, using immune system checkpoint inhibitors against CTLA-4 and/or PD-1, and other novel checkpoints. The pathologist has a central role to play in the strategies that determine whether these agents are effective, both at the basic science and clinical levels. These roles include studies assessing various molecular pathways in melanoma pathogenesis, the assessment of the expression of various proteins, and other factors in excised human melanomas, selecting appropriate tissue for formal molecular testing, assessing response to treatments, and determining whether individual tumors display a phenotype that predicts likely response to treatment (such as expression of various molecular targets).
The Concept of the Cancer Stem Cell
The presence of stem cells has been documented in most somatic tissues. These cells exhibit the capacity for self-renewal and also can support the growth of progenitor cells that will differentiate into the tissue of origin. These normal adult stem cells have been found to be able to withstand cytotoxic agents. This function is related to the robust expression of ABC transporters. The capacity of stem cells to persist derives from the interplay of principally the Wnt , Notch, and hedgehog pathways that interact with the stem cell niche in the tissue of origin (Jones and Wagers 2008). This interaction determines whether the stem cell renews itself or differentiates. Cancer stem cells were first described in hematologic malignancies and now have been described in other tumors including breast and melanoma. The origin of cancer stem cells is not fully understood but is under intensive investigation. It may involve several “unorthodox” mechanisms such as cell-cell fusion and horizontal gene transfer (Bjerkvig et al. 2005). However, has been a population of human melanoma initiating cells has been identified that are defined by the presence of ABCB5, a transporter related to the documented resistance to cytotoxic agents found in normal and cancer stem cells (Schatton et al. 2008). The importance of this finding, namely the discovery of cells that are self-renewing in tumors, is that such cells are an appropriate and potentially rewarding target for therapy (and at the present time, likely a barrier to therapeutic success).
The Metastatic Niche Concept
A very important aspect of stem cell research relates to the discovery of the metastatic niche. The niche, as now clearly found in normal tissues, such as the bulge of the hair follicle, is defined as a specific anatomic location that presents all the elements necessary for the survival of normal stem cells in different tissues (Jones and Wagers 2008). Recent evidence indicates that there is also a niche for metastatic cancer cells. Through elegant experiments it has been demonstrated that malignant cells are capable of recruiting hematopoietic stem cells. These prepare favorable conditions in specific tissue sites through upregulation of adhesion molecules and other factors that modify the microenvironment. These modifications make this site favorable for the implantation of malignant cells, creating a metastatic niche. This process starts before the malignant cells arrive at the designated site and involves molecular cross-talk between neoplastic cells and the recruited hematopoietic stem cells (Kaplan et al. 2005; Li et al. 2007).
The unraveling of metastatic mechanisms, along with the identification of cancer stem cells, will have a tremendous impact on cancer therapy allowing for different approaches to tackle this complex and challenging group of illnesses. This new direction is clearly a challenge for molecular pathology as it offers great opportunities, not only for understanding malignancy and metastasis but also for therapeutic maneuvers to impede such events.
Closing Remarks
Histopathology remains the gold standard for the diagnosis of melanoma. The pathology report should not only document the key characteristics upon which the diagnosis was based but also pathological factors that are important for determining the patient’s prognosis and for guiding the next stages in their management. The pathological classification of melanoma, now supported by recent molecular findings, has provided important insights into the pathogenesis of this malignancy. There are many potential pitfalls for those diagnosing pigmented lesions, and, in many instances, accurate diagnosis requires interpretation of the pathological features in the context of pertinent clinical information such as the site of the lesion and age of the patient. Molecular pathology evaluation can provide useful adjunct information for diagnosing difficult to classify borderline primary melanocytic tumors including utilizing techniques such as fluorescent in situ hybridization, comparative genomic hybridization, and next-generation sequencing. For patients with metastatic melanoma, molecular pathology testing of their tumor to identify driver mutations that are susceptible to molecular targeting by drug therapy is now an important part of routine care. In the future, molecular testing to identify gene signatures, tumor mutation burden, or features of the tumor microenvironment is likely to be utilized for selecting optimal personalized therapies for melanoma patients.
References
Abernethy JL, Soyer HP, Kerl H, Jorizzo JL, White WL (1994) Epidermotropic metastatic malignant melanoma simulating melanoma in situ. A report of 10 examples from two patients. Am J Surg Pathol 18(11):1140–1149
Acker SM, Shidham VB, Kouzova M (2001) Discrimination between chronically sun damaged skin, solar lentigo and melanoma using immunomorphometry. Lab Investig 81:65A
Ackerman AB, David KM (1986) A unifying concept of malignant melanoma: biologic aspects. Hum Pathol 17(5):438–440
Allen AC, Spitz S (1954) Histogenesis and clinicopathologic correlation of nevi and malignant melanomas; current status. AMA Arch Derm Syphilol 69(2):150–171
Amersi FF, Terando AM, Goto Y, Scolyer RA, Thompson JF, Tran AN, Faries MB, Morton DL, Hoon DS (2008) Activation of CCR9/CCL25 in cutaneous melanoma mediates preferential metastasis to the small intestine. Clin Cancer Res 14(3):638–645
Amin MB, Edge SB (2017) AJCC Cancer staging manual. Springer, New York
Antonescu CR, Tschernyavsky SJ, Woodruff JM, Jungbluth AA, Brennan MF, Ladanyi M (2002) Molecular diagnosis of clear cell sarcoma: detection of EWS-ATF1 and MITF-M transcripts and histopathological and ultrastructural analysis of 12 cases. J Mol Diagn 4(1):44–52
Arora A, Lowe L, Su L, Rees R, Bradford C, Cimmino VC, Chang AE, Johnson TM, Sabel MS (2005) Wide excision without radiation for desmoplastic melanoma. Cancer 104(7):1462–1467
Aung PP, Nagarajan P, Prieto VG (2017) Regression in primary cutaneous melanoma: etiopathogenesis and clinical significance. Lab Investig 97(6):657
Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, Saw RP, Thompson JF (2012) Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol 30(21):2678–2683
Azzola MF, Shaw HM, Thompson JF, Soong SJ, Scolyer RA, Watson GF, Colman MH, Zhang Y (2003) Tumor mitotic rate is a more powerful prognostic indicator than ulceration in patients with primary cutaneous melanoma: an analysis of 3661 patients from a single center. Cancer 97(6):1488–1498
Baer SC, Schultz D, Synnestvedt M, Elder DE (1995) Desmoplasia and neurotropism. Prognostic variables in patients with stage I melanoma. Cancer 76(11):2242–2247
Bahrami S, Cheng L, Wang M, Jones TD, Malone JC, Billings SD (2007) Clonal relationships between epidermotropic metastatic melanomas and their primary lesions: a loss of heterozygosity and X-chromosome inactivation-based analysis. Mod Pathol 20(8):821–827
Bahwan J (1997) Mel-5: a novel antibody for differential diagnosis of epidermal pigmented lesions of the skin in paraffin-embedded sections. Melanoma Res 7:43
Balch CM (2002) American joint committee on Cancer. Cancer staging manual, 6th edn. Springer, New York
Balch CM, Murad TM, Soong SJ, Ingalls AL, Halpern NB, Maddox WA (1978) A multifactorial analysis of melanoma: prognostic histopathological features comparing Clark’s and Breslow’s staging methods. Ann Surg 188(6):732–742
Balch CM, Wilkerson JA, Murad TM, Soong SJ, Ingalls AL, Maddox WA (1980) The prognostic significance of ulceration of cutaneous melanoma. Cancer 45(12):3012–3017
Balch CM, Soong SJ, Milton GW, Shaw HM, McGovern VJ, Murad TM, McCarthy WH, Maddox WA (1982) A comparison of prognostic factors and surgical results in 1,786 patients with localized (stage I) melanoma treated in Alabama, USA, and New South Wales, Australia. Ann Surg 196(6):677–684
Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A Jr, Kirkwood JM, McMasters KM, Mihm MF, Morton DL, Reintgen DS, Ross MI, Sober A, Thompson JA, Thompson JF (2001a) Final version of the American joint committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 19(16):3635–3648
Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, Urist M, McMasters KM, Ross MI, Kirkwood JM, Atkins MB, Thompson JA, Coit DG, Byrd D, Desmond R, Zhang Y, Liu PY, Lyman GH, Morabito A (2001b) Prognostic factors analysis of 17,600 melanoma patients: validation of the American joint committee on Cancer melanoma staging system. J Clin Oncol 19(16):3622–3634
Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG (1999) LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol 144(4):789–801
Barnhill R (1995) Pathology of Melanocytiv nevi and malignant melanoma. Butterworth-Heinemann, Boston
Barnhill R (1998a) Tumors of melanocytes. In: Barnhill R, Crowson AN, Busam K, Granter SR (eds) Textbook of dermatopathology. McGraw-Hill, New York, p 537
Barnhill RL (1998b) Childhood melanoma. Semin Diagn Pathol 15(3):189–194
Barnhill RL (2001) The biology of melanoma micrometastases. Recent Results Cancer Res 158:3–13
Barnhill RL, Lugassy C (2004) Angiotropic malignant melanoma and extravascular migratory metastasis: description of 36 cases with emphasis on a new mechanism of tumour spread. Pathology 36(5):485–490
Barnhill RL, Flotte TJ, Fleischli M, Perez-Atayde A (1995) Cutaneous melanoma and atypical Spitz tumors in childhood. Cancer 76(10):1833–1845
Barnhill RL, Fine JA, Roush GC, Berwick M (1996) Predicting five-year outcome for patients with cutaneous melanoma in a population-based study. Cancer 78(3):427–432
Barnhill RL, Argenyi ZB, From L, Glass LF, Maize JC, Mihm MC Jr, Rabkin MS, Ronan SG, White WL, Piepkorn M (1999) Atypical Spitz nevi/tumors: lack of consensus for diagnosis, discrimination from melanoma, and prediction of outcome. Hum Pathol 30(5):513–520
Barnhill R, Dy K, Lugassy C (2002) Angiotropism in cutaneous melanoma: a prognostic factor strongly predicting risk for metastasis. J Invest Dermatol 119(3):705–706
Barnhill RL, Katzen J, Spatz A, Fine J, Berwick M (2005) The importance of mitotic rate as a prognostic factor for localized cutaneous melanoma. J Cutan Pathol 32(4):268–273
Barnhill RL, Argenyi Z, Berwick M, Duray PH, Erickson L, Guitart J, Horenstein MG, Lowe L, Messina J, Paine S, Piepkorn MW, Prieto V, Rabkin MS, Schmidt B, Selim A, Shea CR, Trotter MJ (2008) Atypical cellular blue nevi (cellular blue nevi with atypical features): lack of consensus for diagnosis and distinction from cellular blue nevi and malignant melanoma (“malignant blue nevus”). Am J Surg Pathol 32(1):36–44
Bastian BC (2014) The molecular pathology of melanoma: an integrated taxonomy of melanocytic neoplasia. Annu Rev Pathol 9:239–271
Bastian BC, Kashani-Sabet M, Hamm H, Godfrey T, Moore DH 2nd, Brocker EB, LeBoit PE, Pinkel D (2000) Gene amplifications characterize acral melanoma and permit the detection of occult tumor cells in the surrounding skin. Cancer Res 60(7):1968–1973
Bastian B, de la Fouchardiere A, Elder D, Gerami P, Lazar A, Massi D, Nagore E, Scolyer R, Yun S (2018) Genomic landscapes of melanoma. In: Elder D, Massi D, Scolyer R, Willemze R (eds) World Health Organisation classification of skin tumours, 4th edn., International Agency for Research on Cancer, Lyon, pp 72–75
Berg P, Lindelof B (1997) Differences in malignant melanoma between children and adolescents. A 35-year epidemiological study. Arch Dermatol 133(3):295–297
Berk DR, LaBuz E, Dadras SS, Johnson DL, Swetter SM (2010) Melanoma and melanocytic tumors of uncertain malignant potential in children, adolescents and young adults – the Stanford experience 1995–2008. Pediatr Dermatol 27(3):244–254. https://doi.org/10.1111/j.1525-1470.2009.01078.x
Berman DM (2006) Lack of agreement on predictors for metastasizing thin melanomas. Histopathology 48(2):217–219
Biddle DA, Evans HL, Kemp BL, El-Naggar AK, Harvell JD, White WL, Iskandar SS, Prieto VG (2003) Intraparenchymal nevus cell aggregates in lymph nodes: a possible diagnostic pitfall with malignant melanoma and carcinoma. Am J Surg Pathol 27(5):673–681
Bjerkvig R, Tysnes BB, Aboody KS, Najbauer J, Terzis AJ (2005) Opinion: the origin of the cancer stem cell: current controversies and new insights. Nat Rev Cancer 5(11):899–904
Blessing K, Grant JJ, Sanders DS, Kennedy MM, Husain A, Coburn P (2000) Small cell malignant melanoma: a variant of naevoid melanoma. Clinicopathological features and histological differential diagnosis. J Clin Pathol 53(8):591–595
Breiteneder-Geleff S, Matsui K, Soleiman A, Meraner P, Poczewski H, Kalt R, Schaffner G, Kerjaschki D (1997) Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am J Pathol 151(4):1141–1152
Breslow A (1970) Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg 172(5):902–908
Bridge JA, Sreekantaiah C, Neff JR, Sandberg AA (1991) Cytogenetic findings in clear cell sarcoma of tendons and aponeuroses. Malignant melanoma of soft parts. Cancer Genet Cytogenet 52(1):101–106
Busam KJ (1999) Metastatic melanoma to the skin simulating blue nevus. Am J Surg Pathol 23(3):276–282
Busam KJ (2005) Cutaneous desmoplastic melanoma. Adv Anat Pathol 12(2):92–102
Busam KJ, Barnhill RL (1995) Spitz nevus and variants. In: Barnhill RL, Piepkorn M, Busam KJ (eds) Pathology of melanocytic nevi and malignant melanoma. Butterworth-Heinemann, Boston, p 195
Busam KJ, Iversen K, Coplan KC, Jungbluth AA (2001) Analysis of microphthalmia transcription factor expression in normal tissues and tumors, and comparison of its expression with S-100 protein, gp100, and tyrosinase in desmoplastic malignant melanoma. Am J Surg Pathol 25(2):197–204
Busam KJ, Mujumdar U, Hummer AJ, Nobrega J, Hawkins WG, Coit DG, Brady MS (2004) Cutaneous desmoplastic melanoma: reappraisal of morphologic heterogeneity and prognostic factors. Am J Surg Pathol 28(11):1518–1525
Buzaid AC, Ross MI, Balch CM, Soong S, McCarthy WH, Tinoco L, Mansfield P, Lee JE, Bedikian A, Eton O, Plager C, Papadopoulos N, Legha SS, Benjamin RS (1997) Critical analysis of the current American joint committee on Cancer staging system for cutaneous melanoma and proposal of a new staging system. J Clin Oncol 15(3):1039–1051
Callery C, Cochran AJ, Roe DJ, Rees W, Nathanson SD, Benedetti JK, Elashoff RM, Morton DL (1982) Factors prognostic for survival in patients with malignant melanoma spread to the regional lymph nodes. Ann Surg 196(1):69–75
Carlson JA, Mihm MC (1997) Vulvar nevi, lichen sclerosus et atrophicus, and vitiligo. Arch Dermatol 133(10):1314–1316
Carlson JA, Dickersin GR, Sober AJ, Barnhill RL (1995) Desmoplastic neurotropic melanoma. A clinicopathologic analysis of 28 cases. Cancer 75(2):478–494
Carlson JA, Mu XC, Slominski A, Weismann K, Crowson AN, Malfetano J, Prieto VG, Mihm MC Jr (2002) Melanocytic proliferations associated with lichen sclerosus. Arch Dermatol 138(1):77–87
Carney JA, Ferreiro JA (1996) The epithelioid blue nevus. A multicentric familial tumor with important associations, including cardiac myxoma and psammomatous melanotic schwannoma. Am J Surg Pathol 20(3):259–272
Carson KF, Wen DR, Li PX, Lana AM, Bailly C, Morton DL, Cochran AJ (1996) Nodal nevi and cutaneous melanomas. Am J Surg Pathol 20(7):834–840
Cascinelli N, Zurrida S, Galimberti V, Bartoli C, Bufalino R, Del Prato I, Mascheroni L, Testori A, Clemente C (1994) Acral lentiginous melanoma. A histological type without prognostic significance. J Dermatol Surg Oncol 20(12):817–822
Cassarino DS, Cabral ES, Kartha RV, Swetter SM (2008) Primary dermal melanoma: distinct immunohistochemical findings and clinical outcome compared with nodular and metastatic melanoma. Arch Dermatol 144(1):49–56
Ceballos PI, Ruiz-Maldonado R, Mihm MC Jr (1995) Melanoma in children. N Engl J Med 332(10):656–662
Cellier L, Perron E, Pissaloux D, Karanian M, Haddad V, Alberti L, de la Fouchardiere A (2018) Cutaneous Melanocytoma with CRTC1-TRIM11 fusion: report of 5 cases resembling clear cell sarcoma. Am J Surg Pathol 42(3):382–391. https://doi.org/10.1097/PAS.0000000000000996
Cerroni L, Kerl H (2001) Tutorial on melanocytic lesions. Am J Dermatopathol 23(3):237–241
Chakera AH, Hesse B, Burak Z, Ballinger JR, Britten A, Caracò C, Cochran AJ, Cook MG, Drzewiecki KT, Essner R (2009) EANM-EORTC general recommendations for sentinel node diagnostics in melanoma. Eur J Nucl Med Mol Imaging 36(10):1713–1742
Chang KL, Folpe AL (2001) Diagnostic utility of microphthalmia transcription factor in malignant melanoma and other tumors. Adv Anat Pathol 8(5):273–275
Chang AE, Karnell LH, Menck HR (1998) The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 83(8):1664–1678
Cho KH, Han KH, Minn KW (1998) Superficial spreading melanoma arising in a longstanding melanocytic nevus on the sole. J Dermatol 25(5):337–340
Chorny JA, Barr RJ (2002) S100-positive spindle cells in scars: a diagnostic pitfall in the re-excision of desmoplastic melanoma. Am J Dermatopathol 24(4):309–312
Chun K, Vazquez M, Sanchez JL (1993) Malignant melanoma in children. Int J Dermatol 32(1):41–43
Chung EB, Enzinger FM (1983) Malignant melanoma of soft parts. A reassessment of clear cell sarcoma. Am J Surg Pathol 7(5):405–413
Clark WH Jr (1967) A classification of malignant melanoma in man correlated with histogenesis and biological behaviour. In: Montagna W, Hu F (eds) Advances in the biology of the skin. Pergamon, New York, p 621
Clark WH Jr, Mihm MC Jr (1969) Lentigo maligna and lentigo-maligna melanoma. Am J Pathol 55(1):39–67
Clark WH Jr, From L, Bernardino EA, Mihm MC (1969) The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res 29(3):705–727
Clark WH Jr, Reimer RR, Greene M, Ainsworth AM, Mastrangelo MJ (1978) Origin of familial malignant melanomas from heritable melanocytic lesions. ‘The B-K mole syndrome’. Arch Dermatol 114(5):732–738
Clark WH Jr, Elder DE, Guerry D, Epstein MN, Greene MH, Van Horn M (1984) A study of tumor progression: the precursor lesions of superficial spreading and nodular melanoma. Hum Pathol 15(12):1147–1165
Clark WH Jr, Elder DE, Van Horn M (1986) The biologic forms of malignant melanoma. Hum Pathol 17(5):443–450
Clark WH Jr, Elder DE, Guerry D, Braitman LE, Trock BJ, Schultz D, Synnestvedt M, Halpern AC (1989) Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst 81(24):1893–1904
Clark WH Jr, Hood AF, Tucker MA, Jampel RM (1998) Atypical melanocytic nevi of the genital type with a discussion of reciprocal parenchymal-stromal interactions in the biology of neoplasia. Hum Pathol 29(1 Suppl 1):S1–S24
Clemente CG, Mihm MC Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N (1996) Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 77(7):1303–1310
Cochran AJ (2000) The pathologist’s role in sentinel lymph node evaluation. Semin Nucl Med 30(1):11–17
Cochran AJ, Wen DR, Morton DL (1988) Occult tumor cells in the lymph nodes of patients with pathological stage I malignant melanoma. An immunohistological study. Am J Surg Pathol 12(8):612–618
Cochran AJ, Wen DR, Morton DL (1992) Management of the regional lymph nodes in patients with cutaneous malignant melanoma. World J Surg 16(2):214–221
Cochran AJ, Bailly C, Cook M, Crotty K, McCarthy S, Mihm M, Mooi W, Sagebiel R (1998) Recommendations for the reporting of tissues removed as part of the surgical treatment of cutaneous melanoma. The Association of Directors of anatomic and surgical pathology. Am J Clin Pathol 110(6):719–722
Cochran AJ, Bhuta S, Paul E, Ribas A (2000) The shifting patterns of metastatic melanoma. Clin Lab Med 20(4):759–783, vii
Cochran AJ, Bailly C, Paul E (2003) Optimal surgery for cutaneous melanoma requires accurate and complete pathologic information. Facial Plast Surg Clin North Am 11(1):23–32
Cochran AJ, Roberts A, Wen DR, Huang RR, Itakura E, Luo F, Binder SW (2004a) Optimized assessment of sentinel lymph nodes for metastatic melanoma: implications for regional surgery and overall treatment planning. Ann Surg Oncol 11(3 Suppl):156S–161S
Cochran AJ, Wen DR, Huang RR, Wang HJ, Elashoff R, Morton DL (2004b) Prediction of metastatic melanoma in nonsentinel nodes and clinical outcome based on the primary melanoma and the sentinel node. Mod Pathol 17(7):747–755
Cohen LM (1995) Lentigo maligna and lentigo maligna melanoma. J Am Acad Dermatol 33(6):923–936; quiz 937–940
Cohen LM (1996) The starburst giant cell is useful for distinguishing lentigo maligna from photodamaged skin. J Am Acad Dermatol 35(6):962–968
Cohen LM, McCall MW, Hodge SJ, Freedman JD, Callen JP, Zax RH (1994) Successful treatment of lentigo maligna and lentigo maligna melanoma with Mohs’ micrographic surgery aided by rush permanent sections. Cancer 73(12):2964–2970
Cohen JN, Joseph NM, North JP, Onodera C, Zembowicz A, LeBoit PE (2017) Genomic analysis of pigmented epithelioid melanocytomas reveals recurrent alterations in PRKAR1A, and PRKCA genes. Am J Surg Pathol 41(10):1333–1346
Conley J, Lattes R, Orr W (1971) Desmoplastic malignant melanoma (a rare variant of spindle cell melanoma). Cancer 28(4):914–936
Cook MG, Spatz A, Brocker EB, Ruiter DJ (2002) Identification of histological features associated with metastatic potential in thin (<1.0 mm) cutaneous melanoma with metastases. A study on behalf of the EORTC melanoma group. J Pathol 197(2):188–193
Cook MG, Green MA, Anderson B, Eggermont AM, Ruiter DJ, Spatz A, Kissin MW, Powell BW (2003) The development of optimal pathological assessment of sentinel lymph nodes for melanoma. J Pathol 200(3):314–319
Costa S, Byrne M, Pissaloux D, Haddad V, Paindavoine S, Thomas L, Aubin F, Lesimple T, Grange F, Bonniaud B (2016) Melanomas associated with blue nevi or mimicking cellular blue nevi. Am J Surg Pathol 40(3):368–377
Cox NH, Aitchison TC, Sirel JM, MacKie RM (1996) Comparison between lentigo maligna melanoma and other histogenetic types of malignant melanoma of the head and neck. Scottish melanoma group. Br J Cancer 73(7):940–944
Cox NH, Aitchison TC, MacKie RM (1998) Extrafacial lentigo maligna melanoma: analysis of 71 cases and comparison with lentigo maligna melanoma of the head and neck. Br J Dermatol 139(3):439–443
Crawford RI, Tron VA, Ma R, Rivers JK (1995) Sinonasal malignant melanoma – a clinicopathologic analysis of 18 cases. Melanoma Res 5(4):261–265
Crotty KA (1997) Spitz naevus: histological features and distinction from malignant melanoma. Australas J Dermatol 38(Suppl 1):S49–S53
Crotty KA, Menzies SW (2004) Dermoscopy and its role in diagnosing melanocytic lesions: a guide for pathologists. Pathology 36(5):470–477
Crotty KA, McCarthy SW, Palmer AA, Ng AB, Thompson JF, Gianoutsos MP, Shaw HM (1992) Malignant melanoma in childhood: a clinicopathologic study of 13 cases and comparison with Spitz nevi. World J Surg 16(2):179–185
Crotty KA, Scolyer RA, Li L, Palmer AA, Wang L, McCarthy SW (2002) Spitz naevus versus Spitzoid melanoma: when and how can they be distinguished? Pathology 34(1):6–12
Crowson AN, Magro CM, Mihm MC Jr (1999) Malignant melanoma with prominent pigment synthesis: “animal type” melanoma – a clinical and histological study of six cases with a consideration of other melanocytic neoplasms with prominent pigment synthesis. Hum Pathol 30(5):543–550
Crowson AN, Magro CH, Mihm MC (2001a) The melanocytic proliferations: a comprehensive textbook of pigmented lesions. Wiley, New York
Crowson AN, Magro CM, Mihm MC (2001b) Malignant melanoma. In: The melanocytic proliferation: a comprehensive textbook of pigmented lesions. Wiley, New York, p 281
Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Brocker EB, LeBoit PE, Pinkel D, Bastian BC (2005) Distinct sets of genetic alterations in melanoma. N Engl J Med 353(20):2135–2147
Dadras SS, Paul T, Bertoncini J, Brown LF, Muzikansky A, Jackson DG, Ellwanger U, Garbe C, Mihm MC, Detmar M (2003) Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol 162(6):1951–1960
Dadras SS, Lange-Asschenfeldt B, Velasco P, Nguyen L, Vora A, Muzikansky A, Jahnke K, Hauschild A, Hirakawa S, Mihm MC, Detmar M (2005) Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod Pathol 18(9):1232–1242
Dahlstrom JE, Scolyer RA, Thompson JF, Jain S (2004) Spitz naevus: diagnostic problems and their management implications. Pathology 36(5):452–457
Darier J (1925) Le melanome malin mesenchymateaux ou melano-sarcome. Bull Assoc Fr Cancer 14:221
Day CL Jr, Harrist TJ, Gorstein F, Sober AJ, Lew RA, Friedman RJ, Pasternack BS, Kopf AW, Fitzpatrick TB, Mihm MC Jr (1981) Malignant melanoma. Prognostic significance of “microscopic satellites” in the reticular dermis and subcutaneous fat. Ann Surg 194(1):108–112
Day CL Jr, Lew RA, Mihm MC Jr, Sober AJ, Harris MN, Kopf AW, Fitzpatrick TB, Harrist TJ, Golomb FM, Postel A, Hennessey P, Gumport SL, Raker JW, Malt RA, Cosimi AB, Wood WC, Roses DF, Gorstein F, Rigel D, Friedman RJ, Mintzis MM, Grier RW (1982a) A multivariate analysis of prognostic factors for melanoma patients with lesions greater than or equal to 3.65 mm in thickness. The importance of revealing alternative Cox models. Ann Surg 195(1):44–49
Day CL Jr, Mihm MC Jr, Lew RA, Harris MN, Kopf AW, Fitzpatrick TB, Harrist TJ, Golomb FM, Postel A, Hennessey P, Gumport SL, Raker JW, Malt RA, Cosimi AB, Wood WC, Roses DF, Gorstein F, Rigel D, Friedman RJ, Mintzis MM, Sober AJ (1982b) Prognostic factors for patients with clinical stage I melanoma of intermediate thickness (1.51–3.39 mm). A conceptual model for tumor growth and metastasis. Ann Surg 195(1):35–43
Day CL Jr, Mihm MC Jr, Sober AJ, Harris MN, Kopf AW, Fitzpatrick TB, Lew RA, Harrist TJ, Golomb FM, Postel A, Hennessey P, Gumport SL, Raker JW, Malt RA, Cosimi AB, Wood WC, Roses DF, Gorstein F, Rigel D, Friedman RJ, Mintzis MM (1982c) Prognostic factors for melanoma patients with lesions 0.76–1.69 mm in thickness. An appraisal of “thin” level IV lesions. Ann Surg 195(1):30–34
DeMatos P, Tyler D, Seigler HF (1998) Mucosal melanoma of the female genitalia: a clinicopathologic study of forty-three cases at Duke University Medical Center. Surgery 124(1):38–48
Dewar DJ, Newell B, Green MA, Topping AP, Powell BW, Cook MG (2004) The microanatomic location of metastatic melanoma in sentinel lymph nodes predicts nonsentinel lymph node involvement. J Clin Oncol 22(16):3345–3349
Dodds TJ, Lo S, Jackett L, Nieweg O, Thompson JF, Scolyer RA (2018) Prognostic significance of Periadnexal extension in cutaneous melanoma and its implications for pathologic reporting and staging. Am J Surg Pathol 42(3):359–366
Dodds TJ, Wilmott JS, Jackett LA, Lo SN, Long GV, Thompson JF, Scolyer RA (2019) Primary anorectal melanoma: clinical, immunohistology and DNA analysis of 43 cases. Pathology 51(1):39–45
Doubrovsky A, Scolyer RA, Murali R, McKenzie PR, Watson GF, Lee CS, McLeod DJ, McCarthy WH, Uren RF, Stretch JR, Saw RP, Thompson JF (2008) Diagnostic accuracy of fine needle biopsy for metastatic melanoma and its implications for patient management. Ann Surg Oncol 15(1):323–332
Dubreuilh MW (1894) Lentigo malin des vieillards. Ann Dermatol Syphiligr 5:1092
Dunton CJ, Berd D (1999) Vulvar melanoma, biologically different from other cutaneous melanomas. Lancet 354(9195):2013–2014
Dymock RB, Menz J (1986) Recurrent melanocytic naevi following partial removal (pseudomelanoma). Australas J Dermatol 27(2):67–69
Egan CA, Bradley RR, Logsdon VK, Summers BK, Hunter GR, Vanderhooft SL (1997) Vulvar melanoma in childhood. Arch Dermatol 133(3):345–348
Elder DE (1995) Skin cancer. Melanoma and other specific nonmelanoma skin cancers. Cancer 75(1 Suppl):245–256
Elder DE (2006) Pathology of melanoma. Clin Cancer Res 12(7 Pt 2):2308s–2311s
Elder DE, Xu X (2004) The approach to the patient with a difficult melanocytic lesion. Pathology 36(5):428–434
Elder DE, Jucovy PM, Tuthill RJ, Clark WH Jr (1980) The classification of malignant melanoma. Am J Dermatopathol 2(4):315–320
Elder DE, Guerry D, Epstein MN, Zehngebot L, Lusk E, Van Horn M, Clark WH Jr (1984) Invasive malignant melanomas lacking competence for metastasis. Am J Dermatopathol 6(Suppl):55–61
Ellis PS, Whitehead R (1981) Mitosis counting – a need for reappraisal. Hum Pathol 12(1):3–4
Enzinger FM (1965) Clear-cell sarcoma of tendons and aponeuroses. An analysis of 21 cases. Cancer 18:1163–1174
Eroglu Z, Zaretsky JM, Hu-Lieskovan S, Kim DW, Algazi A, Johnson DB, Liniker E, Ben K, Munhoz R, Rapisuwon S, Gherardini PF, Chmielowski B, Wang X, Shintaku IP, Wei C, Sosman JA, Joseph RW, Postow MA, Carlino MS, Hwu WJ, Scolyer RA, Messina J, Cochran AJ, Long GV, Ribas A (2018) High response rate to PD-1 blockade in desmoplastic melanomas. Nature 553(7688):347–350. https://doi.org/10.1038/nature25187
Faries MB, Thompson JF, Cochran AJ, Andtbacka RH, Mozzillo N, Zager JS, Jahkola T, Bowles TL, Testori A, Beitsch PD, Hoekstra HJ, Moncrieff M, Ingvar C, Wouters M, Sabel MS, Levine EA, Agnese D, Henderson M, Dummer R, Rossi CR, Neves RI, Trocha SD, Wright F, Byrd DR, Matter M, Hsueh E, MacKenzie-Ross A, Johnson DB, Terheyden P, Berger AC, Huston TL, Wayne JD, Smithers BM, Neuman HB, Schneebaum S, Gershenwald JE, Ariyan CE, Desai DC, Jacobs L, McMasters KM, Gesierich A, Hersey P, Bines SD, Kane JM, Barth RJ, McKinnon G, Farma JM, Schultz E, Vidal-Sicart S, Hoefer RA, Lewis JM, Scheri R, Kelley MC, Nieweg OE, Noyes RD, Hoon DSB, Wang HJ, Elashoff DA, Elashoff RM (2017) Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med 376(23):2211–2222. https://doi.org/10.1056/NEJMoa1613210
Farrahi F, Egbert BM, Swetter SM (2005) Histologic similarities between lentigo maligna and dysplastic nevus: importance of clinicopathologic distinction. J Cutan Pathol 32(6):405–412
Finan MC, Perry HO (1982) Lentigo maligna: a form of malignant melanoma in situ. Geriatrics 37(12):113–115
Fletcher CDM (2000) Diagnostic histopathology of tumors. Churchill Livingstone, New York
Folberg R, McLean IW, Zimmerman LE (1985) Malignant melanoma of the conjunctiva. Hum Pathol 16(2):136–143
Folberg R, Jakobiec FA, McLean IW, Zimmerman LE (1992) Is primary acquired melanosis of the conjunctiva equivalent to melanoma in situ? Mod Pathol 5(1):2–5; discussion 6–8
Francken AB, Shaw HM, Thompson JF, Soong SJ, Accortt NA, Azzola MF, Scolyer RA, Milton GW, McCarthy WH, Colman MH, McGovern VJ (2004) The prognostic importance of tumor mitotic rate confirmed in 1317 patients with primary cutaneous melanoma and long follow-up. Ann Surg Oncol 11(4):426–433
Friedmann I (1998) Osteoid and bone formation in a nasal mucosal melanoma and its metastasis. Histopathology 33(1):88
Gerami P, Busam K, Cochran A, Cook MG, Duncan LM, Elder DE, Fullen DR, Guitart J, LeBoit PE, Mihm MC Jr (2014) Histomorphologic assessment and interobserver diagnostic reproducibility of atypical spitzoid melanocytic neoplasms with long-term follow-up. Am J Surg Pathol 38(7):934–940
Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, Lazar AJ, Faries MB, Kirkwood JM, McArthur GA (2017) Melanoma staging: evidence-based changes in the American joint committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 67(6):472–492
Gilchrest BA, Blog FB, Szabo G (1979) Effects of aging and chronic sun exposure on melanocytes in human skin. J Invest Dermatol 73(2):141–143
Gilchrist KW, Gilbert E, Metter G, Powers D (1977) Importance of microscopic vascular invasion in primary cutaneous malignant melanoma. Surg Gynecol Obstet 145(4):559–561
Gimotty PA, Van Belle P, Elder DE, Murry T, Montone KT, Xu X, Hotz S, Raines S, Ming ME, Wahl P, Guerry D (2005) Biologic and prognostic significance of dermal Ki67 expression, mitoses, and tumorigenicity in thin invasive cutaneous melanoma. J Clin Oncol 23(31):8048–8056
Gimotty PA, Elder DE, Fraker DL, Botbyl J, Sellers K, Elenitsas R, Ming ME, Schuchter L, Spitz FR, Czerniecki BJ, Guerry D (2007) Identification of high-risk patients among those diagnosed with thin cutaneous melanomas. J Clin Oncol 25(9):1129–1134
Gleason BC, Hirsch MS, Nucci MR, Schmidt BA, Zembowicz A, Mihm MC Jr, McKee PH, Brenn T (2008) Atypical genital nevi. A clinicopathologic analysis of 56 cases. Am J Surg Pathol 32(1):51–57
Gonzalez-Bosquet J, Garcia Jimenez A, Gil Moreno A, Xercavins J (1997) Malignant vulvo-vaginal melanoma: a report of 7 cases. Eur J Gynaecol Oncol 18(1):63–67
Gorsky M, Epstein JB (1998) Melanoma arising from the mucosal surfaces of the head and neck. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 86(6):715–719
Grande Sarpa H, Reinke K, Shaikh L, Leong SP, Miller JR 3rd, Sagebiel RW, Kashani-Sabet M (2006) Prognostic significance of extent of ulceration in primary cutaneous melanoma. Am J Surg Pathol 30(11):1396–1400
Granter SR, McKee PH, Calonje E, Mihm MC Jr, Busam K (2001) Melanoma associated with blue nevus and melanoma mimicking cellular blue nevus: a clinicopathologic study of 10 cases on the spectrum of so-called ‘malignant blue nevus’. Am J Surg Pathol 25(3):316–323
Grin JM, Grant-Kels JM, Grin CM, Berke A, Kels BD (1998) Ocular melanomas and melanocytic lesions of the eye. J Am Acad Dermatol 38(5 Pt 1):716–730
Grogan J, Cooper CL, Dodds TJ, Guitera P, Menzies SW, Scolyer RA (2018) Punch ‘scoring’: a technique that facilitates melanoma diagnosis of clinically suspicious pigmented lesions. Histopathology 72(2):294–304
Gross EA, Andersen WK, Rogers GS (1999) Mohs micrographic excision of lentigo maligna using Mel-5 for margin control. Arch Dermatol 135(1):15–17
Guerriere-Kovach PM, Hunt EL, Patterson JW, Glembocki DJ, English JC 3rd, Wick MR (2004) Primary melanoma of the skin and cutaneous melanomatous metastases: comparative histologic features and immunophenotypes. Am J Clin Pathol 122(1):70–77
Guerry DI, Synnestvedt M, Elder D, Schultz D (1993) Lessons from tumor progression: the invasive RGP of melanoma is common, incapable of metastasis, and indolent. J Invest Dermatol 100:342S
Guillen FJ, Albert DM, Mihm MC Jr (1985) Pigmented melanocytic lesions of the conjunctiva – a new approach to their classification. Pathology 17(2):275–280
Guitart J, Lowe L, Piepkorn M, Prieto VG, Rabkin MS, Ronan SG, Shea CR, Tron VA, White W, Barnhill RL (2002) Histological characteristics of metastasizing thin melanomas: a case-control study of 43 cases. Arch Dermatol 138(5):603–608
Gyorki DE, Busam K, Panageas K, Brady MS, Coit DG (2003) Sentinel lymph node biopsy for patients with cutaneous desmoplastic melanoma. Ann Surg Oncol 10(4):403–407
Haigh PI, Lucci A, Turner RR, Bostick PJ, Krasne DL, Stern SL, Morton DL (2001) Carbon dye histologically confirms the identity of sentinel lymph nodes in cutaneous melanoma. Cancer 92(3):535–541
Harmelin ES, Holcombe RN, Goggin JP, Carbonell J, Wellens T (1998) Acral lentiginous melanoma. J Foot Ankle Surg 37(6):540–545
Harris GR, Shea CR, Horenstein MG, Reed JA, Burchette JL Jr, Prieto VG (1999) Desmoplastic (sclerotic) nevus: an underrecognized entity that resembles dermatofibroma and desmoplastic melanoma. Am J Surg Pathol 23(7):786
Harrist TJ, Rigel DS, Day CL Jr, Sober AJ, Lew RA, Rhodes AR, Harris MN, Kopf AW, Friedman RJ, Golomb FM et al (1984) “Microscopic satellites” are more highly associated with regional lymph node metastases than is primary melanoma thickness. Cancer 53(10):2183–2187
Haupt HM, Stern JB (1995) Pagetoid melanocytosis. Histologic features in benign and malignant lesions. Am J Surg Pathol 19(7):792–797
Hawkins WG, Busam KJ, Ben-Porat L, Panageas KS, Coit DG, Gyorki DE, Linehan DC, Brady MS (2005) Desmoplastic melanoma: a pathologically and clinically distinct form of cutaneous melanoma. Ann Surg Oncol 12(3):207–213
Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, Patch A-M, Kakavand H, Alexandrov LB, Burke H (2017) Whole-genome landscapes of major melanoma subtypes. Nature 545(7653):175
Herlyn M, Thurin J, Balaban G, Bennicelli JL, Herlyn D, Elder DE, Bondi E, Guerry D, Nowell P, Clark WH et al (1985) Characteristics of cultured human melanocytes isolated from different stages of tumor progression. Cancer Res 45(11 Pt 2):5670–5676
Holman CD, Armstrong BK (1984) Cutaneous malignant melanoma and indicators of total accumulated exposure to the sun: an analysis separating histogenetic types. J Natl Cancer Inst 73(1):75–82
Holman CD, Armstrong BK, Heenan PJ (1983) A theory of the etiology and pathogenesis of human cutaneous malignant melanoma. J Natl Cancer Inst 71(4):651–656
Hoorweg JJ, Loftus BM, Hilgers FJ (1997) Osteoid and bone formation in a nasal mucosal melanoma and its metastasis. Histopathology 31(5):465–468
Hung T, Piris A, Lobo A, Mihm MC Jr, Sober AJ, Tsao H, Tanabe KK, Duncan LM (2013) Sentinel lymph node metastasis is not predictive of poor outcome in patients with problematic spitzoid melanocytic tumors. Hum Pathol 44(1):87–94. https://doi.org/10.1016/j.humpath.2012.04.019
Hutchinson J (1890) Notes on the cancerous process. Arch Surg 2:83
Ishihara Y, Saida T, Miyazaki A, Koga H, Taniguchi A, Tsuchida T, Toyama M, Ohara K (2006) Early acral melanoma in situ: correlation between the parallel ridge pattern on dermoscopy and microscopic features. Am J Dermatopathol 28(1):21–27
Jackett LA, McCarthy SW, Scolyer RA (2016) SOX10 expression in cutaneous scars: a potential diagnostic pitfall in the evaluation of melanoma re-excision specimens. Pathology 48(6):626–628
Jain S, Allen PW (1989) Desmoplastic malignant melanoma and its variants. A study of 45 cases. Am J Surg Pathol 13(5):358–373
Jakobiec FA, Folberg R, Iwamoto T (1989) Clinicopathologic characteristics of premalignant and malignant melanocytic lesions of the conjunctiva. Ophthalmology 96(2):147–166
Jelfs PL, Giles G, Shugg D, Coates M, Durling G, Fitzgerald P, Ring I (1994) Cutaneous malignant melanoma in Australia, 1989. Med J Aust 161(3):182–187
Jimbow K, Takahashi H, Miura S, Ikeda S, Kukita A (1984) Biological behavior and natural course of acral malignant melanoma. Clinical and histologic features and prognosis of palmoplantar, subungual, and other acral malignant melanomas. Am J Dermatopathol 6(Suppl):43–53
Johnson OK Jr, Emrich LJ, Karakousis CP, Rao U, Greco WR (1985) Comparison of prognostic factors for survival and recurrence in malignant melanoma of the skin, clinical stage I. Cancer 55(5):1107–1117
Johnson R, Sviland L (1998) Is extensive histological examination of wide excision specimens necessary following a diagnosis of melanoma. Histopathology 32(4):379–380
Jones DL, Wagers AJ (2008) No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol 9(1):11–21
Kapelanski DP, Block GE, Kaufman M (1979) Characteristics of the primary lesion of malignant melanoma as a guide to prognosis and therapy. Ann Surg 189(2):225–235
Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438(7069):820–827
Karim RZ, van den Berg KS, Colman MH, McCarthy SW, Thompson JF, Scolyer RA (2008) The advantage of using a synoptic pathology report format for cutaneous melanoma. Histopathology 52(2):130–138
Kashani-Sabet M, Sagebiel RW, Ferreira CM, Nosrati M, Miller JR 3rd (2001) Vascular involvement in the prognosis of primary cutaneous melanoma. Arch Dermatol 137(9):1169–1173
Kashani-Sabet M, Sagebiel RW, Ferreira CM, Nosrati M, Miller JR 3rd (2002) Tumor vascularity in the prognostic assessment of primary cutaneous melanoma. J Clin Oncol 20(7):1826–1831
Kato T, Suetake T, Kumasaka N, Tabata N, Sugiyama Y, Tagami H (1995) Nodular melanoma in 62 Japanese patients: influence of initial surgical treatment on local recurrence and prognosis. J Dermatol 22(10):723–728
Kato T, Suetake T, Sugiyama Y, Tabata N, Tagami H (1996) Epidemiology and prognosis of subungual melanoma in 34 Japanese patients. Br J Dermatol 134(3):383–387
Kato T, Suetake T, Tabata N, Takahashi K, Tagami H (1999) Epidemiology and prognosis of plantar melanoma in 62 Japanese patients over a 28-year period. Int J Dermatol 38(7):515–519
Kelley SW, Cockerell CJ (2000) Sentinel lymph node biopsy as an adjunct to management of histologically difficult to diagnose melanocytic lesions: a proposal. J Am Acad Dermatol 42(3):527–530
Kelly JW, Chamberlain AJ, Staples MP, McAvoy B (2003) Nodular melanoma. No longer as simple as ABC. Aust Fam Physician 32(9):706–709
Kim JC, Murphy GF (2000) Dysplastic melanocytic nevi and prognostically indeterminate nevomelanomatoid proliferations. Clin Lab Med 20(4):691–712
Kim SH, Garcia C, Rodriguez J, Coit DG (1999) Prognosis of thick cutaneous melanoma. J Am Coll Surg 188(3):241–247
Kirkham N (1998) What is there to find in malignant melanoma re-excision specimens? Histopathology 32(6):566–567
Kornberg R, Ackerman AB (1975) Pseudomelanoma: recurrent melanocytic nevus following partial surgical removal. Arch Dermatol 111(12):1588–1590
Kossard S, Wilkinson B (1997) Small cell (naevoid) melanoma: a clinicopathologic study of 131 cases. Australas J Dermatol 38(Suppl 1):S54–S58
Krige JE, Hudson DA, Johnson CA, King HS, Chetty R (1995) Subungual melanoma. S Afr J Surg 33(1):10–14
Kuchelmeister C, Schaumburg-Lever G, Garbe C (2000) Acral cutaneous melanoma in caucasians: clinical features, histopathology and prognosis in 112 patients. Br J Dermatol 143(2):275–280
Larsen TE, Grude TH (1979) A retrospective histological study of 669 cases of primary cutaneous malignant melanoma in clinical stage I. The consequences of a reclassification of the original group of lentigo maligna melanomas. Acta Pathol Microbiol Scand A 87A(4):255–260
Larsson KB, Shaw HM, Thompson JF, Harman RC, McCarthy WH (1999) Primary mucosal and glans penis melanomas: the Sydney melanoma unit experience. Aust N Z J Surg 69(2):121–126
Lefevre M, Vergier B, Balme B, Thiebault R, Delaunay M, Thomas L, Beylot-Barry M, Machet L, De Muret A, Bioulac-Sage P, Bailly C (2003) Relevance of vertical growth pattern in thin level II cutaneous superficial spreading melanomas. Am J Surg Pathol 27(6):717–724
Leiter U, Stadler R, Mauch C, Hohenberger W, Brockmeyer N, Berking C, Sunderkotter C, Kaatz M, Schulte KW, Lehmann P, Vogt T, Ulrich J, Herbst R, Gehring W, Simon JC, Keim U, Martus P, Garbe C, German Dermatologic Cooperative Oncology G (2016) Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol 17(6):757–767. https://doi.org/10.1016/S1470-2045(16)00141-8
Leon P, Daly JM, Synnestvedt M, Schultz DJ, Elder DE, Clark WH Jr (1991) The prognostic implications of microscopic satellites in patients with clinical stage I melanoma. Arch Surg 126(12):1461–1468
Levene A (1979) Disseminated dermal melanocytosis terminating in melanoma. A human condition resembling equine melanotic disease. Br J Dermatol 101(2):197–205
Levene A (1980) On the histological diagnosis and prognosis of malignant melanoma. J Clin Pathol 33(2):101–124
Levit EK, Kagen MH, Scher RK, Grossman M, Altman E (2000) The ABC rule for clinical detection of subungual melanoma. J Am Acad Dermatol 42(2 Pt 1):269–274
Li LX, Crotty KA, McCarthy SW, Palmer AA, Kril JJ (2000) A zonal comparison of MIB1-Ki67 immunoreactivity in benign and malignant melanocytic lesions. Am J Dermatopathol 22(6):489–495
Li F, Tiede B, Massague J, Kang Y (2007) Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res 17(1):3–14
Little JH, Holt J, Davis N (1980) Changing epidemiology of malignant melanoma in Queensland. Med J Aust 1(2):66–69
Liu W, Dowling JP, Murray WK, McArthur GA, Thompson JF, Wolfe R, Kelly JW (2006) Rate of growth in melanomas: characteristics and associations of rapidly growing melanomas. Arch Dermatol 142(12):1551–1558
Lohmann CM, Coit DG, Brady MS, Berwick M, Busam KJ (2002) Sentinel lymph node biopsy in patients with diagnostically controversial spitzoid melanocytic tumors. Am J Surg Pathol 26(1):47–55
Lu C, Zhang J, Nagahawatte P, Easton J, Lee S, Liu Z, Ding L, Wyczalkowski MA, Valentine M, Navid F, Mulder H, Tatevossian RG, Dalton J, Davenport J, Yin Z, Edmonson M, Rusch M, Wu G, Li Y, Parker M, Hedlund E, Shurtleff S, Raimondi S, Bhavin V, Donald Y, Mardis ER, Wilson RK, Evans WE, Ellison DW, Pounds S, Dyer M, Downing JR, Pappo A, Bahrami A (2015) The genomic landscape of childhood and adolescent melanoma. J Invest Dermatol 135(3):816–823. https://doi.org/10.1038/jid.2014.425
Ludgate MW, Fullen DR, Lee J, Lowe L, Bradford C, Geiger J, Schwartz J, Johnson TM (2009) The atypical Spitz tumor of uncertain biologic potential: a series of 67 patients from a single institution. Cancer 115(3):631–641. https://doi.org/10.1002/cncr.24047
Magro CM, Crowson AN, Mihm MC (2006) Unusual variants of malignant melanoma. Mod Pathol 19(Suppl 2):S41–S70
Mandal RV, Murali R, Lundquist KF, Ragsdale BD, Heenan P, McCarthy SW, Mihm MC, Scolyer RA, Zembowicz A (2009) Pigmented epithelioid melanocytoma: favorable outcome after 5-year follow-up. Am J Surg Pathol 33(12):1778–1782
Martin HM, Birkin AJ, Theaker JM (1998) Malignant melanoma re-excision specimens – how many blocks? Histopathology 32(4):362–367
Massi G (2007) Melanocytic nevi simulant of melanoma with medicolegal relevance. Virchows Arch 451(3):623–647
Massi D, Franchi A, Borgognoni L, Reali UM, Santucci M (1999) Thin cutaneous malignant melanomas (< or =1.5 mm): identification of risk factors indicative of progression. Cancer 85(5):1067–1076
McCarthy SW, Scolyer RA (2004) Melanocytic lesions of the face: diagnostic pitfalls. Ann Acad Med Singap 33(4 Suppl):3–14
McCarthy SW, Crotty KA, Palmer AA, Ng AB, McCarthy WH, Shaw HM (1994) Cutaneous malignant melanoma in teenagers. Histopathology 24(5):453–461
McCarthy SW, Scolyer RA, Palmer AA (2004) Desmoplastic melanoma: a diagnostic trap for the unwary. Pathology 36(5):445–451
McCarthy SW, Crotty KA, Scolyer RA (2006) Desmoplastic melanoma and desmoplastic neurotropic melanoma. In: LeBoit PE, Burg G, Weedon D, Sarasian A (eds) WHO classification of tumors. Pathology and genetics of skin tumours. IARC Press, Lyon, pp 76–78
McGovern VJ (1970) The classification of melanoma and its relationship with prognosis. Pathology 2(2):85–98
McGovern VJ (1972) Melanoma: growth patterns, muiltiplicity and regression. In: McCarthy WH (ed) Melanoma and skin cancer. Proceedings of the international cancer conference. Blight, Government Printer, Sydney, pp 95–106
McGovern VJ, Mihm MC Jr, Bailly C, Booth JC, Clark WH Jr, Cochran AJ, Hardy EG, Hicks JD, Levene A, Lewis MG, Little JH, Milton GW (1973) The classification of malignant melanoma and its histologic reporting. Cancer 32(6):1446–1457
McGovern VJ, Cochran AJ, Van der Esch EP, Little JH, MacLennan R (1986) The classification of malignant melanoma, its histological reporting and registration: a revision of the 1972 Sydney classification. Pathology 18(1):12–21
McNutt NS (1998) “Triggered trap”: nevoid malignant melanoma. Semin Diagn Pathol 15(3):203–209
McNutt NS, Urmacher C, Hakimian J, Hoss DM, Lugo J (1995) Nevoid malignant melanoma: morphologic patterns and immunohistochemical reactivity. J Cutan Pathol 22(6):502–517
McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, Park JJ, Haydu LE, Spencer C, Wongchenko M, Lane S, Lee DY, Kaper M, McKean M, Beckermann KE, Rubinstein SM, Rooney I, Musib L, Budha N, Hsu J, Nowicki TS, Avila A, Haas T, Puligandla M, Lee S, Fang S, Wargo JA, Gershenwald JE, Lee JE, Hwu P, Chapman PB, Sosman JA, Schadendorf D, Grob JJ, Flaherty KT, Walker D, Yan Y, McKenna E, Legos JJ, Carlino MS, Ribas A, Kirkwood JM, Long GV, Johnson DB, Menzies AM, Davies MA (2018) Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol 19(3):310–322. https://doi.org/10.1016/S1470-2045(18)30078-0
Melnik MK, Urdaneta LF, Al-Jurf AS, Foucar E, Jochimsen PR, Soper RT (1986) Malignant melanoma in childhood and adolescence. Am Surg 52(3):142–147
Menzies SW, Liyanarachi S, Coates E, Smith A, Cooke-Yarborough C, Lo S, Armstrong BK, Scolyer RA, Guitera P (2019) Estimated risk of progression of lentigo maligna to lentigo maligna melanoma. Melanoma Res
Merkow LP, Burt RC, Hayeslip DW, Newton FJ, Slifkin M, Pardo M (1969) A cellular and malignant blue nevus: a light and electron microscopic study. Cancer 24(5):888–896
Mihm MC, Googe PB (1990) Problematic pigmented lesions. A case method approach. Lea & Febiger, Philadelphia
Mihm MC Jr, Clemente CG, Cascinelli N (1996) Tumor infiltrating lymphocytes in lymph node melanoma metastases: a histopathologic prognostic indicator and an expression of local immune response. Lab Investig 74(1):43–47
Montagna W, Kirchner S, Carlisle K (1989) Histology of sun-damaged human skin. J Am Acad Dermatol 21(5 Pt 1):907–918
Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, Foshag LJ, Cochran AJ (1992) Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg 127(4):392–399
Morton DL, Hoon DS, Cochran AJ, Turner RR, Essner R, Takeuchi H, Wanek LA, Glass E, Foshag LJ, Hsueh EC, Bilchik AJ, Elashoff D, Elashoff R (2003) Lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: therapeutic utility and implications of nodal microanatomy and molecular staging for improving the accuracy of detection of nodal micrometastases. Ann Surg 238(4):538–549; discussion 549–550
Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Elashoff R, Essner R, Nieweg OE, Roses DF, Hoekstra HJ, Karakousis CP, Reintgen DS, Coventry BJ, Glass EC, Wang HJ (2006) Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med 355(13):1307–1317
Morton DL, Thompson JF, Cochran AJ, Mozzillo N, Nieweg OE, Roses DF, Hoekstra HJ, Karakousis CP, Puleo CA, Coventry BJ (2014) Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med 370(7):599–609
Mraz-Gernhard S, Sagebiel RW, Kashani-Sabet M, Miller JR 3rd, Leong SP (1998) Prediction of sentinel lymph node micrometastasis by histological features in primary cutaneous malignant melanoma. Arch Dermatol 134(8):983–987
Mrozek K, Karakousis CP, Perez-Mesa C, Bloomfield CD (1993) Translocation t(12;22)(q13;q12.2-12.3) in a clear cell sarcoma of tendons and aponeuroses. Genes Chromosomes Cancer 6(4):249–252
Murali R, Doubrovsky A, Watson GF, McKenzie PR, Lee CS, McLeod DJ, Uren RF, Stretch JR, Saw RP, Thompson JF, Scolyer RA (2007) Diagnosis of metastatic melanoma by fine-needle biopsy: analysis of 2,204 cases. Am J Clin Pathol 127(3):385–397
Murali R, Sharma RN, Thompson JF, Stretch JR, Lee CS, McCarthy SW, Scolyer RA (2008) Sentinel lymph node biopsy in histologically ambiguous melanocytic tumors with spitzoid features (so-called atypical spitzoid tumors). Ann Surg Oncol 15(1):302–309. https://doi.org/10.1245/s10434-007-9577-3
Nagore E, Oliver V, Botella-Estrada R, Moreno-Picot S, Insa A, Fortea JM (2005) Prognostic factors in localized invasive cutaneous melanoma: high value of mitotic rate, vascular invasion and microscopic satellitosis. Melanoma Res 15(3):169–177
North JP, Kageshita T, Pinkel D, Leboit PE, Bastian BC (2008) Distribution and significance of occult Intraepidermal tumor cells surrounding primary melanoma. J Invest Dermatol 128:2024
Ohsie SJ, Binder SW, Cochran AJ (2008) Immunohistochemical characteristics of melanoma. J Cutan Pathol 35:433
Ostmeier H, Fuchs B, Otto F, Mawick R, Lippold A, Krieg V, Suter L (1999) Can immunohistochemical markers and mitotic rate improve prognostic precision in patients with primary melanoma? Cancer 85(11):2391–2399
Ozgur F, Akyurek M, Kayikcioglu A, Barista I, Gokoz A (1997) Metastatic malignant blue nevus: a case report. Ann Plast Surg 39(4):411–415
Pandey M, Mathew A, Abraham EK, Ahamed IM, Nair KM (1998) Primary malignant melanoma of the mucous membranes. Eur J Surg Oncol 24(4):303–307
Paradela S, Fonseca E, Pita-Fernández S, Kantrow SM, Diwan AH, Herzog C, Prieto VG (2010) Prognostic factors for melanoma in children and adolescents: a clinicopathologic, single-center study of 137 patients. Cancer 116(18):4334–4344
Pawlik TM, Ross MI, Prieto VG, Ballo MT, Johnson MM, Mansfield PF, Lee JE, Cormier JN, Gershenwald JE (2006) Assessment of the role of sentinel lymph node biopsy for primary cutaneous desmoplastic melanoma. Cancer 106(4):900–906
Petronic-Rosic V, Shea CR, Krausz T (2004) Pagetoid melanocytosis: when is it significant? Pathology 36(5):435–444
Piura B, Rabinovich A, Dgani R (1999) Malignant melanoma of the vulva: report of six cases and review of the literature. Eur J Gynaecol Oncol 20(3):182–186
Plaza JA, Torres-Cabala C, Evans H, Diwan HA, Suster S, Prieto VG (2010) Cutaneous metastases of malignant melanoma: a clinicopathologic study of 192 cases with emphasis on the morphologic spectrum. Am J Dermatopathol 32(2):129–136
Prehn RT (1996) The paradoxical association of regression with a poor prognosis in melanoma contrasted with a good prognosis in keratoacanthoma. Cancer Res 56(5):937–940
Prieto V (2017) Sentinel lymph nodes in cutaneous melanoma. Clin Lab Med 37:417–430
Prieto VGC, Sandra H (2002) Processing of sentinel lymph nodes for detection of metastatic melanoma. Ann Diagn Pathol 6(4):257–264
Prieto-Granada CN, Lezcano C, Scolyer RA, Mihm MC Jr, Piris A (2016a) Lethal melanoma in children: a clinicopathological study of 12 cases. Pathology 48(7):705–711. https://doi.org/10.1016/j.pathol.2016.08.008
Prieto-Granada CN, Wiesner T, Messina JL, Jungbluth AA, Chi P, Antonescu CR (2016b) Loss of H3K27me3 expression is a highly sensitive marker for sporadic and radiation-induced MPNST. Am J Surg Pathol 40(4):479
Quinn MJ, Crotty KA, Thompson JF, Coates AS, O’Brien CJ, McCarthy WH (1998) Desmoplastic and desmoplastic neurotropic melanoma: experience with 280 patients. Cancer 83(6):1128–1135
Raber G, Mempel V, Jackisch C, Hundeiker M, Heinecke A, Kurzl R, Glaubitz M, Rompel R, Schneider HP (1996) Malignant melanoma of the vulva. Report of 89 patients. Cancer 78(11):2353–2358
Ragnarsson-Olding B, Johansson H, Rutqvist LE, Ringborg U (1993) Malignant melanoma of the vulva and vagina. Trends in incidence, age distribution, and long-term survival among 245 consecutive cases in Sweden 1960–1984. Cancer 71(5):1893–1897
Ragnarsson-Olding BK, Kanter-Lewensohn LR, Lagerlof B, Nilsson BR, Ringborg UK (1999a) Malignant melanoma of the vulva in a nationwide, 25-year study of 219 Swedish females: clinical observations and histopathologic features. Cancer 86(7):1273–1284
Ragnarsson-Olding BK, Nilsson BR, Kanter-Lewensohn LR, Lagerlof B, Ringborg UK (1999b) Malignant melanoma of the vulva in a nationwide, 25-year study of 219 Swedish females: predictors of survival. Cancer 86(7):1285–1293
Ranieri JM, Wagner JD, Azuaje R, Davidson D, Wenck S, Fyffe J, Coleman JJ 3rd (2002) Prognostic importance of lymph node tumor burden in melanoma patients staged by sentinel node biopsy. Ann Surg Oncol 9(10):975–981
Rao BN, Hayes FA, Pratt CB, Fleming ID, Kumar AP, Lobe T, Dilawari R, Meyer W, Parham D, Custer MD (1990) Malignant melanoma in children: its management and prognosis. J Pediatr Surg 25(2):198–203
Rapini RP (1999) Spitz nevus or melanoma? Semin Cutan Med Surg 18(1):56–63
Raspagliesi F, Ditto A, Paladini D, Fontanelli R, Stefanon B, Dipalma S, De Palo G (2000) Prognostic indicators in melanoma of the vulva. Ann Surg Oncol 7(10):738–742
Reed D, Kudchadkar R, Zager JS, Sondak VK, Messina JL (2013) Controversies in the evaluation and management of atypical melanocytic proliferations in children, adolescents, and young adults. J Natl Compr Cancer Netw 11(6):679–686
Reeves BR, Fletcher CD, Gusterson BA (1992) Translocation t(12;22)(q13;q13) is a nonrandom rearrangement in clear cell sarcoma. Cancer Genet Cytogenet 64(2):101–103
Reintgen DS, Vollmer R, Seigler HF (1989) Juvenile malignant melanoma. Surg Gynecol Obstet 168(3):249–253
Retsas S, Henry K, Mohammed MQ, MacRae K (2002) Prognostic factors of cutaneous melanoma and a new staging system proposed by the American joint committee on Cancer (AJCC): validation in a cohort of 1284 patients. Eur J Cancer 38(4):511–516
Riber-Hansen R, Nyengaard JR, Hamilton-Dutoit SJ, Steiniche T (2009) The nodal location of metastases in melanoma sentinel lymph nodes. Am J Surg Pathol 33(10):1522–1528
Ridgeway CA, Hieken TJ, Ronan SG, Kim DK, Das Gupta TK (1995) Acral lentiginous melanoma. Arch Surg 130(1):88–92
Robson A, Allen P, Hollowood K (2001) S100 expression in cutaneous scars: a potential diagnostic pitfall in the diagnosis of desmoplastic melanoma. Histopathology 38(2):135–140
Ronan SG, Eng AM, Briele HA, Shioura NN, Das Gupta TK (1987) Thin malignant melanomas with regression and metastases. Arch Dermatol 123(10):1326–1330
Ruhoy SM, Prieto VG, Eliason SL, Grichnik JM, Burchette JL Jr, Shea CR (2000) Malignant melanoma with paradoxical maturation. Am J Surg Pathol 24(12):1600–1614
Ruiter DJ, Spatz A, van den Oord JJ, Cook MG (2002) Pathologic staging of melanoma. Semin Oncol 29(4):370–381
Saida T (2000) Malignant melanoma on the sole: how to detect the early lesions efficiently. Pigment Cell Res 13(Suppl 8):135–139
Saldanha G, Potter L, Daforno P, Pringle JH (2006) Cutaneous melanoma subtypes show different BRAF and NRAS mutation frequencies. Clin Cancer Res 12(15):4499–4505
Saldanha G, Flatman K, Teo KW, Bamford M (2017) A novel numerical scoring system for melanoma tumour infiltrating lymphocytes has better prognostic value than standard scoring. Am J Surg Pathol 41(7):906
Sander B, Karlsson P, Rosdahl I, Westermark P, Boeryd B (1999) Cutaneous malignant melanoma in Swedish children and teenagers 1973–1992: a clinico-pathological study of 130 cases. Int J Cancer 80(5):646–651
Savar A, Ross MI, Prieto VG, Ivan D, Kim S, Esmaeli B (2009) Sentinel lymph node biopsy for ocular adnexal melanoma: experience in 30 patients. Ophthalmology 116(11):2217–2223
Scalzo DA, Hida CA, Toth G, Sober AJ, Mihm MC Jr (1997) Childhood melanoma: a clinicopathological study of 22 cases. Melanoma Res 7(1):63–68
Schaefer I-M, Fletcher CD, Hornick JL (2016) Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol 29(1):4
Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, Fuhlbrigge RC, Kupper TS, Sayegh MH, Frank MH (2008) Identification of cells initiating human melanomas. Nature 451(7176):345–349
Schmoeckel C, Bockelbrink A, Bockelbrink H, Koutsis J, Braun-Falco O (1983) Low- and high-risk malignant melanoma – I. evaluation of clinical and histological prognosticators in 585 cases. Eur J Cancer Clin Oncol 19(2):227–235
Schmoeckel C, Castro CE, Braun-Falco O (1985) Nevoid malignant melanoma. Arch Dermatol Res 277(5):362–369
Scolyer RA, Crotty KA, Palmer AA, McCarthy SW (2002) Pagetoid melanocytosis in Spitz naevi (reply). Pathology 34:592
Scolyer RA, Shaw HM, Thompson JF, Li LX, Colman MH, Lo SK, McCarthy SW, Palmer AA, Nicoll KD, Dutta B, Slobedman E, Watson GF, Stretch JR (2003) Interobserver reproducibility of histopathologic prognostic variables in primary cutaneous melanomas. Am J Surg Pathol 27(12):1571–1576
Scolyer RA, Thompson JF, Stretch JR, Sharma R, McCarthy SW (2004a) Pathology of melanocytic lesions: new, controversial, and clinically important issues. J Surg Oncol 86(4):200–211
Scolyer RA, Thompson JF, Warnke K, McCarthy SW (2004b) Pigmented epithelioid melanocytoma. Am J Surg Pathol 28(8):1114–1115; author reply 1115–1116
Scolyer RA, Thompson JF, Stretch JR, McCarthy SW (2005) Collaboration between clinicians and pathologists: a necessity for the optimal management of melanoma patients. Cancer Forum 29:76–81
Scolyer RA, Thompson JF, McCarthy SW, Strutton GM, Elder DE (2006) Incomplete biopsy of melanocytic lesions can impair the accuracy of pathological diagnosis. Australas J Dermatol 47(1):71–73; author reply 74–75
Scolyer RA, Murali R, McCarthy SW, Thompson JF (2010) Histologically ambiguous (“borderline”) primary cutaneous melanocytic tumors: approaches to patient management including the roles of molecular testing and sentinel lymph node biopsy. Arch Pathol Lab Med 134(12):1770–1777. https://doi.org/10.1043/2009-0612-RAR.1
Scolyer RA, Long GV, Thompson JF (2011) Evolving concepts in melanoma classification and their relevance to multidisciplinary melanoma patient care. Mol Oncol 5(2):124–136. https://doi.org/10.1016/j.molonc.2011.03.002
Scolyer RA, Soyer HP, Kelly JW, James C, McLean CA, Coventry BJ, Ferguson PM, Rawson RV, Mar VJ, de Menezes SL, Fisburn O, Stretch JR, Lee S, Thompson JF (2019) Improving diagnostic accuracy for suspicious melanocytic skin lesions: new Australian melanoma clinical practice guidelines stress the importance of clinician/pathologist communication. Aust J Gen Pract 48:357
Seifried S, Haydu LE, Quinn MJ, Scolyer RA, Stretch JR, Thompson JF (2015) Melanoma of the vulva and vagina: principles of staging and their relevance to management based on a clinicopathologic analysis of 85 cases. Ann Surg Oncol 22(6):1959–1966
Sepehr A, Chao E, Trefrey B, Blackford A, Duncan LM, Flotte TJ, Sober A, Mihm MC Jr, Tsao H (2011) Long-term outcome of Spitz-type melanocytic tumors. Arch Dermatol 147(10):1173–1179. https://doi.org/10.1001/archdermatol.2011.170
Shaikh L, Sagebiel RW, Ferreira CM, Nosrati M, Miller JR 3rd, Kashani-Sabet M (2005) The role of microsatellites as a prognostic factor in primary malignant melanoma. Arch Dermatol 141(6):739–742
Shaw HM, Quinn MJ, Scolyer RA, Thompson JF (2006) Survival in patients with desmoplastic melanoma. J Clin Oncol 24(8):e12; author reply e13
Skelton HG, Maceira J, Smith KJ, McCarthy WF, Lupton GP, Graham JH (1997) HMB45 negative spindle cell malignant melanoma. Am J Dermatopathol 19(6):580–584
Skov-Jensen T, Hastrup J, Lambrethsen E (1966) Malignant melanoma in children. Cancer 19(5):620–626
Smithers BM, McLeod GR, Little JH (1990) Desmoplastic, neural transforming and neurotropic melanoma: a review of 45 cases. Aust N Z J Surg 60(12):967–972
Spanknebel K, Coit DG, Bieligk SC, Gonen M, Rosai J, Klimstra DS (2005) Characterization of micrometastatic disease in melanoma sentinel lymph nodes by enhanced pathology: recommendations for standardizing pathologic analysis. Am J Surg Pathol 29(3):305–317
Spatz A, Calonje E, Handfield-Jones S, Barnhill RL (1999) Spitz tumors in children: a grading system for risk stratification. Arch Dermatol 135(3):282–285
Speleman F, Delattre O, Peter M, Hauben E, Van Roy N, Van Marck E (1997) Malignant melanoma of the soft parts (clear-cell sarcoma): confirmation of EWS and ATF-1 gene fusion caused by a t(12;22) translocation. Mod Pathol 10(5):496–499
Spitz S (1948) Melanomas in childhood. Semin Diagn Pathol 24:591
Starz H, Balda BR, Kramer KU, Buchels H, Wang H (2001) A micromorphometry-based concept for routine classification of sentinel lymph node metastases and its clinical relevance for patients with melanoma. Cancer 91(11):2110–2121
Starz H, Haas CJ, Schulz GM, Balda BR (2003) Tyrosinase RT-PCR as a supplement to histology for detecting melanoma and nevus cells in paraffin sections of sentinel lymph nodes. Mod Pathol 16(9):920–929
Su LD, Fullen DR, Sondak VK, Johnson TM, Lowe L (2003) Sentinel lymph node biopsy for patients with problematic spitzoid melanocytic lesions: a report on 18 patients. Cancer 97(2):499–507
Sugiura M, Colby KA, Mihm MC Jr, Zembowicz A (2007) Low-risk and high-risk histologic features in conjunctival primary acquired melanosis with atypia: clinicopathologic analysis of 29 cases. Am J Surg Pathol 31(2):185–192
Suster S (1986) Pseudomelanoma. A pathologist’s perspective. Int J Dermatol 25(8):506–507
Suster S, Ronnen M, Bubis JJ (1987) Verrucous pseudonevoid melanoma. J Surg Oncol 36(2):134–137
Swetter SM, Ecker PM, Johnson DL, Harvell JD (2004) Primary dermal melanoma: a distinct subtype of melanoma. Arch Dermatol 140(1):99–103
Swetter SM, Boldrick JC, Jung SY, Egbert BM, Harvell JD (2005) Increasing incidence of lentigo maligna melanoma subtypes: northern California and national trends 1990–2000. J Invest Dermatol 125(4):685–691
Swetter SM, Tsao H, Bichakjian CK, Curiel-Lewandrowski C, Elder DE, Gershenwald JE, Guild V, Grant-Kels JM, Halpern AC, Johnson TM, Sober AJ, Thompson JA, Wisco OJ, Wyatt S, Hu S, Lamina T (2019) Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol 80(1):208–250. https://doi.org/10.1016/j.jaad.2018.08.055
Tannous ZS, Lerner LH, Duncan LM, Mihm MC Jr, Flotte TJ (2000) Progression to invasive melanoma from malignant melanoma in situ, lentigo maligna type. Hum Pathol 31(6):705–708
Taran JM, Heenan PJ (2001) Clinical and histologic features of level 2 cutaneous malignant melanoma associated with metastasis. Cancer 91(9):1822–1825
Tasseron EW, van der Esch EP, Hart AA, Brutel de la Riviere G, Aartsen EJ (1992) A clinicopathological study of 30 melanomas of the vulva. Gynecol Oncol 46(2):170–175
Tate PS, Ronan SG, Feucht KA, Eng AM, Das Gupta TK (1993) Melanoma in childhood and adolescence: clinical and pathological features of 48 cases. J Pediatr Surg 28(2):217–222
Temple WJ, Mulloy RH, Alexander F, Marx LH, Jenkins M, Jerry LM (1991) Childhood melanoma. J Pediatr Surg 26(2):135–137
Thompson JF, Scolyer RA (2004) Cooperation between surgical oncologists and pathologists: a key element of multidisciplinary care for patients with cancer. Pathology 36(5):496–503
Thompson JF, Scolyer RA, Kefford RF (2005) Cutaneous melanoma. Lancet 365(9460):687–701
Thompson JF, Soong SJ, Balch CM, Gershenwald JE, Ding S, Coit DG, Flaherty KT, Gimotty PA, Johnson T, Johnson MM, Leong SP, Ross MI, Byrd DR, Cascinelli N, Cochran AJ, Eggermont AM, McMasters KM, Mihm MC Jr, Morton DL, Sondak VK (2011) Prognostic significance of mitotic rate in localized primary cutaneous melanoma: an analysis of patients in the multi-institutional American joint committee on Cancer melanoma staging database. J Clin Oncol 29(16):2199–2205. https://doi.org/10.1200/JCO.2010.31.5812
Troxel DB (2003) Pitfalls in the diagnosis of malignant melanoma: findings of a risk management panel study. Am J Surg Pathol 27(9):1278–1283
Troxel DB (2006) Medicolegal aspects of error in pathology. Arch Pathol Lab Med 130(5):617–619
Trozak DJ, Rowland WD, Hu F (1975) Metastatic malignant melanoma in prepubertal children. Pediatrics 55(2):191–204
van Dijk MC, Aben KK, van Hees F, Klaasen A, Blokx WA, Kiemeney LA, Ruiter DJ (2008) Expert review remains important in the histopathological diagnosis of cutaneous melanocytic lesions. Histopathology 52(2):139–146
Van Es SL, Colman MH, Thompson JF, McCarthy SW, Scolyer RA (2008) Angiotropism is an independent predictor of local recurrence and in-transit metastasis in primary cutaneous melanoma. Am J Surg Pathol 32(9):1396–1403
Varey AH, Goumas C, Hong AM, Mann GJ, Fogarty GB, Stretch JR, Saw RP, Spillane AJ, Shannon KF, Lee KJ (2017) Neurotropic melanoma: an analysis of the clinicopathological features, management strategies and survival outcomes for 671 patients treated at a tertiary referral center. Mod Pathol 30(11):1538
Veenhuizen KC, De Wit PE, Mooi WJ, Scheffer E, Verbeek AL, Ruiter DJ (1997) Quality assessment by expert opinion in melanoma pathology: experience of the pathology panel of the Dutch melanoma working party. J Pathol 182(3):266–272
Verzi AE, Bubley JA, Haugh AM, Zhang B, Wagner A, Kruse L, West DP, Wayne J, Guitart J, Gerami P (2017) A single-institution assessment of superficial spreading melanoma (SSM) in the pediatric population: molecular and histopathologic features compared with adult SSM. J Am Acad Dermatol 77(5):886–892. https://doi.org/10.1016/j.jaad.2017.05.051
Viros A, Fridlyand J, Bauer J, Lasithiotakis K, Garbe C, Pinkel D, Bastian BC (2008) Improving melanoma classification by integrating genetic and morphologic features. PLoS Med 5(6):e120. https://doi.org/10.1371/journal.pmed.0050120
Vollmer RT, Seigler HF (2000) A model for pretest probability of lymph node metastasis from cutaneous melanoma. Am J Clin Pathol 114(6):875–879
Wagner JD, Davidson D, Coleman JJ 3rd, Hutchins G, Schauwecker D, Park HM, Havlik RJ (1999) Lymph node tumor volumes in patients undergoing sentinel lymph node biopsy for cutaneous melanoma. Ann Surg Oncol 6(4):398–404
Walsh N, Crotty K, Palmer A, McCarthy S (1998) Spitz nevus versus spitzoid malignant melanoma: an evaluation of the current distinguishing histopathologic criteria. Hum Pathol 29(10):1105–1112
Wechter ME, Gruber SB, Haefner HK, Lowe L, Schwartz JL, Reynolds KR, Johnston CM, Johnson TM (2004) Vulvar melanoma: a report of 20 cases and review of the literature. J Am Acad Dermatol 50(4):554–562
Weedon D, Little JH (1977) Spindle and epithelioid cell nevi in children and adults. A review of 211 cases of the Spitz nevus. Cancer 40(1):217–225
Weinstock MA, Sober AJ (1987) The risk of progression of lentigo maligna to lentigo maligna melanoma. Br J Dermatol 116(3):303–310
Wen DR, Huang RR, Binder SW, Morton DL, Cochran AJ (2004) Evaluation of melanoma in non-sentinel nodes: how much is enough? Mod Pathol 17:100S
White WL, Hitchcock MG (1998) Dying dogma: the pathological diagnosis of epidermotropic metastatic malignant melanoma. Semin Diagn Pathol 15(3):176–188
Whiteman DC, Milligan A, Welch J, Green AC, Hayward NK (1997) Germline CDKN2A mutations in childhood melanoma. J Natl Cancer Inst 89(19):1460
Wiesner T, Obenauf AC, Murali R, Fried I, Griewank KG, Ulz P, Windpassinger C, Wackernagel W, Loy S, Wolf I (2011) Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet 43(10):1018
Wilmott JS, Johansson PA, Newell F, Waddell N, Ferguson P, Quek C, Patch AM, Nones K, Shang P, Pritchard AL, Kazakoff S, Holmes O, Leonard C, Wood S, Xu Q, Saw RPM, Spillane AJ, Stretch JR, Shannon KF, Kefford RF, Menzies AM, Long GV, Thompson JF, Pearson JV, Mann GJ, Hayward NK, Scolyer RA (2019) Whole genome sequencing of melanomas in adolescent and young adults reveals distinct mutation landscapes and the potential role of germline variants in disease susceptibility. Int J Cancer 144(5):1049–1060. https://doi.org/10.1002/ijc.31791
Wong TY, Duncan LM, Mihm MC Jr (1993) Melanoma mimicking dermal and Spitz’s nevus (“nevoid” melanoma). Semin Surg Oncol 9(3):188–193
Wong TY, Suster S, Duncan LM, Mihm MC Jr (1995) Nevoid melanoma: a clinicopathological study of seven cases of malignant melanoma mimicking spindle and epithelioid cell nevus and verrucous dermal nevus. Hum Pathol 26(2):171–179
Yasuoka N, Ueda M, Ohgami Y, Hayashi K, Ichihashi M (1999) Amelanotic acral lentiginous malignant melanoma. Br J Dermatol 141(2):370–372
Yeh I, Lang UE, Durieux E, Tee MK, Jorapur A, Shain AH, Haddad V, Pissaloux D, Chen X, Cerroni L (2017) Combined activation of MAP kinase pathway and β-catenin signaling cause deep penetrating nevi. Nat Commun 8(1):644
Yun SJ, Gimotty PA, Hwang WT, Dawson P, Van Belle P, Elder DE, Elenitsas R, Schuchter L, Zhang PJ, Guerry D, Xu X (2011) High lymphatic vessel density and lymphatic invasion underlie the adverse prognostic effect of radial growth phase regression in melanoma. Am J Surg Pathol 35(2):235–242. https://doi.org/10.1097/PAS.0b013e3182036ccd
Zembowicz A, McCusker M, Chiarelli C, Dei Tos AP, Granter SR, Calonje E, McKee PH (2001) Morphological analysis of nevoid melanoma: a study of 20 cases with a review of the literature. Am J Dermatopathol 23(3):167–175
Zembowicz A, Carney JA, Mihm MC (2004) Pigmented epithelioid melanocytoma: a low-grade melanocytic tumor with metastatic potential indistinguishable from animal-type melanoma and epithelioid blue nevus. Am J Surg Pathol 28(1):31–40
Zembowicz A, Knoepp SM, Bei T, Stergiopoulos S, Eng C, Mihm MC, Stratakis CA (2007) Loss of expression of protein kinase a regulatory subunit 1alpha in pigmented epithelioid melanocytoma but not in melanoma or other melanocytic lesions. Am J Surg Pathol 31(11):1764–1775
Zuckerman R, Maier JP, Guiney WB Jr, Huntsman WT, Mooney EK (2001) Pediatric melanoma: confirming the diagnosis with sentinel node biopsy. Ann Plast Surg 46(4):394–399
Zucman J, Delattre O, Desmaze C, Epstein AL, Stenman G, Speleman F, Fletchers CD, Aurias A, Thomas G (1993) EWS and ATF-1 gene fusion induced by t(12;22) translocation in malignant melanoma of soft parts. Nat Genet 4(4):341–345
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this entry
Cite this entry
Scolyer, R.A., Prieto, V.G., Elder, D.E., Cochran, A.J., Mihm, M.C. (2020). Classification and Histopathology of Melanoma. In: Balch, C., et al. Cutaneous Melanoma. Springer, Cham. https://doi.org/10.1007/978-3-030-05070-2_49
Download citation
DOI: https://doi.org/10.1007/978-3-030-05070-2_49
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-05068-9
Online ISBN: 978-3-030-05070-2
eBook Packages: MedicineReference Module Medicine