Abstract
The discovery of dopamine as a brain neurotransmitter by Arvid Carlsson and colleagues about 50 years ago contributed to better understand some of the brain diseases. Some of the drugs that are most widely used in neurologic and psychiatry illnesses, such as levodopa and antipsychotic drugs, act on dopaminergic mechanism. The discovery that the motor impairments of Parkinson’s disease patients improved after restoring the physiological levels of striatal dopamine with levodopa attracted the attention of the neuroscience community for the role of this neurotransmitter in motor and brain functions. In the last decades, the knowledge has also been challenged by evidence that Parkinson’s disease also affects cognitive and affective functions. Shortly after the introduction of levodopa as a therapy, a complex set of secondary phenomena such as dyskinesia was observed following repeated administration of the dopamine precursor. Information of dopaminergic cells and circuits has been enriched by findings obtained with several and highly sensitive technology in cellular biology, with sophisticated behavioral analyses of transgenic animals and functional neuroimaging. The present chapter attempts to review results reported in different clinical studies and animal models to provide a comprehensive picture of the role of dopamine in Parkinson’s disease. Treatments have successfully been translated from preclinical to pharmacotherapeutic arsenal increasing clinical settings.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Basal ganglia
- Dopamine
- Dopamine receptors
- Dopaminergic drugs
- Levodopa
- Mesocortical system
- Mesolimbic system
- Nigrostriatal system
- Nitric oxide
- Parkinson’s disease
1 Introduction
At the beginning of the nineteenth century, when James Parkinson published the book entitled “Essay on the Shaking Palsy” (Parkinson 2002), life expectation was no longer than 45 years old. The “shaking palsy,” later named Parkinson’s disease (PD) by Charcot, affects mainly aged people. Nowadays, the prevalence of PD in industrialized countries is generally estimated at 0.3 % of the entire population and about 1 % in people over 60 years of age (Nussbaum and Ellis 2003). Age disease incidence ranges from 110 to 330 per 100,000 individuals over 50 years old, and its incidence increases with increasing age throughout the life span (Mayeux 2003). Now, in the beginning of the twenty-first century, life expectancy is near 80 years old, and the incidence rate of PD increases to 400–500 individuals per 100,000 annually (Mayeux 2003). Therefore, there are much more parkinsonian patients nowadays than two centuries ago, and with the increasing age of the general population, the prevalence of PD will rise constantly in the future.
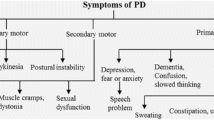
PD is characterized clinically by bradykinesia, rest tremor, muscle rigidity, and postural reflex impairment. The discovery that the motor impairments of PD patients improved after restoring the physiological levels of striatal dopamine with levodopa attracted the attention of the neuroscience community for the role of this neurotransmitter in motor functions (Olanow and Tatton 1999). Since dopamine is the main modulator of the basal ganglia, their components have long been seen as part of the motor system (Graybiel et al. 1994). In the last decades, this view has been challenged by evidence that PD also affects cognitive and affective functions (Dubois and Pillon 1997; Chaudhuri et al. 2006; Da Cunha et al. 2009). In addition, dysfunction of dopamine neurotransmission has been implicated in many other psychiatric and neurological diseases, such as attention deficit hyperactivity disorder (ADHD), drug addiction, Tourette syndrome, schizophrenia, and obsessive-compulsive syndrome. Furthermore, a growing body of evidence has been accumulated supporting the view that some kinds of cognitive processes, such as instrumental learning, decision-making, and other executive functions, depend critically on the dopamine signaling (see Da Cunha et al. 2009).
The present chapter attempts to review results reported in different clinical studies and animal models to provide a comprehensive picture of the role of dopamine signaling in PD.
2 Dopamine Neurotransmission System: Pathways, Receptors, and Signaling
A detailed review of the history and neurobiology of the dopamine signaling is beyond the scope of this chapter and can be found in very interesting reviews by Hornykiewicz (2002), Iversen and Iversen (2007), and Fahn (2008). The neurotransmitter dopamine plays a crucial role in motor, motivational, and reward-related functions of the central nervous system (CNS). Our understanding of neuropsychiatric illnesses and the modern psychopharmacology was greatly influenced by the discovery of dopamine as a neurotransmitter in the brain by Arvid Carlsson and colleagues about 50 years ago. Some of the drugs that are most widely used in the treatment of neurologic and psychiatry illnesses, such as levodopa, methylphenidate, and antipsychotic drugs, act on dopaminergic mechanisms. Therefore, dopamine neurotransmitter system has been one of the major and fertile fields of behavioral neuroscience and its research culminated in the award of the Nobel Prize in 2000 to three scientists, Carlsson, Greengard, and Kandel, for their important contributions to this field.
Dopamine was the last catecholamine discovered, and Sir Henry Dale, in 1952, suggested dopamine as the current name of 3,4-dihydroxyphenethylamine, also known as 3-hydroxytyramine. Its biosynthesis begins with dietary amino acid tyrosine actively transported into the brain. Tyrosine hydroxylase (TH), the rate-limiting enzyme in catecholamine synthesis, is regulated by multiple mechanisms (Molinoff and Axelrod 1971). Rapid activation of TH activity occurs via its phosphorylation by several protein kinases, including protein kinase A, Ca2+/calmodulin-dependent protein kinase II, and protein kinase C. Possibly, its phosphorylation induces conformational change that results in a lower affinity of TH for catecholamines that trigger end-product inhibition of this enzyme. The end result is an increase of TH catalytic activity (Nestler et al. 2009). Several drugs can up- or downregulate TH expression, and extracellular stimuli may induce longer-term changes in TH activity through transcriptional regulation of its gene.
Dopamine is synthesized in the cytosol and transported into synaptic vesicles via the vesicular monoamine transporter 2 (VMAT2). From the synaptic vesicles, dopamine is released in a quantal manner when a nerve impulse traveling down the axon reaches the nerve terminal. Once in the synaptic cleft, dopamine can act on both postsynaptic and presynaptic dopamine receptors. Dopamine could be metabolized to homovanillic acid (HVA); 3,4-dihydroxyphenylacetic acid (DOPAC) via monoamine oxidase (MAO), an enzyme considered to be mostly intraneuronal; and 3-methoxytyramine (3-MT) via catechol-O-methyltransferase (COMT), an enzyme considered mostly extraneuronal. Entacapone and tolcapone are reversible COMT inhibitors which have been approved for clinical use in patients with PD as adjuvant to levodopa, significantly improving the clinical benefits of this therapy (Bonifácio et al. 2007).
Dopamine signal ends by spontaneous amine diffusion, local enzymatic degradation, and amine reuptake in the presynaptic neurons by dopamine transporter (DAT) (Cragg and Rice 2004; Nestler et al. 2009; Eriksen et al. 2010). This dopamine transporter seemingly play a key role in the dopamine transmission if we consider the behavioral consequences of their inhibition with blocking agents known to be powerful psychostimulants or drugs of abuse, as amphetamine or cocaine (see Nestler et al. 2009; Fig. 1).
Dopamine synthesis, release, signaling, and reuptake and pharmacological approaches currently available for Parkinson’s disease. Dopamine synthesis originates from the amino acid tyrosine, which is converted to DOPA by the enzyme tyrosine hydroxylase (TH) (a rate-limiting enzyme). Subsequently, DOPA is decarboxylated, by the enzyme l-aromatic amino acid decarboxylase (DOPA decarboxylase), to form dopamine. l-dopa that is used in the Parkinson’s disease treatment bypasses the rate-limiting enzymatic step and is decarboxylated by DOPA decarboxylase to form dopamine. The most significant mechanism by which the synaptic actions of dopamine is terminated is the reuptake into the nerve terminal via specific dopamine transporter (DAT) expressed on the membrane of presynaptic terminal. Dopamine is also catabolized by the enzymes monoamine oxidase (MAO) and catechol-O-methyltransferase (COMT). Possibly, MAO exists in mitochondria within dopaminergic nerve terminals, on extracellular space, and also in glia proximate to dopamine synapses. After its synthesis, dopamine acts on pre- and postsynaptic receptors from D1 or D2 dopamine receptor families, which, respectively, activates Gs protein and increases cAMP production or Gi protein and decreases cAMP production. Generally drugs including l-DOPA, carbidopa, amantadine, and COMT and MAO-B inhibitors revert temporarily the dopamine dysfunction caused by dopaminergic neurodegeneration in the basal ganglia of Parkinson’s disease patients
Due to their diverse anatomy and disparate functions, different populations of dopamine neurons have been specifically implicated in a variety of neurological disorders. Biochemical characterization of dopamine in the brain revealed two families of dopamine receptors: D1-like receptors that also comprises D5 receptor and are able to stimulate adenylyl cyclase and increase cAMP production and D2-like receptors that comprises D3 and D4 receptors and are negatively coupled to adenylyl cyclase activity and inhibit cAMP production. In mammals, all of these receptors belong to the superfamily of GPCRs with seven transmembrane domains. D1 family is coupled to stimulatory Gs or the related Golf, and the D2 family is coupled to inhibitory Gi/Go (for review, see Herve and Girault 2005). D1 receptor activation stimulates the cAMP increase leading to the activation of protein kinase that have a broad array of cellular targets, including transcription factors, voltage-dependent ion channels, and glutamate receptors (Svenningsson et al. 2004).
Dopaminergic neurons and pathways were first described using classical histofluorescence techniques during the 1960s and 1970s (Carlsson et al. 1962). Knowledge of dopaminergic cells and circuits has been enriched by findings obtained with several and highly-sensitive techniques, as immunohistochemistry (mainly to dopamine essential synthetic enzyme TH), cellular biology, transgenic mice or conditional mutants, functional anatomy, electrophysiological studies, sophisticated behavioral analyses, and functional neuroimaging. Altogether, these studies have also led to new concepts of healthy and pathological functions of dopaminergic circuits at the molecular and more integrated levels (Björklund and Dunnett 2007).
In the ventral mesencephalic tegmentum, dopaminergic cell bodies are very well organized in the following groups: the substantia nigra pars compacta (SNc) (A9), the ventral tegmental area (VTA-A10), and the retrorubral area (A8) (Fig. 2). Dahlström and Fuxe (1964) defined the nomenclature of dopaminergic system in the rat midbrain: A8, A9 (substantia nigra), and A10 cell groups. The classification is nonetheless widely used.
Rat brain sagittal view (adult, Wistar) of the tyrosine hydroxylase immunocytochemistry showing the distribution of dopaminergic neurons. Abbreviations: aca anterior comm, anterior part, AcbC accumbens nucleus, core, AcbSh accumbens nucleus, shell, cc corpus callosum, CPu caudate putamen (striatum), LC locus coeruleus, LV lateral ventricle, MFB medial forebrain bundle, RRF retrorubral field, SN substantia nigra, VTA ventral tegmental area
Ascending dopaminergic pathways in the mammalian CNS can be simplified divided into three major systems. The two largest groups are located in the midbrain: the nigrostriatal system that originates in the substantia nigra (A9) and innervates predominantly the dorsal striatum and the mesocortical and mesolimbic systems that arise from the VTA and project to the cortical or several limbic areas (Bjorklund and Lindvall 1984; Krimer et al. 1997). The cell group of retrorubral area that forms a dorsal and caudal extension of the VTA contains cells that project to striatal, limbic, and cortical areas (Bentivoglio and Morelli 2005).
2.1 Nigrostriatal System
Since the first detailed studies of the SN by Cajal (1911), two main subdivisions were recognized. The SNc is characterized by densely packed neurons, and the substantia nigra pars reticulata (SNr) is marked by sparse dopaminergic cells, enmeshed in dopaminergic dendrites (Fig. 3). Most of the properties of dopamine neurotransmission in vivo are known from studies of the nigrostriatal dopamine pathway extending from the SNc to the dorsal striatum through the median forebrain bundle. Somato-dendritic release of dopamine in the SNc and axonal dopamine release in the dorsal striatum are both necessary for the expression of basal ganglia-mediated motor behaviors and various cognitive functions. In both rat and monkey, the striatal dopamine innervation is derived not only from SNc but also from cells located in the lateral VTA and retrorubral area. For this reason, the term “mesostriatal dopamine pathway” sometimes is used to include all components of the midbrain dopamine system projecting to the striatum (Björklund and Dunnett 2007).
Tyrosine hydroxylase immunocytochemistry of the regions of the human brain presenting positive reaction in the substantia nigra compacta (a, b; SNc), reticulata (a SNr,), and the striatum (c; caudate). Observe a high density of TH-positive neurons in the SNc, in contrast to sparse distribution in the SNr. TH- positive neurons are detected in the human and non human -primate striatum and and also in mice
The identification of cells of the SN as dopaminergic and of the dopaminergic innervation of the striatum through the nigrostriatal tract is inseparably intertwined with methodological achievements in experimental and chemical neuroanatomy in the 1960s and 1970s. These discoveries were intrinsically correlated to the histopathology of the midbrain dopaminergic system in PD in the 1960s (Bentivoglio and Morelli 2005).
2.2 Mesocortical and Mesolimbic Systems
Both of these systems connect the ventral tegmental dopamine cells (A8, A10) to prefrontal and cingulate cortex or to nucleus accumbens, amygdaloid complex, hippocampus, and piriform cortex. Ventral tegmental dopaminergic neurons play a critical role in motivation, reward-related behavior, attention, and multiple forms of memory (Nestler et al. 2009).
A third population of midbrain dopamine neurons resides in the retrorubral field and projects primarily to the dorsal striatum and the pontomedullary reticular formation; it is thought to play a role in orofacial movements. There are also connections between the retrorubral field and SN/VTA dopamine neurons. There are at least four groups of dopamine neurons in the hypothalamus that are intimately involved in neuroendocrine, hormonal, and arousal processes. Finally, there are populations of dopaminergic amacrine cells in the retina that contribute to neural adaptation to light and also some dopamine cells in the olfactory bulb (Nestler et al. 2009).
The striatum receives input from a number of regions, including the motor, sensory, and frontal cortex, integrates this information under the modulatory influence of dopamine, and uses this information to guide motor behavior. A cardinal feature of the striatum – the main integrator of cortical and thalamic information reaching the basal ganglia – is its dense dopamine innervation, which arises from the midbrain nigrostriatal pathway. Nigrostriatal dopaminergic neurons form highly branched networks within their striatal targets. The full extent of this arborization was elegantly demonstrated by Matsuda and colleagues (2009). A single dopaminergic neuron innervated up to 5.7 % of the neostriatum, while adjacent input dopaminergic neurons showed high degrees of overlapping with targets. It was calculated that a single postsynaptic medium spiny neuron (MSN) could be influenced by up to 194 dopaminergic inputs. As a result of this redundancy, clinical signs of PD only develop after up to 70 % of SNc neurons are lost. Each striatal MSN receives input from several thousand different cortical neurons on its spines. The input from each synapse is weak and many inputs are needed to discharge the neuron (Rothwell 2011).
Anatomically, striatal cells fall into two main classes: (a) spiny projection neurons and (b) aspiny interneurons. Spiny projection neurons, also known as medium spiny neurons (MSNs), represent the vast majority of striatal neurons. MSNs receive glutamatergic inputs from cortex and thalamus that terminate predominantly on spines (Kemp and Powell 1971). In addition, they are a main target of midbrain dopaminergic neuron axons that form synapses on MSN dendrites and spine necks (Smith et al. 1994).
One of the major targets of this innervation is the principal neuron of the striatum: GABAergic spiny projection neurons. Spiny projection neurons constitute as much as 90 % of the striatal neuron population. They can be divided into two approximately equally sized populations based on axonal projections. One population – direct pathway spiny projection neurons – projects axons to the nuclei at the interface between the basal ganglia and the rest of the brain, whereas the other population, indirect pathway spiny projection neurons, projects only indirectly to the interface nuclei. The dopamine D1 receptor is expressed selectively by direct pathway spiny projection neurons. Also, direct pathway spiny projection neurons express high levels of substance P (SP) and dynorphin (DYN). The dopamine D2 receptor is expressed by indirect pathway spiny projection neurons, which also express enkephalin (ENK). These two G protein-coupled receptors (GPCRs) have distinctive intracellular signaling cascades and targets, leading to fundamentally different cellular responses to extracellular dopamine (see below).
There are four well-characterized classes of striatal interneuron: cholinergic interneuron, parvalbumin-expressing GABAergic interneuron, calretinin-expressing GABAergic interneuron, and neuropeptide Y (NPY)/nitric oxide-expressing GABAergic interneuron (Tepper et al. 2008). Together, these interneurons constitute 5–10 % of all striatal neurons. One of them, the cholinergic interneuron, co expresses dopamine D2 and D5 receptors (Bergson et al. 1995) and modulates both spiny projection neurons populations through muscarinic receptors (Yan and Surmeier 1996). Two other prominent interneurons, the somatostatin (SOM)/NPY-expressing GABAergic interneuron and the parvalbumin (PV) GABAergic interneuron, express dopamine D5 receptors (Centonze et al. 2003). The PV interneuron is strongly innervated by globus pallidus neurons that express dopamine D2 receptors (Bevan et al. 1998), creating a microcircuit that is influenced by both dopamine D1 and D2 receptors.
According to the classic model of basal ganglia function (see Fig. 4; DeLong 1990), movements occur during pauses in the tonic inhibitory activity of the basal ganglia output interface, generated by activity in the direct pathway. This model had its origins in neurophysiologic studies showing that corticostriatal activation of the direct striatonigral pathway results in pauses of the tonic activity of GABAergic neurons in the SNr (Chevalier et al. 1985). The opposing role of the indirect pathway in suppressing movements was originally proposed on the basis of studies in animal models of PD. Mink (2003) reviewed the classic model, proposing that the activity of direct and indirect pathways is coordinated to select particular motor programs and to inhibit competing motor programs. This model predicts that during ongoing behavior, there will be increased activity in neuronal ensembles that are part of direct and indirect pathway circuits, rather than one or the other. The execution of a movement sequence would then generate a complex pattern of activity in specific neuronal ensembles. Recent work using optogenetic or genetic approaches supports the general tenets of the model (Hikida et al. 2010; Kravitz et al. 2010). Although this model has proven to be of considerable clinical value, it fails to account for the great diversity and complexity of the decision-making process in action selection (Cisek and Kalaska 2010).
Overview of schematic representation of the main basal ganglia-thalamocortical circuits (left). Normal status: the two output routes (“indirect” and “direct”) are in balance at the level of the output structures (the GPi and the SNr) (right). Neurodegenerative changes in the central nervous system in Parkinson’s disease (PD): depletion of dopamine in the striatum leads to imbalance in the two output routes and suppression of thalamocortical activity. The thickness of the arrows indicates the level of activity in the pathways. For some time, the symptoms of PD have been described as a loss of balance between the direct and indirect striatal output pathways. The indirect pathway is attenuated primarily by D2 receptor stimulation, whereas the direct pathway is activated via D1 receptor stimulation. With normal tonic/phasic balance, cortical activation of the indirect pathway (attenuated by tonic D2 stimulation) is in balance with other presumably motor cortical inputs influencing the direct pathway (augmented by phasic D1 stimulation). However, in PD, this balance is disrupted, with the loss of DA resulting in hyperactivity (with akinesia) in the indirect pathway and depression (with loss of voluntary movement) within the direct pathway. The model predicts that neuronal firing in the STN and GPi is increased in the parkinsonian state, leading to excessive inhibition of brain stem and thalamocortical neurons with the development of parkinsonian motor features. In contrast, the model proposes that dyskinesia is related to the decreased firing in the STN and GPi, with reduced inhibition of thalamic and cortical motor regions. Abbreviations: D dopamine, GPe external segment of globus pallidus, GPi internal segment of globus pallidus, MD mediodorsal nucleus, SNr reticular part of the substantia nigra, SNc pars compacta of the substantia nigra, STN subthalamic nucleus, VA/VL ventral anterior/ventral lateral thalamic nuclei (Modified from Hauser 2009)
Dopamine acted by stimulating the MSNs of the direct and inhibiting those of the indirect pathway (Albin et al. 1989; Gerfen et al. 1990; Gerfen and Surmeier 2011). These opposing effects were suggested to depend on the expression of dopamine D1 receptors in the MSNs of the direct pathway and of dopamine D2 receptors in the MSNs of the indirect pathway. These receptors have distinctive intracellular signaling cascades and targets, leading to fundamentally different cellular responses to extracellular dopamine (Gerfen and Surmeier 2011). Also, direct pathway expresses high levels of substance P and dynorphin, whereas indirect pathway expresses enkephalin. Direct and indirect pathways are representing on Fig. 4.
Dopamine stimulation of D1 receptors acts via cAMP and protein kinase A to phosphorylate dopamine- and cAMP-regulated phosphoprotein, Mr 32 kDa (DARPP-32), an important mediator of cAMP signaling and striatal excitability. The DARPP-32 signaling pathway has a central role in mediating signal transduction within MSNs in the striatum, and the function of this phosphoprotein depends on its relative state of phosphorylation at two main regulatory sites, threonine 34 (T34) and threonine T75 (T75). When DARPP-32 is phosphorylated at T34 by protein kinase A, it becomes a potent inhibitor of protein phosphatase 1, which in turn regulates the phosphorylation state of several classes of effector proteins, including transcription factors, ionotropic receptors, and ion channels (Greengard et al. 1999; Grace 2002). It has been shown that DARPP-32 is also present in other non-D1-containing neurons as well, including the enkephalin-containing striatal neurons (Langley et al. 1997). In this case, in contrast, stimulation of dopamine D2 receptors causes a dephosphorylation of DARPP-32 via calcineurin activation by calcium influx (Grace 2002).
Dopamine release appears to occur via two functionally distinct components. One is known as phasic component of dopamine release and is believed to underlie most of the behavioral indices of this transmitter. Phasic dopamine release occurs in a high-amplitude, brief pulsatile manner by means of action potentials and then is rapidly removed from the synaptic cleft via reuptake (Grace 1991, 2002). The other component is known as tonic dopamine release that occurs in very low concentration but is sufficient to activate extrasynaptic receptors, including dopamine terminal autoreceptors. D2 and D3 receptors act as inhibitory presynaptic autoreceptors and as postsynaptic receptors. In addition, D2 receptors have two splice variants, D2short and D2long (Nestler et al. 2009). Autoreceptors can exist on most portions of dopamine cells including the soma, dendrites, and nerve terminals. Stimulation of dopamine autoreceptors slows or inhibits dopamine synthesis or release. Thus, autoreceptors are supposed to exert a tonic downregulation of dopamine neuron activity, maintaining their firing within a stable range of activity (Harden and Grace 1995).
3 Dopamine-Related Disease: Parkinson’s Disease
Several important diseases of the CNS are associated with dysfunctions of the dopamine system, ranging from PD to schizophrenia and drug addiction. In this review, we shall focus only the Parkinson’s disease and some consequences for human beings. The discovery of mechanism of action of several drugs that act on dopamine synapses really improved the knowledge about the dopaminergic system and its related diseases.
PD is characterized by complex motor symptomatology, including tremor at rest, bradykinesia, rigidity, postural instability, stooped posture, and freezing that occur by hypofunctional dopamine states when nigrostriatal pathway is degenerated. Dopamine became clinically relevant when dopamine depletion in the caudate nucleus of patients with PD was discovered, whereas intravenous (i.v.) administration of l-dihydroxyphenylalanine (l-DOPA), the amino acid precursor of dopamine, dramatically and rapidly alleviated parkinsonian symptoms (Hornykiewicz 2010). Studies with animal models of PD provided the first clear indication of the dichotomy in the regulation of the direct and indirect pathways, suggesting that their excitability shifts in opposite directions following the loss of dopamine, creating an imbalance in the regulation of the motor thalamus favoring suppression of movement (Albin et al. 1989). Specifically, direct pathway was suggested to spike less in the PD state, whereas indirect pathway was thought to spike more (Gerfen and Surmeier 2011).
Besides motor symptoms, dopaminergic-related non-motor symptoms, as depression, apathy, and sleep disorders, are part of the clinical spectrum of PD. There is increasing evidence that such symptoms are treatable, at least in part, with various dopaminergic agents (Chaudhuri and Schapira 2009).
One current drawback of dopaminergic therapy is that it cannot be targeted to a specific subpopulation of dopamine neurons. A consequence of this is significant dopamine-related side effects, such as hallucinations or compulsive gambling during treatment for PD or extrapyramidal and sexual side effects caused by antipsychotics. In addition, from the standpoint of pathogenesis, it is clear that dopaminergic neurotransmission is not the sole dysfunction in this neurological disease.
4 Dopamine Signaling in Parkinson’s Disease
4.1 The Neuropathology of Parkinson’s Disease: A Brief Historical Overview
The association of PD with the mesencephalic dopaminergic zone was hypothesized by Edouard Brissaud in 1893, based on an autopsy case of unilateral Parkinsonism associated with a tumor confined in the SN (see Parent and Parent 2010). The hypothesis was validated by Konstantin Tretiakoff in 1919, in his doctoral thesis, reporting the marked loss of neurons in the SNc and the presence of intracytoplasmic inclusions which he called “corps de Lewy” (reference to Friedrich Lewy’s description of these pathological findings) in the surviving neurons of PD patients (Lees et al. 2008), after confirmed by Hassler in 1938, which found a constant cell loss in the SNc of 10 PD patients (Hassler 1938). Just on the beginning of 1960, Hornykiewicz described the presence of dopamine in SN and that it is reduced in the midbrain and in the striatum of parkinsonian patients, suggesting the possible existence of a link between SN and the corpus striatum (Hornykiewicz 1963).
Using new fluorescence techniques, Dahlström and Fuxe (1964) described firstly, in rat, the occurrence of catecholaminergic cell in the midbrain and lower brain stem, and Andén et al. (1964) noticed that lesions of SNc caused a substantial loss of the catecholaminergic fluorescence in the striatum, helping the acceptance of the existence of a nigrostriatal fiber system originating from dopaminergic midbrain neurons. The utilization of immunohistochemistry labeling of enzymes participating in the metabolism was very useful to study the catecholaminergic neurons of midbrain, and Hökfelt et al. (1973) were the first to identify dopaminergic neurons in midbrain using antibodies to aromatic acid decarboxylase, followed by the immunohistochemical revelation of the rate-limiting enzyme of dopamine synthesis, the TH, done by Ljungdahl et al. (1975), confirming the dopaminergic nature of nigrostriatal pathway and starting a new era for basal ganglia research.
Jakes and co-workers described two proteins of synuclein family, the alpha- and beta-synuclein, in human brain (Jakes et al. 1994). After some descriptions of familial cases of PD associated with mutation on alpha-synuclein (AS) (Polymeropoulos et al. 1997; Goedert 1997), Spillantini et al. (1997) reported, for the first time, the strong immunolabeling of Lewy bodies (LB) and neurites (LN) with AS in PD and dementia with Lewy body (DLB), followed by several publications which reinforced the relevance of AS on PD pathogenesis.
4.2 The Topographic Braak Staging of Parkinson’s Disease
After the recent observations on relationship of AS and PD, Del Tredici et al. (2002) evaluated the presence of AS in brain stem of subjects without history of neurologic or psychiatric disease. They described that all patients with AS inclusions in dorsal motor nucleus of vagus (DMV) and in intermediate reticular zone (IRtZ) presented also AS-immunoreactivity in other brain stem nuclei, as the locus coeruleus (LC) and the SN, suggesting that the pathological progression of PD could begin in the medulla and spread to the whole brain. The absence of clinical signs of PD in these individuals, even with AS pathology, can be explained by the existence of a presymptomatic phase before the onset of motor dysfunction.
The study of Braak et al. (2003) changed the classic concepts of an essentially dopaminergic dysfunction in PD. Using the same methods of Del Tredici et al. (2002) in 3 groups of (1) 41 individuals with clinical diagnosis of PD, (2) 69 individuals without neurological or psychiatric disease but with AS brain pathology, and (3) 58 individuals without neurological or psychiatric disease nor AS pathology, they showed that LB and LN marked with AS were present in nigral and extranigral areas, with a particular vulnerability and independent of neurotransmitter produced by the neurons, which became involved at different times in the progression of the disease, in a pattern of topographical caudorostral progression, as follows:
-
Stage 1: The presence of LN and LB was detected in two major nuclei: DMV and the IRtZ. At this stage LN was observed first than LB; however, in advanced cases, the LN outnumbers the LB in these areas. This pattern was seen at all stages of disease progression. Some neuromelanin-laden nerve cells in these nuclei do not show LB/LN. The anterior olfactory structures (olfactory bulb/stalk and the anterior olfactory nucleus) are also affected by AS inclusions. This stage of pathology spares the pontine melanized neurons, as the LC.
-
Stage 2: The more accentuated presence of LB/LN in DMV and IRtZ and the loss of neurons are seen at this stage. Furthermore, the pathological LB/LN appears in pontine areas of caudal raphe nuclei, the gigantocellular reticular nucleus and the coeruleus-subcoeruleus complex, but the latter nucleus has unaffected neurons. The midbrain nuclei remain uninvolved.
-
Stage 3: There is worsening of lesions on affected nuclei in medulla and pons, and the LB/LN is seen in midbrain structures and nuclei from the basal forebrain. In the midbrain, the presence of LB/LN in SN is observed initially in melanized projection neurons of posterolateral and posteromedial subnuclei, without macroscopically detectable depigmentation. The compact portion of pedunculopontine tegmental nucleus is affected only by LN in stage 3. The magnocellular nuclei of the basal forebrain (medial septal nucleus, interstitial nucleus of diagonal band, basal nucleus of Meynert) develop only a network of LN. There is no pathological alteration in temporal mesocortex or neocortex, but LNs are seen in anterior olfactory nucleus and second sector of Ammon’s horn (CA2) (for a wide review about extranigral brain stem nuclei affected by AS pathology, see Grinberg et al. 2010).
-
Stage 4: The midbrain shows accentuated involvement of neuromelanin-laden neurons of SNc, more significant in posterior region of pars compacta; the depigmentation is macroscopically seen at this stage and is observed in the extracellular melanin granules in SNc. Other mesencephalic regions are affected, such as the paranigral and parabrachial nucleus, and the compact portion of pedunculopontine tegmental nucleus (PPN) is more affected by LB and LN. The basal forebrain becomes severely involved, and areas as stria terminalis, central nucleus of amygdala, and ventral claustrum show LB. The anterior olfactory nucleus is much damaged. This stage is characterized by AS accumulation in the anteromedial temporal mesocortex, with outer layers developing networks of LN and LB within inner layers.
-
Stage 5: The last two stages indicate diffuse AS immunolabeling, including the neocortex. The SN is almost without melanin, and LB develops in melanized nerve cells as DMV and reticular formation. The network of LN in CA2 extends into adjacent sectors, comprising large portions of hippocampus. From the anteromedial temporal mesocortex (the periallocortical transentorhinal region and the proneocortical entorhinal area), the pathological progression moves toward the sensory association areas, anterior cingulate cortex, and prefrontal area, with LB in infragranular layers and LN in supragranular layers. The primary neocortical areas are spared.
-
Stage 6: The final stage encloses the entire neocortex, but the pathological changes on premotor area, primary motor field, and primary sensory area are mild.
The authors of this study suggested that this staging model permits the distinction of clinically manifested cases, even as non-motor initial PD patients, as REM sleep disorders, psychiatric diseases, intestinal constipation, olfactory dysfunctions, and dysautonomia. The Braak hypothesis shows a correlation among pathological and clinical phenotypes. Jellinger (2003) observed that some individuals from Braak’s study had stages 3–4 PD pathology, but no motor symptoms were diagnosed, in which he called incident Lewy body pathology (iLBP). Furthermore, as noticed by Parkkinen et al. (2003) and Ishizawa et al. 2003, other diseases as DLB and AD showed LB with AS-immunoreactivity in the brain, reducing the specificity of Braak scheme for PD. After the publication of those works, the Braak staging model had gained acceptance rapidly. However, there are no conclusive proofs confirming a caudal-to-rostral temporal progression in PD pathology. Additional studies are required to elucidate these questions.
4.3 The Human Midbrain Dopaminergic System and Parkinson’s Disease
Classically, the bradykinesia, rigidity, and tremor in parkinsonian syndromes are attributed to mesencephalic dysfunctions, and diseases as strokes and tumors localized in SN can cause a rigid-akinetic phenotype (Garcia de Yebenes et al. 1982; Akyol et al. 2006; Orta Daniel and Ulises 2008). PD is modernly considered as a multisystem neurodegenerative illness, affecting dopaminergic and non-dopaminergic neurons (serotoninergic, glutamatergic, cholinergic, nitrergic) as seen on Braak hypothesis. However, the major motor symptoms (bradykinesia and rigidity) and the motor complications (dyskinesia, wearing off phenomenon) are associated to dopaminergic transmission, justifying the special attention given to the nigrostriatal degeneration. There are other dopamine-rich areas in CNS, as the hypothalamus and retina, but its involvement in PD is controversial (for review see Archibald et al. 2009).
Based on the immunoreactivity to calbindin, the midbrain dopaminergic system can be divided into two tiers: the dorsal and the ventral tier. The dorsal tier encompasses the retrorubral groups and the VTA, its neurons are calbindin-positive, and they have horizontal orientation and low expression of DAT and D2 receptors. The ventral tier is composed by SNc, with calbindin-negative and ventrally oriented neurons, showing high amounts of DAT and D2 receptors. This cytochemical phenotypic classification has a practical application: the ventral tier is particularly vulnerable to the neurodegeneration of PD but with selective sparing of the dorsal tier (Haber and Gdowski 2004). However, the neuromelanin-laden neuron loss in SNc is not necessarily uniform (Ma et al. 1996).
Fearnley and Lees (1991) studied the micro-architecture of SN and the rates of neuron loss in normal aging and parkinsonian syndromes, and there was a linear reduction of pigmented neurons in advancing aging, more affected in dorsal tier. In opposite pattern, the PD individuals had 45 % of neuron loss in the first decade of disease, being greatest in the lateral ventral tier (average loss 91 %), followed by the medial ventral tier (71 %) and the dorsal tier (56 %). These results show a specific pattern of nerve cell loss in SNc on PD (Fearnley and Lees 1991). Ma et al. (1996) performed a morphometric analysis of SNc in PD individuals, and they described a marked reduction in pigmented neuron area, perimeter, diameter, and number in PD cases.
Damier et al. (1999a, b) developed a new method to analyze the SNc, based in immunolabeling to calbindin, identifying calbindin-rich regions (“matrix”) and calbindin-poor islands (“nigrosomes”), and these pocket zone patterns were recognized in all brain as five nigrosomes. The nigrosome 1, localized in the ventral third and the lateral two-thirds of the calbindin-rich neuropil, corresponds to the ventral tier (Damier et al. 1999a). In another study (Damier et al. 1999b), the same authors used this SN analysis in PD subjects, and the neuronal depletion was maximum (98 %) in nigrosome 1, corroborating the findings of Fearnley and Lees (1991). A spatiotemporal progression of cell loss on PD was suggested, beginning in nigrosome 1, after spreading to other nigrosomes and to the matrix.
Another method used for analysis of dopamine depletion in parkinsonian syndromes is the radionuclide imaging with positive emission tomography (PET) and single-photon emission computed tomography (SPECT), applied as biomarkers for early disease and study of disease progression, mostly the presynaptic dopamine function, of the nigrostriatal pathway. There are four major ways to examine the dopaminergic terminals function: (1) the18F-dopa in SPECT and11C-dopa in PET, measuring the DOPA-decarboxylase activity; (2) molecules as11C or18F (for PET), or123I or99mTc (for SPECT, as99mTc-TRODAT-1) quantify the availability of DATs; (3) 11C-dihydrotetrabenazine or its 18F-labeled analogue measures the VMAT2 density; and (4) the ability of dopaminergic terminals to release dopamine after pharmacological stimuli (e.g., amphetamine) can be evaluated by changes in dopamine D2 receptor availability with11C-raclopride PET or123I-iodobenzamide SPECT (Brooks 2007; Stoessl et al. 2011).
It is known that18F-dopa uptake has correlation with nigral dopaminergic cell counts and dopamine concentrations in striatum of humans and nonhuman primates (Pate et al. 1993; Snow et al. 1993). In unilateral Hoehn & Yahr stage 1 PD patients, the dorsal posterior putamen18F-dopa uptake is first reduced, even contralateral to the asymptomatic limb, corresponding to the evidences that ventrolateral nigral dopaminergic projections to the dorsal putamen are more affected in the onset of clinical phase of PD (Kish et al. 1988).
The subsequent PD progression does not reduce the18F-dopa uptake in the first 2 years (Whone et al. 2003), but after that the functional loss occurs more rapidly in PD than aged controls (Brooks 2003), and the initial posterior-anterior gradient of striatal involvement is substituted by similar rates of decline in the whole striatum. Furthermore, the PET/SPECT measures show inverse correlation with severity of rigidity and bradykinesia in PD but poorly with tremor, suggesting the parkinsonian tremor may be associated with extranigral mechanisms (Vingerhoets et al. 1997; Benamer et al. 2000). Another finding is the increase of18F-dopa uptake into the internal globus pallidus in initial PD as a possible compensatory action, but this phenomenon vanishes with disease progression, simultaneously with onset of fluctuating responses to levodopa (Whone et al. 2003).
The motor complications on PD are also associated with radioimaging changes. PD patients with fluctuating responses to levodopa present 20 % reduction in18F-dopa uptake than those with sustained therapeutic responses (de la Fuente-Fernandez et al. 2000), and the11C-raclopride binding shows a mean 10 % fall in posterior putamen of advanced PD subjects compared with early-stage patients (Torstenson et al. 1997). Nevertheless, PET studies with dyskinetic individuals show similar levels of striatal dopamine D1 and D2 receptor availability (Turjanski et al. 1997). The dementia in PD, a frequent non-motor complication of advanced stages, shows a reduction in18F-dopa uptake in cingulate and medial prefrontal areas, suggesting the involvement of the mesocortical dopaminergic pathway on cognitive decline in PD (Ito et al. 2002).
In conclusion, the major importance of midbrain dopaminergic neuronal degeneration on symptomatic progression of PD is unquestionable. However, it must be emphasized that there are several other non-dopaminergic neurons involved in its pathogenesis, as shown by Braak hypothesis, and the AS aggregation and accumulation can be the common link between these vulnerable nerve cells.
4.4 Dopamine-Related Treatments of Parkinson’s Disease
The treatment of PD is very complex due to the progressive nature of the disease and the array of motor and non-motor features combined with early and late side effects associated with therapeutic interventions. While no treatments have yet been shown conclusively to slow the progression of the disease, a growing number of symptomatic pharmacologic therapies are available, as well as several forms of surgery and numerous non-pharmacological approaches.
Among dopaminergic drugs, l-DOPA standard and controlled-release formulations are marketed as fixed associations with a DOPA-decarboxylase inhibitor carbidopa or benzeraside. There are several dopamine agonists, with different receptor binding, pharmacokinetic profiles, and routes of administration and two types of indirect dopamine transmission enhancers, monoamine oxidase-B (MAO-B) and COMT inhibitors. Among non-dopaminergic medications, there are several anticholinergics, although amantadine is the only drug widely available for anti-glutamatergic effects. Functional neurosurgery for PD attempts to restore functional balance in basal ganglia relays. There are currently three targets: the ventral intermediate nucleus of the thalamus, the internal segment of the globus pallidus, and the subthalamic nucleus. Either CNS lesions (thalamotomy, pallidotomy, or subthalamic nucleus lesions) or implants of chronic stimulating electrodes at these sites (deep brain stimulation) can be used. Reconstructive surgery involves the implantation of human fetal mesencephalic cells (fetal transplant) or other dopamine-producing cells into the striatum. Rehabilitation for PD (speech, occupational, and physical therapies) has been applied empirically based on its accepted use in other chronic disorders.
It is important to consider that while drug treatments available for PD increased in the last 30 years, the first dopaminergic drug l-DOPA remains the more effective. Actually, the more recent drugs refined the treatment but they do not alter the clinical aspect and the evolution of the disease, and everyone can only temporary control and ameliorate the motor symptoms. The increasing evidences that cognitive dysfunctions, depression, constipation, pain, and nocturnal incapacity can play important role in the quality of life of patients and the benefits of any new treatment should be evaluated in their impact in the well-bearing, mental health, and others deficiencies of the patients. A summarized review of the pharmacological approaches currently available for PD is illustrated in Table 1.
All the dopaminergic therapies are based in the pathway of catecholamine synthesis in the body, as presented in Fig. 1. Since most parkinsonian motor symptoms occur as a consequence of dopamine depletion in the basal ganglia, dopamine replacement strategies represent the main therapeutic approach used to counteract PD motor impairment.
4.4.1 3,4-Dihydroxyphenylalanine (l-DOPA)
Since its introduction in the routine treatment of PD at the beginning of the 1970s (Cotzias 1971), l-DOPA (l-3,4-dihydroxyphenylalanine), a dopamine metabolic precursor, still remains as the most effective treatment for the treatment of the classical motor parkinsonian signs. l-DOPA, the naturally occurring isomer of the amino acid 3,4-dihydroxyphenylalanine, was first isolated in 1913 from legumes (seedlings of Vicia faba) by Marcus Guggenheim (Hauser 2009). Already 2 years earlier, Casimir Funk had synthesized D,l-DOPA in the laboratory (Hauser 2009). Funk and Guggenheim considered the amino acid as a possible parent compound of adrenaline. The discovery by Peter Holtz (1959) of an enzyme, DOPA decarboxylase, in mammalian tissue (kidney) extracts that converted l-DOPA to the corresponding – biologically active – amine, that is, dopamine, represented a turning point in catecholamine research (Hauser 2009). After the discovery of dopamine occurrence in the body, many biochemical studies were performed in an attempt to provide experimental support for the proposed biosynthesis of the catecholamines, mainly dopamine, from the amino acid l-DOPA (Hornykiewicz 2002).
The introduction of high dosage of l-DOPA by George Cotzias revolutionized the treatment of PD (Singh et al. 2007). As a prodrug of dopamine, l-DOPA crosses the blood-brain barrier, and it is decarboxylated to dopamine in the nigrostriatal pathways by brain enzymes Nowadays, l-DOPA is always given with carbidopa or benserazide, peripheral DOPA-decarboxylase inhibitors, which prevents peripheral metabolism of l-DOPA and allows a higher percentage of a dose to cross the blood-brain barrier. Primarily, they block l-DOPA metabolism in the periphery, thereby reducing the rate of the first-pass metabolism, and slowing the plasma clearance of l-DOPA; however, the enzyme inhibitors do not cross the blood-brain barrier.
The effects of the dopamine replacement therapy are predictable (as are the side effects), and none of the more recently introduced synthetic dopamine agonists has surpassed the clinical benefit derived from l-DOPA. l-DOPA has a long-duration response in early disease that enables adequate symptomatic control with dosage schedules (Table 1). This is a consequence of the ability of the nigrostriatal system to convert l-DOPA to dopamine, store it in presynaptic vesicles, and release it in response to physiological stimuli. However, as the disease progresses, the conversion of l-DOPA to dopamine in dopaminergic neurons becomes limited.
4.4.2 l-DOPA and Dyskinesia
Although the precise mechanisms of dyskinesia have remained elusive, three main risk factors for this side effect have been conclusively identified in clinical studies, namely, a young age at disease onset, disease severity (reflecting the extent of putaminal dopamine denervation), and high doses of l-DOPA (Jenner 2008; Stocchi 2009; see a recent review of Huot et al. 2013). These risk factors are reproduced in both nonhuman primate and rodent species by applying neurotoxic lesions to the nigrostriatal dopamine pathway, followed by daily administration of l-DOPA at a sufficient dosage.
Although initially effective, dopaminergic therapies are eventually complicated by motor fluctuations, including off-time (periods of return of PD symptoms when medication effect wears off) and dyskinesia (drug-induced involuntary movements including chorea and dystonia) in most patients (Jenner 2008; Stocchi 2009). The mechanisms by which dyskinesia develop are not completely understood, but pulsatile stimulation of dopamine receptors by short-acting agents as l-DOPA and the degree of striatal denervation have been implicated (Obeso et al. 2000; Stocchi 2009). Dyskinesia may occur at the time of maximal clinical benefit and peak concentration of l-DOPA (peak-dose dyskinesia) or may appear at the beginning and/or at the end of the l-DOPA effect (diphasic dyskinesia, off-dystonia, off-dyskinesia). The progressive reduction in the duration of the clinical effect is called “wearing off.” This change in the response to l-DOPA is more related to the disease progression and loss of buffering capacity of the presynaptic terminals to keep constant the levels of dopamine in the striatum (Chase 1998). When wearing off begins, it required modification of dosage and/or dose frequency of l-DOPA or the introduction of additional therapies. Interestingly, at this stage of the disease, so long as the plasma l-DOPA concentration is kept constant, the clinical response will persist (Mouradian et al. 1990; Schapira et al. 2009) and “wearing off” does not occur if the drug is given by continuous infusion (Nutt et al. 1989; Schapira et al. 2009). Motor complications can be an important source of disability for some patients who cycle between “on” periods, which are complicated by dyskinesia and “off” periods in which they suffer severe motor disability (Schapira et al. 2009).
Nonhuman primates have been used to produce models of l-DOPA-induced dyskinesia since the late 1980s (Crossman 1987). Following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) lesion and subsequent treatment with l-DOPA, nonhuman primates (and macaques in particular) exhibit choreiform and dystonic movements that look very similar to those seen in dyskinetic PD patients. These models have been extensively used to define the basic pharmacological features of l-DOPA-induced dyskinesia (Jenner 2003; Cenci et al. 2009). Rats with 6-hydroxydopamine (6-OHDA) lesions have been the most widely used animal model of PD since the 1970s, but the idea that these animals can exhibit l-DOPA-induced dyskinesia has become accepted only recently (Henry et al. 1998; Cenci et al. 2009). For many years, drug-induced rotation remained the only available method to study motor effects of dopaminergic therapies in this animal model. When given to unilaterally 6-OHDA-lesioned rats, l-DOPA or dopamine receptor agonists induce the animals to turn in the direction contralateral to the lesion, a behavior traditionally regarded as a correlate of the antiparkinsonian activity of these drugs. Later on, it was realized that contralateral rotation shows sensitization during chronic dopaminergic treatment (Henry et al. 1998) – a feature reminiscent of the priming for dyskinesia described in nonhuman primates and supposed to occur in PD patients. About 10 years ago came the first reports that 6-OHDA-lesioned rats treated with l-DOPA exhibit movements with dystonic and hyperkinetic features engaging the forelimb contralateral to the lesion, as well as axial and orofacial muscles (Lundblad et al. 2002; Winkler et al. 2002). These movements interfere with the rat’s physiological motor activities, and they are reduced by treatments that have antidyskinetic efficacy in nonhuman primates and PD patients. Rating scales for rodent abnormal involuntary movements (AIMs) were developed to reflect the topographic distribution, frequency, duration, and amplitude of dyskinetic behaviors induced by l-DOPA (Dekundy et al. 2007; Cenci et al. 2009). Producing and validating an animal model is a fundamental task for investigators involved in translational PD research.
Nevertheless, it must be emphasized that l-DOPA remains the “gold standard” medication in the treatment of PD. The use of other dopaminergic drugs at the beginning of the treatment helps to delay the onset of the motor complications in patients with PD. However, at some time when the disability of the patient increases, l-DOPA needs to be introduced (Olanow et al. 2009b). l-DOPA use is appropriate when all other reasonable strategies fail to produce adequate control of symptoms or when patient’s symptoms pose an imminent danger to them (e.g., loss of postural reflexes with high risk for falling).
Recently, there was a growing discussion about a possible neurotoxic effect of l-DOPA that could increase the disease progression, and many patients and doctors became afraid to use the drug and delayed its use at the most (Kurlan 2005). However, a critical evaluation of older and most recent studies fails to describe a deleterious effect of l-DOPA in the evolution of the degenerative process (Olanow et al. 2004). In contrast our work showed that chronic l-DOPA treatment to partial 6-OHDA-lesioned rats induces partial recovery of the dopaminergic system suggesting their trophic effect which was confirmed by the increase of the trophic factor pleiotrophin (Murer et al. 1998; Ferrario et al. 2004).
Therefore, in the initial stages of the disease, l-DOPA therapy is the most effective for improving motor symptoms in PD. However, long-term treatment is accompanied by fluctuations in motor performance, dyskinesia, and neuropsychiatric complications. Furthermore, as PD progresses, patients develop features that do not respond well to l-DOPA therapy, such as freezing episodes, autonomic dysfunction, falling, and dementia. The increasingly diverse possibilities in the therapy of PD, and the many side effects and complications of therapy, require the formulation of reliable standards for patient care that are based on current scientific knowledge.
4.4.3 Catechol-O-Methyltransferase Inhibitors (COMT)
l-DOPA is usually combined with a DOPA-decarboxylase inhibitor (carbidopa or benserazide) to improve absorption and reduce peripheral metabolism (Schapira et al. 2009). However, the majority of l-DOPA is still metabolized in the gut and liver by COMT to form 3-O-methyldopa (3-OMD). 3-OMD accumulates and may interfere with the brain penetration of l-DOPA. COMT inhibitors reduce the metabolism of l-DOPA, extending its plasma half-life by 30 % and prolonging the clinical effect of each l-DOPA dose. This effect was suspected to be of enough magnitude to produce more stable l-DOPA plasma levels if a COMT inhibitor was used with each dose of l-DOPA. The hypothesis was that this combined therapy could delay the appearance of motor complications because of producing more continuous dopaminergic stimulation. However, clinical studies were unable to show this positive effect, and in opposition, the combined therapy led to earlier appearance of dyskinesia (Klivenyi and Vecsei 2010). This unexpected effect may be explained by the potentializing effect of the COMT inhibitors in the l-DOPA effect, since its dosage, or effect, is associated with earlier beginning of motor complications.
Entacapone and tolcapone are the two selective COMT inhibitors currently in use for the treatment of PD (see Table 1), while newer molecules are now in clinical evaluation. Both COMT inhibitors only have observable clinical effects when used in association to l-DOPA. The most significant clinical effect is the reduction in the daily off-time, by small prolongation in the time of l-DOPA effect (Olanow et al. 2004; Jankovic and Stacy 2007). The COMT inhibitors also potentialize the effect of l-DOPA, and they can be used to achieve a stronger symptomatic effect (Gallagher and Schrag 2008). Tolcapone is a central and peripheral COMT inhibitor and is apparently more efficacious than entacapone that only inhibits peripheral metabolism, but there are some concerns about its safety and in particular to its potential hepatotoxicity (Assal et al. 1998). Entacapone is much more widely used and appears to be less hepatotoxic (Gershanik et al. 2003; Schrag 2005). The main adverse effects are related to its potentializing dopaminergic effects and include dyskinesia, orthostatic hypotension, nausea, vomiting, and confusion. Other important side effects are the occurrence of diarrhea and urinary discoloration. The control of hepatic enzymes is always indicated during the use of tolcapone and eventually during the use of entacapone. The association of entacapone in a single tablet with l-DOPA and carbidopa is now available. Another effect of the COMT inhibitor is the reduction in the systemic levels of 3-OMD that was supposed to interfere with l-DOPA metabolism in the central nervous system. However, any clinical benefit could be proved to be due to this property (Muller and Kuhn 2006). Another systemic effect of the COMT inhibitors is the reduction in the blood levels of homocysteine in treated PD patients (Muller and Kuhn 2006; Muller and Muhlack 2009). This could be a hypothetical benefit in the long term, since homocysteine could mediate the progression of neuronal degeneration and increase the risk for development of dementia, vascular disease, and polyneuropathy in levodopa-treated PD patients (Muller and Muhlack 2009).
Recently, it was established that a continuous duodenal l-DOPA infusion may be an efficient strategy to obtain continuous dopaminergic stimulation and to decrease the complications of the therapy (Clarke et al. 2009). Other ways to obtain a stable plasma level of l-DOPA were under research and include the development of new controlled-release tablets and cutaneous patches of the drug.
4.4.4 Monoamine Oxidase Isoenzyme Type B (MAO-B) Inhibitor
Substantial experimental evidences indicate that the oxidative stress is an important pathogenic mechanism in PD (Yacoubian and Standaert 2009). There are many possible mechanisms associated to the generation of free radicals in PD. The own dopamine metabolism produces free radicals in excess. Blocking the first step of dopamine metabolism by inactivating MAO-B reduces the production of free radicals. Selegiline and rasagiline are selective MAO-B inhibitors currently in clinical use (see Table 1). MAO-B inhibition may increase the concentration of dopamine in the synaptic cleft due to the inhibition in the dopamine degradations and also produce an antiparkinsonian effect (Wu et al. 2000). Unlike selegiline, rasagiline is not metabolized to amphetamine and has no sympathomimetic activity. Rasagiline has also shown neuroprotective effects in in vitro and in vivo models of PD (Jenner 2004).
Selective MAO inhibitors become nonselective at doses greater than those recommended. In this condition, selegiline and rasagiline may increase the risk of a hypertensive crisis if used in combination with tyramine-containing foods (e.g., fermented cheese, aged meats) or amine-containing medications (e.g., over-the-counter cough/cold medicines). Serotoninergic crisis also has been associated with the use of MAO-B inhibitors in association with antidepressive drugs, since they can increase brain serotonin levels. The motor symptomatic effect of the MAO-B inhibitors is only modest, and these drugs are most commonly used to treat patients at the earlier stage of the disease. However, the MAO-B inhibitors may also be used at the later stages to increase the daily on-time. Despite recent clinical studies with rasagiline suggested that the drug may also have a neuroprotective effect (Olanow et al. 2009a), this is still a matter of controversy (see Ahlskog and Uitti 2010).
4.4.5 Dopamine Receptor Agonists
Dopamine agonists directly stimulate dopamine receptors. Of the nine dopamine agonists marketed for the treatment of PD, five were ergot derivatives (bromocriptine, cabergoline, dihydroergocryptine, lisuride, and pergolide) and four are non-ergot derivatives (apomorphine, piribedil, pramipexole, and ropinirole) (see Table 1). Pergolide was retired of the market of most countries due to tendency to induce cardiac valvar fibrosis; however, the use of ergot-derived dopamine agonists (pergolide and cabergoline) in patients with PD was associated with increased risk of cardiac valvar regurgitation (Steiger et al. 2009). The acute side effects of dopamine agonists are similar to those observed with l-DOPA and include nausea, vomiting, and postural hypotension. It is generally accepted that the shared D2-like receptor agonistic activity produces the antiparkinsonian effect. This D2 effect also explains peripheral (gastrointestinal nausea and vomiting), cardiovascular (orthostatic hypotension), and neuropsychiatric (somnolence, psychosis, and hallucinations) side effects. Even at lower doses, patients experience orthostatic hypotension, constipation, dyskinesia, confusion, and excessive somnolence with these agents (Wong et al. 2003). These adverse effects tend to occur with the initiation of treatment and to abate as tolerance develops usually over the ensuing days to weeks. Other problems related to the use of dopamine agonists include weight gain (possibly related to overeating) and edema (especially in the lower extremities). More recently, attention has been focused on the association of dopamine agonist treatment with the development of pathologic gambling, hypersexuality, and compulsive eating and shopping. This has attracted considerable scientific interest because of the known relationship between dopamine and reward (Obeso et al. 2000).
Almost all currently used dopamine agonists are able to protect dopamine neurons from death in many in vitro and in vivo experiments. This neuroprotective effect may be the result of different mechanisms: antioxidation, scavenging of free radicals, suppression of lipid peroxidation, and inhibition of apoptosis. Dopamine agonists have a significant clinical effect in the motor parkinsonian signs, but most of them were not comparable to that observed with l-DOPA. The use of dopamine agonists at the beginning of the treatment of PD to spare l-DOPA is indicated specially for younger patients. The monotherapy with this class of drugs is associated with lower incidence of dyskinesia and wearing off but with a lower antiparkinsonian effect than l-DOPA (Hitzeman and Rafii 2009). One explanation for their lower risk to induce motor complications may be due to the long half-life of these drugs in relation to the l-DOPA. One exception for this is apomorphine that has a pharmacokinetic curve very similar to the l-DOPA and is the only dopamine agonist which a similar symptomatic effect to it. The main limitation for the clinical use of this drug is the administration route that must be subcutaneous. In some countries apomorphine is infused subcutaneously and continuously by a pump mechanism in patients with PD. The clinical effect is significant and persistent (Clarke et al. 2009). This is now recognized as a potential continuous dopaminergic stimulation strategy and reduces functional impairment and motor complications.
New delivery ways for dopamine agonist molecule are in development. Subcutaneous patches of rotigotine seem to be efficient and may produce more continuous dopaminergic stimulation (Chen et al. 2009). Long-release tablets of non-ergot dopamine agonists are now in evaluation and may be useful to increase adherence of the patient to the therapeutics.
4.4.6 Is There Nitric Oxide Inhibition Utility in the Dopamine Replacement Therapy?
Notwithstanding recent advances in the management of PD, l-DOPA-induced dyskinesia continues to be a clinical and therapeutic challenge (Cenci and Lindgren 2007; Jenner 2008; Santini et al. 2008). However, many other neurotransmitters may play a central role in the neurobiology and management of l-DOPA-induced dyskinesia. Nitric oxide is a free radical that can also act as an atypical neurotransmitter and influence dopamine-mediated neurotransmission. Nitric oxide also regulates synaptic and neural plasticity being enrolled in the regulation of motor activity (Pierucci et al. 2011; Del Bel et al. 2005, 2011). There is evidence whereby communication between nitrergic and dopaminergic systems plays an essential role in the control of the nigrostriatal pathway. The dual localization of immunoreactivity for nitric oxide synthase (NOS) and tyrosine hydroxylase, enzymes responsible for the synthesis of nitric oxide and dopamine, respectively, proposes a close anatomical link between the two neurotransmitters (Mitkovski et al. 2012).
Recently, our group (Padovan-Neto et al. 2009, 2011; Novaretti et al. 2010; Del Bel et al. 2011) and other laboratories (Takuma et al. 2012; Yuste et al. 2011) described that nitric oxide synthase inhibitors are able to decrease the motor changes related to prolonged administration of l-DOPA in rodent models of PD. Similar results were described, in latest meeting communication, for nonhuman primates by Iravani et al. (2008) and Yuste et al. (2011). Postmortem studies in human brain of patients with Parkinson’s disease have greatly contributed to figure out the disease. In spite of this, few human brain studies have focused on the relationship between the development of dyskinesia and biochemical changes in the human brain. It limits the possible understanding of the nitric oxide system in the treatment of Parkinson’s disease. But Parkinsonian patients presented in serum a marked rise of the production of the nitric oxide second messenger cyclic guanosine monophosphate (cGMP) levels (Chalimoniuk et al. 2004) induced by l-DOPA. Similar result was described in the cerebellum (Gumulka et al. 1976). Comparable result was described in animals (Itokawa et al. 2006). In mice, cGMP is regulated by l-DOPA (Chalimoniuk and Langfort 2007). In addition, Sanchez and cols. (2002) showed that nitric oxide stimulates l-DOPA release in the striatum in a time- and concentration-dependent manner.
A role for nitrergic neurotransmission in the pathophysiology of dyskinesia and as a potential drug target for new therapeutic has been proposed also by others (Iravani and Jenner 2011; Pierucci et al. 2011; Huot et al. 2013). NOS inhibitor treatment was able to prevent abnormal involuntary movements without significantly changing normal motor (Padovan-Neto et al. 2009). Different from the cataleptic effects of these drugs, there was no tolerance to the antidyskinetic effects of NOS inhibitors (Novaretti et al. 2010). The antidyskinetic effects of 7-nitroindazole, a preferential neuronal nitric oxide synthase (nNOS) inhibitor, seem to involve reduction in gene and protein expression in denervated striatal neurons (Padovan-Neto et al. 2011). l-DOPA increased nNOS mRNA levels in the contra- and ipsilateral side to the dopamine lesion of the frontal cortex but did not produce any further increases of nNOS protein in the striatum compared to the 6-OHDA-induced increase (Padovan-Neto et al. 2011).
Despite promising studies, efforts in synthesizing highly selective nNOS inhibitors have been difficult and have limited the development of such drugs (Hobbs et al. 1999; Vallance and Leiper 2002). To date, clinical studies of NOS inhibitors have been limited by the small number of potent and selective drugs that are safe for human administration (Vallance 2003; Petros et al. 1994: Rees 1995; Ashina et al. 1999). The recent discovery that multiple subunits of NOS exist, with apparent unique anatomic distribution and functional and pharmacological diversity (for review see Garthwaite 2008), holds considerable therapeutic potential. One novel therapeutic approach could be to manipulate the levels of endogenous NOS inhibitors. NOS inhibitors are also likely synthesized within the body. l-NMMA (NG-monomethylarginine) and asymmetric dimethylarginine are synthesized by methylation of arginine residues in proteins (Leiper and Vallance 1999). Once the proteins are broken down, the free methylarginines are released into the cell cytosol and act as competitive inhibitors of all NOS isoforms. They are metabolized to citrulline by the action of dimethylarginine dimethylaminohydrolase (Leiper and Vallance 1999; MacAllister et al. 1996). Inhibition of this enzyme leads to the accumulation of methylarginines and thereby blocks nitric oxide production. Antagonists might be developed to target-specific pathways within the basal ganglia.
A novel class of drugs named membrane-associated guanylate kinase inhibitors has recently been proposed in treatment of excitotoxicity (Lau and Tymianski 2010; Doucet et al. 2012). These peptides are competitive antagonists designed to bind postsynaptic scaffolding proteins (as, e.g., PSD-95, postsynaptic density 95 kDa), aimed at dissociating the spatial relationship between nNOS and NMDA (N-methyl-d-aspartate) receptors (NMDA/PSD-95/nNOS receptor complex) (Zhou et al. 2010; Doucet et al. 2012). The association of nNOS with NMDARs, via PSD-95 (Christopherson et al. 1999), plays an important role in a range of normal neuronal functions including synaptic plasticity (Garthwaite et al. 1988; Steinert et al. 2010) as well as pathophysiological disorders of the brain (Aarts et al. 2002; Sun et al. 2008; Zhou et al. 2010; Doucet et al. 2012). Since (a) NMDARs couple to nNOS and (b) NMDAR antagonists (amantadine) and nNOS inhibitors possess antidyskinetic properties, the disruption of the NMDAR/PSD-95/nNOS complex may possibly represent an alternative approach to specifically block nitrergic signaling coupled to NMDA receptor activity. It may prevent the problems associated general reduction of NMDA or nNOS function. To date, PDZ-based interactions represent a drug target that still remains largely unused (Dev 2004). They are however currently undergoing phase II clinical trials (Lau and Tymianski 2010).
The reduction in l-DOPA-induced dyskinesia in rodents and primates treated with NOS inhibitors suggests the involvement of nitric oxide in this altered behavior. These preclinical findings suggest that nitric oxide is a promising therapeutic target for the reduction of l-DOPA-induced dyskinesia.
5 Conclusion
The discoveries of dopamine as a neurotransmitter in the brain, its depletion in patients with PD, and its replacement with l-DOPA therapy were major revolutionary events in the rise to effective therapy for patients with PD. However, although drugs that act on the dopaminergic system provide effective symptomatic control for the classic parkinsonian motor features, the patients’ quality of life continues to deteriorate as a consequence of gait and balance difficulties, autonomic dysfunction, and cognitive impairment. l-DOPA remains at present the most powerful symptomatic drug for the treatment of this condition. However, motor complications of chronic l-DOPA treatment have emerged as a major limitation of this therapy. Nonetheless, the importance of dopamine neurotransmitter system in a broad array of human disorders ranging from PD to schizophrenia has driven an intensive array of investigations oriented toward increasing our understanding of this complex system in normal conditions as well as disease states. Knowledge of dopaminergic cells and circuits has been enriched by findings obtained with several and highly sensitive techniques, as immunohistochemistry, cellular biology, transgenic mice or conditional mutants, functional anatomy, electrophysiological studies, sophisticated behavioral analyses, and functional neuroimaging. Altogether, these studies have also led to new concepts of healthy and pathological functions of dopaminergic circuits at the molecular and system levels.
Abbreviations
- 3-OMD:
-
3-O-methyldopa
- 6-OHDA:
-
6-hydroxydopamine
- AIMs:
-
Abnormal involuntary movements
- AS:
-
Alpha-synuclein
- CNS:
-
Central nervous system
- COMT:
-
Catechol-O-methyltransferase
- DARPP-32:
-
Dopamine- and cAMP-regulated phosphoprotein, Mr 32 kDa
- DAT:
-
Dopamine transporter
- DLB:
-
Dementia with Lewy body
- DMV:
-
Dorsal motor nucleus of vagus
- DYN:
-
Dynorphin
- ENK:
-
Enkephalin
- FS:
-
Fast spiking
- GPCRs:
-
G protein-coupled receptors
- GPe:
-
External segment of the globus pallidus
- GPi:
-
Internal segment of the globus pallidus
- iLBP:
-
Incident Lewy body pathology
- IrtZ:
-
Intermediate reticular zone
- i.v.:
-
Intravenous
- LB:
-
Lewy bodies
- LC:
-
Locus coeruleus
- L-DOPA:
-
L-3,4-dihydroxyphenylalanine
- LN:
-
Lewy neurites
- LTS:
-
Low-threshold spiking
- MAO-B:
-
Monoamine oxidase-B
- MPTP:
-
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MSN:
-
Medium spiny neuron
- NPY:
-
Neuropeptide Y
- PD:
-
Parkinson’s disease
- PET:
-
Positive emission tomography
- PV:
-
Parvalbumin
- SN:
-
Substantia nigra
- SNc:
-
Substantia nigra pars compacta
- SNr:
-
Substantia nigra pars reticulata
- SOM:
-
Somatostatin
- SP:
-
Substance P
- SPECT:
-
Single-photon emission computed tomography
- STN:
-
Subthalamic nucleus
- TH:
-
Tyrosine hydroxylase
- VMAT2:
-
Vesicular monoamine transporter 2
- VTA:
-
Ventral tegmental area
References
Aarts, M., Liu, Y., Liu, L., Besshoh, S., Arundine, M., et al. (2002). Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science, 298, 846–850.
Ahlskog, J. E., & Uitti, R. J. (2010). Rasagiline, Parkinson neuroprotection, and delayed-start trials: Still no satisfaction? Neurology, 74, 1143–1148.
Akyol, A., Akyildiz, U. O., & Tataroglu, C. (2006). Vascular Parkinsonism: a case of lacunar infarction localized to mesencephalic substantia nigra. Parkinsonism & Related Disorders, 12, 459–461.
Albin, R. L., Young, A. B., & Penney, J. B. (1989). The functional anatomy of basal ganglia disorders. Trends in Neurosciences, 12, 366–375.
Andén, N. E., Carlsson, A., Dahlström, A., Fuxe, K., Hillarp, N., et al. (1964). Demonstration and mapping out of nigro-neostriatal dopamine neurons. Life Sciences, 3, 523–530.
Archibald, N. K., Clarke, M. P., Mosimann, U. P., & Burn, D. J. (2009). The retina in Parkinson’s disease. Brain, 132, 1128–1145.
Ashina, M., Lassen, L. H., Bendtsen, L., Jensen, R., & Olesen, J. (1999). Effect of inhibition of nitric oxide synthase on chronic tension-type headache: A randomised crossover trial. Lancet, 353, 287–289.
Assal, F., Spahr, L., Hadengue, A., Rubbia-Brandt, L., & Burkhard, P. R. (1998). Tolcapone and fulminant hepatitis. Lancet, 352, 958.
Benamer, H. T., Patterson, J., Wyper, D. J., Hadley, D. M., Macphee, G. J., et al. (2000). Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Movement Disorders, 15, 692–698.
Bentivoglio, M., & Morelli, M. (2005). The organisation and circuits of mesencephalic dopaminergic neurons and the distribution of dopamine receptors in the brain. In S. B. Dunnett, M. Bentivoglio, A. Björklund, & T. Hokfelt (Eds.), Handbook of chemical neuroanatomy (Dopamine, Vol. 21, pp. 1–107). Amsterdam, Boston: Elsevier.
Bergson, C., Mrzljak, L., Smiley, J. F., Pappy, M., Levenson, R., et al. (1995). Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. Journal of Neuroscience, 15, 7821–7836.
Bevan, M. D., Booth, P. A., Eaton, S. A., & Bolam, J. P. (1998). Selective innervation of neostriatal interneurons by a subclass of neuron in the globus pallidus of the rat. Journal of Neuroscience, 18, 9438–9452.
Björklund, A., & Dunnett, S. B. (2007). Dopamine neuron systems in the brain: an update. Trends in Neurosciences, 30, 194–202.
Björklund, A., & Lindvall, O. (1984). Dopamine-containing systems in the CNS. In A. Björklund & T. Hökfelt (Eds.), Handbook of chemical neuroanatomy (Classical transmitters in the CNS, Vol. 2, pp. 55–122). Amsterdam: Elsevier.
Bonifácio, M. J., Palma, P. N., Almeida, L., & Soares-da-Silva, P. (2007). Catechol-O-methyltransferase and its inhibitors in Parkinson’s disease. CNS Drug Reviews, 13, 352–379.
Braak, H., Del Tredici, K., Rub, U., de Vos, R. A., Jansen Steur, E. N., et al. (2003). Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging, 24, 197–211.
Brooks, D. J. (2003). Imaging end points for monitoring neuroprotection in Parkinson’s disease. Annals of Neurology, 53, 110–118.
Brooks, D. J. (2007). Functional neuroimaging in movement disorders. In J. Jankovic & E. Tolosa (Eds.), Parkinson’s disease & movement disorders (5th ed.). Philadelphia, PA: Lippincott Williams & Wilkins.
Carlsson, A., Falck, B., & Hillarp, N. A. (1962). Cellular localization of brain monoamines. Acta Physiologica Scandinavica, 56, 1–28.
Cenci, M. A., & Lindgren, H. S. (2007). Advances in understanding l-DOPA-induced dyskinesia. Current Opinion in Neurobiology, 7, 665–671.
Cenci, M. A., Ohlin, K. E., & Rylander, D. (2009). Plastic effects of l-DOPA treatment in the basal ganglia and their relevance to the development of dyskinesia. Parkinsonism & Related Disorders, 15, 59–63.
Centonze, D., Grande, C., Usiello, A., Gubellini, P., Erbs, E., et al. (2003). Receptor subtypes involved in the presynaptic and postsynaptic actions of dopamine on striatal interneurons. Journal of Neuroscience, 23, 6245–6254.
Chalimoniuk, M., & Langfort, J. (2007). The effect of subchronic, intermittent l-DOPA treatment on neuronal nitric oxide synthase and soluble guanylyl cyclase expression and activity in the striatum and midbrain of normal and MPTP-treated mice. Neurochemistry International, 50, 821–833.
Chalimoniuk, M., Stepień, A., & Strosznajder, J. B. (2004). Pergolide mesylate, a dopaminergic receptor agonist, applied with l-DOPA enhances serum antioxidant enzyme activity in Parkinson disease. Clinical Neuropharmacology, 27, 223–229.
Chase, T. N. (1998). The significance of continuous dopaminergic stimulation in the treatment of Parkinson’s disease. Drugs, 55, 1–9.
Chaudhuri, R., & Schapira, A. (2009). Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurology, 8, 464–474.
Chaudhuri, K. R., Healy, D. G., & Schapira, A. H. (2006). Non-motor symptoms of Parkinson’s disease: Diagnosis and management. Lancet Neurology, 5, 235–245.
Chen, J. J., Swope, D. M., Dashtipour, K., & Lyons, K. E. (2009). Transdermal rotigotine: A clinically innovative dopamine-receptor agonist for the management of Parkinson’s disease. Pharmacotherapy, 29, 1452–1467.
Chevalier, G., Vacher, S., Deniau, J. M., & Desban, M. (1985). Disinhibition as a basic process in the expression of striatal functions. I. The striato-nigral influence on tecto-spinal/tecto-diencephalic neurons. Brain Research, 334, 215–226.
Christopherson, K. S., Hillier, B. J., Lim, W. A., & Bredt, D. S. (1999). PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. Journal of Biological Chemistry, 274, 27467–27473.
Cisek, P., & Kalaska, J. F. (2010). Neural mechanisms for interacting with a world full of action choices. Annual Review of Neuroscience, 33, 269–298.
Clarke, C. E., Worth, P., Grosset, D., & Stewart, D. (2009). Systematic review of apomorphine infusion, levodopa infusion and deep brain stimulation in advanced Parkinson’s disease. Parkinsonism & Related Disorders, 15, 728–741.
Cotzias, G. C. (1971). Levodopa in the treatment of Parkinsonism. JAMA: The Journal of the American Medical Association, 218, 1903–1908.
Cragg, S. J., & Rice, M. E. (2004). DAncing past the DAT at a DA synapse. Trends in Neurosciences, 27, 270–277.
Crossman, A. R. (1987). Primate models of dyskinesia: The experimental approach to the study of basal ganglia-related involuntary movement disorders. Neuroscience, 21, 1–40.
Da Cunha, C., Wietzikoski, E. C., Bortolanza, M., Dombrowski, P., Santos, L. M., et al. (2009). Non-motor function of the midbrain dopaminergic neurons. In G. Di Giovanni, V. Di Matteo, & E. Esposito (Eds.), Birth, life and death of dopaminergic neurons in the substantia nigra. New York: Springer-Verlag/Wien.
Dahlström, A., & Fuxe, K. (1964). Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiologica Scandinavica, 232, 1–55.
Damier, P., Hirsch, E. C., Agid, Y., & Graybiel, A. M. (1999a). The substantia nigra of the human brain. I. Nigrosomes and the nigral matrix, a compartmental organization based on calbindin D(28K) immunohistochemistry. Brain, 122, 1421–1436.
Damier, P., Hirsch, E. C., Agid, Y., & Graybiel, A. M. (1999b). The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain, 122, 1437–1448.
De la Fuente-Fernandez, R., Pal, P. K., Vingerhoets, F. J., Kishore, A., Schulzer, M., et al. (2000). Evidence for impaired presynaptic dopamine function in parkinsonian patients with motor fluctuations. Journal of Neural Transmission, 107, 49–57.
Dekundy, A., Lundblad, M., Danysz, W., & Cenci, M. A. (2007). Modulation of l-DOPA-induced abnormal involuntary movements by clinically tested compounds: Further validation of the rat dyskinesia model. Behavioural Brain Research, 179, 76–89.
Del Bel, E. A., Guimaraes, F. S., Bermudez-Echeverry, M., Gomes, M. Z., Schiaveto-de-Souza, A., et al. (2005). Role of nitric oxide on motor behavior. Cellular and Molecular Neurobiology, 25, 371–392.
Del Bel, E., Padovan-Neto, F. E., Raisman-Vozari, R., & Lazzarini, M. (2011). Role of nitric oxide in motor control: Implications for Parkinson’s disease pathophysiology and treatment. Current Pharmaceutical Design, 17, 471–488.
Del Tredici, K., Rub, U., De Vos, R. A., Bohl, J. R., & Braak, H. (2002). Where does parkinson disease pathology begin in the brain? Journal of Neuropathology and Experimental Neurology, 61, 413–426.
DeLong, M. R. (1990). Primate models of movement disorders of basal ganglia origin. Trends in Neurosciences, 13, 281–285.
Dev, K. K. (2004). Making protein interactions druggable: Targeting PDZ domains. Nature Reviews. Drug Discovery, 3, 1047–1056.
Doucet, M. V., Harkin, A., & Dev, K. K. (2012). The PSD-95/nNOS complex: New drugs for depression? Pharmacology and Therapeutics, 133, 218–229.
Dubois, B., & Pillon, B. (1997). Cognitive deficits in Parkinson’s disease. Journal of Neurology, 244, 2–8.
Eriksen, J., Jørgensen, T. N., & Gether, U. (2010). Regulation of dopamine transporter function by protein-protein interactions: new discoveries and methodological challenges. Journal of Neurochemistry, 113, 27–41.
Fahn, S. (2008). The history of dopamine and levodopa in the treatment of Parkinson's disease. Movement Disorders, 23, 497–508.
Fearnley, J. M., & Lees, A. J. (1991). Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain, 114, 2283–2301.
Ferrario, J. E., Taravini, I. R. E., Mourlevat, S., Stefano, A. V., Delfino, M. A., et al. (2004). Differential gene expresión induced by chronic levodopa treatment in the striatum of rats with lesions of the nigrostriatal system. Journal of Neurochemistry, 90, 1348–1358.
Gallagher, D. A., & Schrag, A. (2008). Impact of newer pharmacological treatments on quality of life in patients with Parkinson’s disease. CNS Drugs, 22, 563–586.
Garcia de Y'ebenes, J., Gervas, J. J., Iglesias, J., Mena, M. A., Martin del Rio, R., et al. (1982). Biochemical findings in a case of parkinsonism secondary to brain tumor. Annals of Neurology, 11, 313–316.
Garthwaite, J. (2008). Concepts of neural nitric oxide-mediated transmission. European Journal of Neuroscience, 27, 2783–2802.
Garthwaite, J., Charles, S. L., & Chess-Williams, R. (1988). Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature, 336, 385–388.
Gerfen, C.R., Engber, T.M., Mahan, L.C., Susel, Z., Chase, T.N., Monsma, F.J. Jr, Sibley, D.R. (1990). D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science, 250(4986), 1429–1432.
Gerfen, C. R., & Surmeier, D. J. (2011). Modulation of striatal projection systems by dopamine. Annual Review of Neuroscience, 34, 441–446.
Gershanik, O., Emre, M., Bernhard, G., & Sauer, D. (2003). Efficacy and safety of levodopa with entacapone in Parkinson’s disease patients suboptimally controlled with levodopa alone, in daily clinical practice: An international, multicentre, open-label study. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 27, 963–971.
Goedert, M. (1997). Familial Parkinson’s disease. The awakening of alpha-synuclein. Nature, 388, 232–233.
Goetz, C. G., Poewe, W., Rascol, O., & Sampaio, C. (2005). Evidence-based medical review update: Pharmacological and surgical treatments of Parkinson’s disease: 2001 to 2004. Movement Disorders, 20, 523–539.
Grace, A. A. (1991). Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: A hypothesis for the etiology of schizophrenia. Neuroscience, 41, 1–24.
Grace, A. A. (2002). Dopamine. In K. L. Davis, D. Charney, J. T. Coyle, & C. Nemeroff Neuropsychopharmacology: The fifth generation of progress editors. Philadelphia, PA: Lippincott, Williams, & Wilkins.
Graybiel, A. M., Aosaki, T., Flaherty, A. W., & Kimura, M. (1994). The basal ganglia and adaptive motor control. Science, 265, 1826–1831.
Greengard, P., Allen, P. B., & Nairn, A. C. (1999). Beyond the dopamine receptor: The DARPP-32/protein phosphatase-1 cascade. Neuron, 23, 435–447.
Grinberg, L. T., Rueb, U., Alho, A. T., & Heinsen, H. (2010). Brainstem pathology and non-motor symptoms in PD. Journal of Neurological Sciences, 289, 81–88.
Gumulka, S. W., Dinnendahl, V., Schönhöfer, P. S., & Stock, K. (1976). Dopaminergic stimulants and cyclic nucleotides in mouse brain. Effects of dopaminergic antagonists, olinolytics, and GABA agonists. Naunyn-Schmiedeberg’s Archives of Pharmacology, 295, 21–26.
Haber, S. N., & Gdowski, M. J. (2004). The basal ganglia. In G. Paxinos & J. K. Mai (Eds.), The human nervous system (2nd ed.). San Diego, CA: Elsevier Academic Press.
Harden, D. G., & Grace, A. A. (1995). Activation of dopamine cell firing by repeated l-DOPA administration to dopamine-depleted rats: Its potential role in mediating the therapeutic response to LDOPA treatment. Journal of Neuroscience, 15, 6157–6166.
Hassler, R. (1938). Zur die Pathologie der Paralysis Agitans and des postenzephalitischen Parkinsonismus. Journal für Psychologie und Neurologie, 48, 387–476.
Hauser, R. A. (2009). Levodopa: Past, present, and future. European Neurology, 62, 1–8.
Henry, B., Crossman, A. R., & Brotchie, J. M. (1998). Characterization of enhanced behavioral responses to l-DOPA following repeated administration in the 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Experimental Neurology, 151, 334–342.
Herve, D., & Girault, J. A. (2005). Signal transduction of dopamine receptors. In S.B. Dunnett, M. Bentivoglio, A. Björklund & Hökfelt T (Eds.), Handbook of chemical neuroanatomy. Dopamine, Vol. 21, pp. 109–151.
Hikida, T., Kimura, K., Wada, N., Funabiki, K., & Nakanishi, S. (2010). Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron, 66, 896–907.
Hitzeman, N., & Rafii, F. (2009). Dopamine agonists for early Parkinson disease. American Family Physician, 80, 28–30.
Hobbs, A. J., Higgs, A., & Moncada, S. (1999). Inhibition of nitric oxide synthase as a potential therapeutic target. Annual Review of Pharmacology and Toxicology, 39, 191–220.
Hokfelt, T., Fuxe, K., & Goldstein, M. (1973). Immunohistochemical studies on monoamine-containing cell systems. Brain Research, 62, 461–469.
Holtz, P. (1959). Role of l-DOPA decarboxylase in the biosynthesis of catecholamines in nervous tissue and the adrenal medulla. Pharmacological Reviews, 11, 317–329.
Hornykiewicz, O. (1963). Die topische Lokalisation und das Verhalten von Noradrenalin und Dopamin (3-Hydroxytyramin) in der Substantia nigra des normalen und Parkinsonkranken Menschen. Wien Klin Wschr, 75, 309–312.
Hornykiewicz, O. (2002). Dopamine miracle: From brain homogenate to dopamine replacement. Movement Disorders, 17, 501–508.
Hornykiewicz, O. (2010). A brief history of levodopa. Journal of Neurology, 257, 249–252.
Huot, P., Johnston, T. H., Koprich, J. B., Fox, S. H., & Brotchie, J. M. (2013). The pharmacology of l-DOPA-induced dyskinesia in Parkinson’s disease. Pharmacological Reviews, 65, 171–222.
Iravani, M. M., & Jenner, P. (2011). Mechanisms underlying the onset and expression of levodopa-induced dyskinesia and their pharmacological manipulation. Journal of Neural Transmission, 118, 1661–1690.
Iravani, M. M., Stockwell, K. A., Tayarani-Binazir, K., Jackson, M. J., Smith, L. A., et al. (2008). Inhibition of neuronal nitric oxide synthase as a novel target for suppression of levodopa-induced dyskinesia in primates. Neuroscience Meeting Planner Society for Neuroscience. Abstract 139.15/M6.
Ishizawa, T., Mattila, P., Davies, P., Wang, D., & Dickson, D. W. (2003). Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. Journal of Neuropathology and Experimental Neurology, 62, 389–397.
Ito, K., Nagano-Saito, A., Kato, T., et al. (2002). Striatal and extrastriatal dysfunction in Parkinson’s disease with dementia: A 6-[18F]fluoro-l-dopa PET study. Brain, 125, 1358–1365.
Itokawa, K., Ohkuma, A., Araki, N., Tamura, N., & Shimazu, K. (2006). Effect of l-DOPA on nitric oxide production in striatum of freely mobile mice. Neuroscience Letters, 402, 142–144.
Iversen, S. D., & Iversen, L. L. (2007). Dopamine: 50 years in perspective. Trends in Neurosciences, 30, 188–193.
Jakes, R., Spillantini, M. G., & Goedert, M. (1994). Identification of two distinct synucleins from human brain. FEBS Letters, 345, 27–32.
Jankovic, J., & Stacy, M. (2007). Medical management of levodopa-associated motor complications in patients with Parkinson’s disease. CNS Drugs, 21, 677–692.
Jellinger, K. A. (2003). Alpha-synuclein pathology in Parkinson’s and Alzheimer’s disease brain: Incidence and topographic distribution–a pilot study. Acta Neuropathologica, 106, 191–201.
Jenner, P. (2003). The MPTP-treated primate as a model of motor complications in PD: Primate model of motor complications. Neurology, 61, 4–11.
Jenner, P. (2004). Preclinical evidence for neuroprotection with monoamine oxidase-B inhibitors in Parkinson’s disease. Neurology, 63, 13–22.
Jenner, P. (2008). Molecular mechanisms of l-DOPA-induced dyskinesia. Nature Reviews Neuroscience, 9, 665–677.
Kemp, J. M., & Powell, T. P. (1971). The connexions of the striatum and globus pallidus: Synthesis and speculation. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 262, 441–457.
Kish, S. J., Shannak, K., & Hornykiewicz, O. (1988). Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. The New England Journal of Medicine, 318, 876–880.
Klivenyi, P., & Vecsei, L. (2010). Novel therapeutic strategies in Parkinson’s disease. European Journal of Clinical Pharmacology, 66, 119–125.
Kravitz, A. V., Freeze, B. S., Parker, P. R., Kay, K., Thwin, M. T., et al. (2010). Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature, 466, 622–626.
Krimer, L. S., Jakab, R. L., & Goldman-Rakic, P. S. (1997). Quantitative three dimensional analysis of the catecholaminergic innervation of identified neurons in the macaque prefrontal cortex. Journal of Neuroscience, 17, 7450–7461.
Kurlan, R. (2005). “Levodopa phobia”: A new iatrogenic cause of disability in Parkinson disease. Neurology, 64, 923–924.
Langley, K. C., Bergson, C., Greengard, P., & Ouimet, C. C. (1997). Co-localization of the D1 dopamine receptor in a subset of DARPP-32-containing neurons in rat caudate-putamen. Neuroscience, 78, 977–983.
Lau, A., & Tymianski, M. (2010). Glutamate receptors, neurotoxicity and neurodegeneration. Pflügers Archiv, 460, 525–542.
Lees, A. J., Selikhova, M., Andrade, L. A., & Duyckaerts, C. (2008). The black stuff and Konstantin Nikolaevich Tretiakoff. Movement Disorders, 23, 777–783.
Leiper, J., & Vallance, P. (1999). Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovascular Research, 43, 542–548.
Ljungdahl, A., Hokfelt, T., Goldstein, M., & Park, D. (1975). Retrograde peroxidase tracing of neurons combined with transmitter histochemistry. Brain Research, 84, 313–319.
Lundblad, M., Andersson, M., Winkler, C., Kirik, D., Wierup, N., et al. (2002). Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. European Journal of Neuroscience, 15, 120–132.
Ma, S. Y., Rinne, J. O., Collan, Y., Roytta, M., & Rinne, U. K. (1996). A quantitative morphometrical study of neuron degeneration in the substantia nigra in Parkinson’s disease. Journal of Neurological Sciences, 140, 40–45.
MacAllister, R. J., Parry, H., Kimoto, M., Ogawa, T., Russell, R. J., Hodson, H., Whitley, G. S., & Vallance, P. (1996). Regulation of nitric oxide synthesis by dimethylarginine dimethylaminohydrolase. British Journal of Pharmacology, 119, 1533–1540.
Matsuda, W., Furuta, T., Nakamura, K., Hioki, H., Fujiyama, F., et al. (2009). Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. Journal of Neuroscience, 29, 444–453.
Mayeux, R. (2003). Epidemiology of neurodegeneration. Annual Review of Neuroscience, 26, 81–104.
Mink, J. W. (2003). The basal ganglia and involuntary movements: Impaired inhibition of competing motor patterns. Archives of Neurology, 60, 1365–1368.
Mitkovski, M., Padovan-Neto, F. E., Raisman-Vozari, R., Ginestet, L., da-Silva, C. A., et al. (2012). Investigations into potential extrasynaptic communication between the dopaminergic and nitrergic systems. Frontiers in Physiology, 3, 372.
Molinoff, P. B., & Axelrod, J. (1971). Biochemistry of catecholamines. Annual Review of Biochemistry, 40, 465–500.
Mouradian, M. M., Heuser, I. J., Baronti, F., & Chase, T. N. (1990). Modification of central dopaminergic mechanisms by continuous levodopa therapy for advanced Parkinson’s disease. Annals of Neurology, 27, 18–23.
Muller, T., & Kuhn, W. (2006). Tolcapone decreases plasma levels of S-adenosyl-l-homocysteine and homocysteine in treated Parkinson’s disease patients. European Journal of Clinical Pharmacology, 62, 447–450.
Muller, T., & Muhlack, S. (2009). Peripheral COMT inhibition prevents levodopa associated homocysteine increase. Journal of Neural Transmission, 116, 1253–1256.
Murer, M. G., Dziewczapolski, G., Menalled, L., Garcia, M., Agid, Y., Gershanik, O. S., et al. (1998). Chronic levodopa is not toxic for remaining dopaminergic neurons, but instead promotes their recovery, in rats with moderate nigrostriatal lesions. Annals of Neurology, 43, 561–575.
Nestler, E. J., Hyman, S. E., & Malenka, R. C. (2009). Molecular neuropharmacology: A foundation for clinical neuroscience. New York: McGraw-Hill Medical.
Novaretti, N., Padovan-Neto, F. E., Tumas, V., da-Silva, C. A., & Del Bel, E. A. (2010). Lack of tolerance for the anti-dyskinetic effects of 7-nitroindazole, a neuronal nitric oxide synthase inhibitor, in rats. Brazilian Journal of Medical and Biological Research, 43(11), 1047–1053.
Nussbaum, R. L., & Ellis, C. E. (2003). Alzheimer’s disease and Parkinson’s disease. The New England Journal of Medicine, 348, 1356–1364.
Nutt, J. G. (1987). On-off phenomenon: Relation to levodopa pharmacokinetics and pharmacodynamics. Annals of Neurology, 22, 535–540.
Nutt, J. G., Gancher, S. T., & Woodward, W. R. (1989). Motor fluctuations in Parkinson’s disease. Annals of Neurology, 25, 633–634.
Obeso, J. A., Rodriguez-Oroz, M. C., Chana, P., Lera, G., Rodriguez, M., et al. (2000). The evolution and origin of motor complications in Parkinson’s disease. Neurology 55, S13–20; discussion S1–3.
Olanow, C. W., & Tatton, W. G. (1999). Etiology and pathogenesis of Parkinson’s disease. Annual Review of Neuroscience, 22, 123–144.
Olanow, C. W., Agid, Y., Mizuno, Y., Albanese, A., Bonuccelli, U., et al. (2004). Levodopa in the treatment of Parkinson’s disease: Current controversies. Movement Disorders, 19, 997–1005.
Olanow, C. W., Rascol, O., Hauser, R., Feigin, P. D., Jankovic, J., ADAGIO Study Investigators, et al. (2009a). A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. The New England Journal of Medicine, 361, 1268–1278.
Olanow, C. W., Stern, M. B., & Sethi, K. (2009b). The scientific and clinical basis for the treatment of Parkinson disease. Neurology, 72, S1–S136.
Orta Daniel, S. J., & Ulises, R. O. (2008). Stroke of the substance nigra and parkinsonism as first manifestation of systemic lupus erythematosus. Parkinsonism & Related Disorders, 14, 367–369.
Padovan-Neto, F. E., Echeverry, M. B., Tumas, V., & Del-Bel, E. A. (2009). Nitric oxide synthase inhibition attenuates l-DOPA-induced dyskinesias in a rodent model of Parkinson’s disease. Neuroscience, 159, 927–935.
Padovan-Neto, F. E., Echeverry, M. B. D., Chiavegatto, S., & Del Bel, E. (2011). Nitric oxide synthase inhibitor improves de novo and long-term l-DOPA-induced dyskinesia in hemiparkinsonian rats. Frontiers in Systems Neuroscience, 5, 40.
Parent, M., & Parent, A. (2010). Substantia nigra and Parkinson’s disease: A brief history of their long and intimate relationship. Canadian Journal of Neurological Sciences, 37, 313–319.
Parkinson, J. (2002). An essay on the shaking palsy. 1817. The Journal of Neuropsychiatry and Clinical Neurosciences, 14, 223–236.
Parkkinen, L., Soininen, H., & Alafuzoff, I. (2003). Regional distribution of alpha-synuclein pathology in unimpaired aging and Alzheimer disease. Journal of Neuropathology and Experimental Neurology, 62, 363–367.
Pate, B. D., Kawamata, T., Yamada, T., McGeer, E. G., Hewitt, K. A., et al. (1993). Correlation of striatal fluorodopa uptake in the MPTP monkey with dopaminergic indices. Annals of Neurology, 34, 331–338.
Petros, A., Lamb, G., Leone, A., Moncada, S., Bennett, D., et al. (1994). Effects of a nitric oxide synthase inhibitor in humans with septic shock. Cardiovascular Research, 28(1), 34–39.
Pierucci, M., Galati, S., Valentino, M., Di Matteo, V., Benigno, A., et al. (2011). Nitric oxide modulation of the basal ganglia circuitry: Therapeutic implication for Parkinson’s disease and other motor disorders. CNS & Neurological Disorders Drug Targets, 10(7), 777–791.
Polymeropoulos, M. H., Lavedan, C., Leroy, E., Ide, S. E., Dehejia, A., et al. (1997). Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science, 276, 2045–2047.
Rees, D. D. (1995). Role of nitric oxide in the vascular dysfunction of septic shock. Biochemical Society Transactions, 23(4), 1025–1029.
Rothwell, J. C. (2011). The motor functions of the basal ganglia. Journal of Integrative Neuroscience, 10, 303–315.
Sanchez, J. J., Abreu, P., & Gonzalez, M. C. (2002). Sodium nitroprusside stimulates l-DOPA release from striatal tissue through nitric oxide and cGMP. European Journal of Pharmacology, 438(1–2), 79–83.
Santini, E., Valjent, E., & Fisone, G. (2008). Parkinson’s disease: Levodopa-induced dyskinesia and signal transduction. FEBS Journal, 275(7), 1392–1399.
Schapira, A. H., Emre, M., Jenner, P., & Poewe, W. (2009). Levodopa in the treatment of Parkinson’s disease. European Journal of Neurology, 16, 982–989.
Schrag, A. (2005). Entacapone in the treatment of Parkinson’s disease. Lancet Neurology, 4, 366–370.
Singh, N., Pillay, V., & Choonara, Y. E. (2007). Advances in the treatment of Parkinson’s disease. Progress in Neurobiology, 81, 29–44.
Smith, Y., Bennett, B. D., Bolam, J. P., Parent, A., & Sadikot, A. F. (1994). Synaptic relationships between dopaminergic afferents and cortical or thalamic input in the sensorimotor territory of the striatum in monkey. The Journal of Comparative Neurology, 344, 1–19.
Snow, B. J., Tooyama, I., McGeer, E. G., et al. (1993). Human positron emission tomographic [18F]fluorodopa studies correlate with dopamine cell counts and levels. Annals of Neurology, 34, 324–330.
Spillantini, M. G., Schmidt, M. L., Lee, V. M., Trojanowski, J. Q., Jakes, R., et al. (1997). Alpha-synuclein in Lewy bodies. Nature, 388, 839–840.
Steiger, M., Jost, W., Grandas, F., & Van Camp, G. (2009). Risk of valvular heart disease associated with the use of dopamine agonists in Parkinson’s disease: A systematic review. Journal of Neural Transmission, 116, 179–191.
Steinert, J. R., Chernova, T., & Forsythe, I. D. (2010). Nitric oxide signaling in brain function, dysfunction, and dementia. The Neuroscientist, 16(4), 435–452.
Stocchi, F. (2009). The therapeutic concept of continuous dopaminergic stimulation (CDS) in the treatment of Parkinson’s disease. Parkinsonism & Related Disorders, 15(Suppl 3), S68–S71.
Stoessl, A. J., Martin, W. W., McKeown, M. J., & Sossi, V. (2011). Advances in imaging in Parkinson’s disease. Lancet Neurology, 10, 987–1001.
Sun, H. S., Doucette, T. A., Liu, Y., Fang, Y., Teves, L., et al. (2008). Effectiveness of PSD95 inhibitors in permanent and transient focal ischemia in the rat. Stroke, 39, 2544–2553.
Svenningsson, P., Nishi, A., Fisone, G., Girault, J. A., Nairn, A. C., et al. (2004). DARPP-32: An integrator of neurotransmission. Annual Review of Pharmacology and Toxicology, 44, 269–296.
Takuma, K., Tanaka, T., Takahashi, T., Hiramatsu, N., Ota, Y., Ago, Y., Matsuda, T. (2012). Neuronal nitric oxide synthase inhibition attenuates the development of L-DOPA-induced dyskinesia in hemi-Parkinsonian rats. European Journal of Pharmacology, 683, 166–173.
Tepper, J. M., Wilson, C. J., & Koós, T. (2008). Feedforward and feedback inhibition in neostriatal GABAergic spiny neurons. Brain Research Reviews, 58, 272–281.
Torstenson, R., Hartvig, P., Langstrom, B., Westerberg, G., & Tedroff, J. (1997). Differential effects of levodopa on dopaminergic function in early and advanced Parkinson’s disease. Annals of Neurology, 41, 334–340.
Turjanski, N., Lees, A. J., & Brooks, D. J. (1997). In vivo studies on striatal dopamine D1 and D2 site binding in l-dopa-treated Parkinson’s disease patients with and without dyskinesias. Neurology, 49, 717–723.
Vallance, P. (2003). Nitric oxide: Therapeutic opportunities. Fundamental and Clinical Pharmacology, 17(1), 1–10.
Vallance, P., & Leiper, J. (2002). Blocking NO synthesis: How, where and why? Nature Reviews. Drug Discovery, 1, 939–950.
Vingerhoets, F. J., Schulzer, M., Calne, D. B., & Snow, B. J. (1997). Which clinical sign of Parkinson’s disease best reflects the nigrostriatal lesion? Annals of Neurology, 41, 58–64.
Whone, A. L., Moore, R. Y., Piccini, P. P., & Brooks, D. J. (2003). Plasticity of the nigropallidal pathway in Parkinson’s disease. Annals of Neurology, 53, 206–213.
Winkler, C., Kirik, D., Bjorklund, A., & Cenci, M. A. (2002). l-DOPA-induced dyskinesia in the intrastriatal 6-hydroxydopamine model of Parkinson’s disease: Relation to motor and cellular parameters of nigrostriatal function. Neurobiology of Disease, 10, 165–186.
Wong, K. S., Lu, C. S., Shan, D. E., Yang, C. C., Tsoi, T. H., & Mok, V. (2003). Efficacy, safety, and tolerability of pramipexole in untreated and levodopa-treated patients with Parkinson’s disease. Journal of Neurological Sciences, 216, 81–87.
Wu, R. M., Chen, R. C., & Chiueh, C. C. (2000). Effect of MAO-B inhibitors on MPP+ toxicity in Vivo. Annals of the New York Academy of Sciences, 899, 255–261.
Yacoubian, T. A., & Standaert, D. G. (2009). Targets for neuroprotection in Parkinson’s disease. Biochimica et Biophysica Acta, 1792, 676–687.
Yan, Z., & Surmeier, D. J. (1996). Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-delimited, G-protein pathway. Journal of Neuroscience, 16, 2592–2604.
Yuste, J. E., Bermúdez, M., Bernal, F. R., Barcia, C., Martin, J., et al. (2011). NOS inhibitors improve l-DOPA-induced dyskinesias in experimental models of Parkinsonism. Movement Disorders, 26(Suppl 2), S257–S258.
Zhou, L., Li, F., Xu, H. B., Luo, C. X., Wu, H. Y., et al. (2010). Treatment of cerebral ischemia by disrupting ischemia-induced interaction of nNOS with PSD-95. Nature Medicine, 16, 1439–1443.
Acknowledgments
This work was partially supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico (CNPQ), Coordenadoria de Aperfeiçoamento de Pessoal (CAPES), Fundação de Apoio à Pesquisa Científica e Tecnológica do Estado de Santa Catarina (FAPESC), CAPES-COFECUB (France/Brazil; 681/2010), FAPESP/INSERM (2008/55092-9), and CAPES/CNPq/FAPs – Linha Pesquisador Visitante Especial/Programa Especial de Cooperacao Internacional/PECI (402658/2012-4). The other authors have no financial or personal conflict of interest related to this study. The authors are grateful to Majid Amar for the preparation of Fig. 2 and to Célia Ap. da Silva and Laure Ginestet for the helpful technical support. We would like to thank MSc Fernando Padovan Neto, MSc Danielle Oliveira Tavares, PhD Roberta Cavalcanti Kwiatkoski, and PhD Nádia Rúbia Ferreira.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this entry
Cite this entry
Prediger, R.D., Bortolanza, M., de Castro Issy, A.C., dos Santos, B.L., Del Bel, E., Raisman-Vozari, R. (2014). Dopaminergic Neurons in Parkinson’s Disease. In: Kostrzewa, R. (eds) Handbook of Neurotoxicity. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-5836-4_7
Download citation
DOI: https://doi.org/10.1007/978-1-4614-5836-4_7
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-5835-7
Online ISBN: 978-1-4614-5836-4
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences