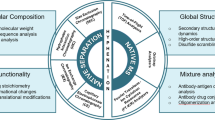
Among all analytical methods used to characterize monoclonal antibodies (mAbs), mass spectrometry plays an increasingly important role for both global and fine structural characterization of therapeutic candidates. In this chapter we discuss and provide detailed protocols for intact antibody and light and heavy chain primary structure characterisation by LC-ESI-TOF, glycosylation and production-system fingerprinting, structural isotyping and disulfide connection determination (humanised IgG1 vs IgG4) and finally non-stable “hot spots” mapping by Mass Spectrometry (de-amidation by MALDI-TOF and LC-IT MS/MS).
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Atmanène C, Wagner-Rousset E, Malissard M, Chol B, Robert A, Corvaïa N, Van Dorsselaer A, Beck A, Sanglier-Cianferani S. (2009) Extending Mass Spectrometry contribution to therapeutic monoclonal antibody lead selection and development: characterization of antigen/antibody immune complexes using non-covalent ESI-MS. Analytical Chemistry. In Press
Banks DD, Gadgil HS, Pipes GD, Bondarenko PV, Hobbs V, Scavezze JL, Kim J, Jiang XR, Mukku V, Dillon TM (2008) Removal of cysteinylation from an unpaired sulfhydryl in the variable region of a recombinant monoclonal IgG1 antibody improves homogeneity, stability, and biological activity. J Pharm Sci 97:775–790
Beck A, Bussat MC, Zorn N, Robillard V, Klinguer-Hamour C, Chenu S, Goetsch L, Corvaia N, Van Dorsselaer A, Haeuw JF (2005) Characterization by liquid chromatography combined with mass spectrometry of monoclonal anti-IGF-1 receptor antibodies produced in CHO and NS0 cells. J Chromatogr B Analyt Technol Biomed Life Sci 819:203–218
Beck A, Klinguer-Hamour C, Bussat MC, Champion T, Haeuw JF, Goetsch L, Wurch T, Sugawara M, Milon A, Van Dorsselaer A, Nguyen T, Corvaia N (2007) Peptides as tools and drugs for immunotherapies. J Pept Sci 13:588–602
Beck A, Wagner-Rousset E, Bussat MC, Lokteff M, Klinguer-Hamour C, Haeuw JF, Goetsch L, Wurch T, Van Dorsselaer A, Corvaia N (2008a) Trends in Glycosylation, Glycoanalysis and Glycoengineering of Therapeutic Antibodies and Fc-Fusion Proteins. Curr Pharm Biotechnol 9:482–501
Beck A, Wagner-Rousset E, Goetsch L, Corvaïa N (2008b) Therapeutic antibodies: structure assessment by mass spectrometry from screening to clinical batches. Screen Trends Drug Discov 9:18–20
Beck A, Wurch T, Corvaïa N (2008c) Editorial: therapeutic antibodies and derivatives: from the bench to the clinic. Curr Pharma Biotechnol 9:421–422
Chelius D, Rehder DS, Bondarenko PV (2005) Identification and characterization of deamidation sites in the conserved regions of human immunoglobulin gamma antibodies. Anal Chem 77:6004–6011
Chelius D, Huff Wimer ME, Bondarenko PV (2006a) Reversed-phase liquid chromatography in-line with negative ionization electrospray mass spectrometry for the characterization of the disulfide-linkages of an immunoglobulin gamma antibody. J Am Soc Mass Spectrom 17:1590–1598
Chelius D, Jing K, Lueras A, Rehder DS, Dillon TM, Vizel A, Rajan RS, Li T, Treuheit MJ, Bondarenko PV (2006b) Formation of pyroglutamic acid from N-terminal glutamic acid in immunoglobulin gamma antibodies. Anal Chem 78:2370–2376
Chu GC, Chelius D, Xiao G, Khor HK, Coulibaly S, Bondarenko PV (2007) Accumulation of succinimide in a recombinant monoclonal antibody in mildly acidic buffers under elevated temperatures. Pharm Res 24:1145–1156
Cohen SL, Price C, Vlasak J (2007) Beta-elimination and peptide bond hydrolysis: two distinct mechanisms of human IgG1 hinge fragmentation upon storage. J Am Chem Soc 129:6976–6977
Cordoba AJ, Shyong BJ, Breen D, Harris RJ (2005) Non-enzymatic hinge region fragmentation of antibodies in solution. J Chromatogr B Analyt Technol Biomed Life Sci 818:115–121
Cox KM, Sterling JD, Regan JT, Gasdaska JR, Frantz KK, Peele CG, Black A, Passmore D, Moldovan-Loomis C, Srinivasan M, Cuison S, Cardarelli PM, Dickey LF (2006) Glycan optimization of a human monoclonal antibody in the aquatic plant Lemna minor. Nat Biotechnol 24:1591–1597
Dick LW Jr, Kim C, Qiu D, Cheng KC (2007) Determination of the origin of the N-terminal pyro-glutamate variation in monoclonal antibodies using model peptides. Biotechnol Bioeng 97:544–553
Dillon TM, Bondarenko PV, Speed RM (2004) Development of an analytical reversed-phase high-performance liquid chromatography-electrospray ionization mass spectrometry method for characterization of recombinant antibodies. J Chromatogr A 1053:299–305
Dillon TM, Bondarenko PV, Rehder DS, Pipes GD, Kleemann GR, Ricci MS (2006) Optimization of a reversed-phase high-performance liquid chromatography/mass spectrometry method for characterizing recombinant antibody heterogeneity and stability. J Chromatogr A 1120:112–120
Gadgil HS, Bondarenko PV, Pipes GD, Dillon TM, Banks D, Abel J, Kleemann GR, Treuheit MJ (2006a) Identification of cysteinylation of a free cysteine in the Fab region of a recombinant monoclonal IgG1 antibody using Lys-C limited proteolysis coupled with LC/MS analysis. Anal Biochem 355:165–174
Gadgil HS, Pipes GD, Dillon TM, Treuheit MJ, Bondarenko PV (2006b) Improving mass accuracy of high performance liquid chromatography/electrospray ionization time-of-flight mass spectrometry of intact antibodies. J Am Soc Mass Spectrom 17:867–872
Gadgil HS, Bondarenko PV, Treuheit MJ, Ren D (2007) Screening and sequencing of glycated proteins by neutral loss scan LC/MS/MS method. Anal Chem 79:5991–5999
Huang LH, Biolsi S, Bales KR, Kuchibhotla U (2006) Impact of variable domain glycosylation on antibody clearance: An LC/MS characterization. Anal Biochem 349:197–207
Johnson KA, Paisley-Flango K, Tangarone BS, Porter TJ, Rouse JC (2007) Cation exchange-HPLC and mass spectrometry reveal C-terminal amidation of an IgG1 heavy chain. Anal Biochem 360:75–83
Le JC, Bondarenko PV (2005) Trap for MAbs: characterization of intact monoclonal antibodies using reversed-phase HPLC on-line with ion-trap mass spectrometry. J Am Soc Mass Spectrom 16:307–311
Liu H, Gaza-Bulseco G, Sun J (2006) Characterization of the stability of a fully human monoclonal IgG after prolonged incubation at elevated temperature. J Chromatogr B Analyt Technol Biomed Life Sci 837:35–43
Liu H, Gaza-Bulseco G, Faldu D, Chumsae C, Sun J (2008a) Heterogeneity of monoclonal antibodies. J Pharm Sci 97:2426–2447
Liu H, Gaza-Bulseco G, Xiang T, Chumsae C (2008b) Structural effect of deglycosylation and methionine oxidation on a recombinant monoclonal antibody. Mol Immunol 45:701–708
Lyubarskaya Y, Houde D, Woodard J, Murphy D, Mhatre R (2006) Analysis of recombinant monoclonal antibody isoforms by electrospray ionization mass spectrometry as a strategy for streamlining characterization of recombinant monoclonal antibody charge heterogeneity. Anal Biochem 348:24–39
Olivova P, Chen W, Chakraborty AB, Gebler JC (2008) Determination of N-glycosylation sites and site heterogeneity in a monoclonal antibody by electrospray quadrupole ion-mobility time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 22:29–40
Qian J, Liu T, Yang L, Daus A, Crowley R, Zhou Q (2007) Structural characterization of N-linked oligosaccharides on monoclonal antibody cetuximab by the combination of orthogonal matrix-assisted laser desorption/ionization hybrid quadrupole-quadrupole time-of-flight tandem mass spectrometry and sequential enzymatic digestion. Anal Biochem 364:8–18
Raju TS, Scallon BJ (2006) Glycosylation in the Fc domain of IgG increases resistance to proteolytic cleavage by papain. Biochem Biophys Res Commun 341:797–803
Rehder DS, Dillon TM, Pipes GD, Bondarenko PV (2006) Reversed-phase liquid chromatography/mass spectrometry analysis of reduced monoclonal antibodies in pharmaceutics. J Chromatogr A 1102:164–175
Siemiatkoski J, Lyubarskaya Y, Houde D, Tep S, Mhatre R (2006) A comparison of three techniques for quantitative carbohydrate analysis used in characterization of therapeutic antibodies. Carbohydr Res 341:410–419
Srebalus Barnes CA, Lim A (2007) Applications of mass spectrometry for the structural characterization of recombinant protein pharmaceuticals. Mass Spectrom Rev 26:370–388
Terashima I, Koga A, Nagai H (2007) Identification of deamidation and isomerization sites on pharmaceutical recombinant antibody using H(2)(18)O. Anal Biochem 368:49–60
Tous GI, Wei ZP, Feng JH, Bilbulian S, Bowen S, Smith J, Strouse R, McGeehan P, Casas-Finet J, Schenerman MA (2005) Characterization of a novel modification to monoclonal antibodies: Thioether cross-link of heavy and light chains. Anal Chem 77:2675–2682
Vlasak J, Ionescu R (2008) Heterogeneity of Monoclonal Antibodies relvealed by charge-sensitive methods. Curr Pharm Biotechnol 9:468–481
Wagner-Rousset E, Bednarczyk A, Bussat MC, Colas O, Corvaia N, Schaeffer C, Van Dorsselaer A, Beck A (2008) The way forward, enhanced characterization of therapeutic antibody glycosylation: comparison of three level mass spectrometry-based strategies. J Chromatogr B Analyt Technol Biomed Life Sci 872:23–37
Wang L, Amphlett G, Lambert JM, Blattler W, Zhang W (2005) Structural characterization of a recombinant monoclonal antibody by electrospray time-of-flight mass spectrometry. Pharm Res 22:1338–1349
Wei Z, Feng J, Lin HY, Mullapudi S, Bishop E, Tous GI, Casas-Finet J, Hakki F, Strouse R, Schenerman MA (2007) Identification of a single tryptophan residue as critical for binding activity in a humanized monoclonal antibody against respiratory syncytial virus. Anal Chem 79:2797–2805
Xiao G, Bondarenko PV, Jacob J, Chu GC, Chelius D (2007) 18O labeling method for identification and quantification of succinimide in proteins. Anal Chem 79:2714–2721
Yan B, Valliere-Douglass J, Brady L, Steen S, Han M, Pace D, Elliott S, Yates Z, Han Y, Balland A, Wang W, Pettit D (2007) Analysis of posttranslational modifications in recombinant monoclonal antibody IgG1 by reversed-phase liquid chromatography/mass spectrometry. J Chromatogr A 1164:153–161
Yang J, Wang S, Liu J, Raghani A (2007) Determination of tryptophan oxidation of monoclonal antibody by reversed phase high performance liquid chromatography. J Chromatogr A 1156:174–182
Ying H, Liu H (2007) Identification of an alternative signal peptide cleavage site of mouse monoclonal antibodies by mass spectrometry. Immunol Lett 111:66–68
Yu L, Vizel A, Huff MB, Young M, Remmele RL Jr, He B (2006) Investigation of N-terminal glutamate cyclization of recombinant monoclonal antibody in formulation development. J Pharm Biomed Anal 42:455–463
Zhang Z, Shah B (2007) Characterization of variable regions of monoclonal antibodies by top-down mass spectrometry. Anal Chem 79:5723–5729
Zhang W, Marzilli LA, Rouse JC, Czupryn MJ (2002) Complete disulfide bond assignment of a recombinant immunoglobulin G4 monoclonal antibody. Anal Biochem 311:1–9
Zhang Z, Pan H, Chen X (2009) Mass spectrometry for structural characterization of therapeutic antibodies. Mass Spectrom Rev 28:147–176
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this protocol
Cite this protocol
Janin-Bussat, MC. et al. (2010). Structural Characterization of Antibodies by Mass Spectrometry. In: Kontermann, R., Dübel, S. (eds) Antibody Engineering. Springer Protocols Handbooks. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-01144-3_39
Download citation
DOI: https://doi.org/10.1007/978-3-642-01144-3_39
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-01143-6
Online ISBN: 978-3-642-01144-3
eBook Packages: Springer Protocols