Abstract
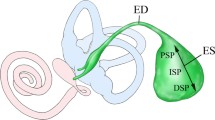
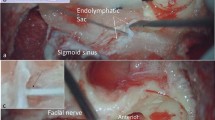
Laser-capture microdissection (LCM) is an excellent tool to selectively obtain target tissue or cells. The endolymphatic sac (ES) is part of the inner ear, and a large part of the ES is located in the temporal bone. The rat ES is conventionally harvested using stereomicroscopy. In this method, contamination is unavoidable because of its size and location; therefore, additional checks, such as in situ hybridization, are necessary to confirm the cellular localization, and quantitative analysis is difficult in the ES. We have shown a selective epithelial tissue method using LCM to obtain RNA without contamination from ES epithelial tissue.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Dahlmann A., von During M. (1995) The endolymphatic duct and sac of the rat: A histological, ultrastructural, and immunocytochemical investigation, Cell Tissue Res. 282: 277–289.
Lo W. W., Daniels D. L., Chakeres D. W et al (1997) The endolymphatic duct and sac, AJNR Am. J. Neuroradiol. 18: 881–887.
R.S. Kimura. (1967) Experimental blockage of the endolymphatic duct and sac and its effect on the inner ear of the guinea pig. A study on endolymphatic hydrops, Ann. Otol. Rhinol. Laryngol. 76: 664–687.
R.S. Kimura. (1982) Animal models of endolymphatic hydrops, Am. J. Otolaryngol. 3: 447–451.
Miyashita T., Tatsumi H., Hayakawa K. et al (2007) Large Na(+) influx and high Na(+), K (+)-ATPase activity in mitochondria-rich epithelial cells of the inner ear endolymphatic sac. Pflugers Arch. 453: 905–13.
Furuta H., Sato C., Kawaguchi Y et al (1999) Expression of mRNAs encoding hormone receptors in the endolymphatic sac of the rat, Acta Otolaryngol. 119: 53–57.
Sawada S., Takeda T., Kitano H et al (2002) Aquaporin-2 regulation by vasopressin in the rat inner ear, Neuroreport 13: 1127–1129.
Murray G. I. (2007) An overview of laser microdissection technologies, acta histochmica. 109: 171–176.
Akiyama K., Miyashita T., Mori T et al (2008) A new approach for selective rat endolymphatic sac epithelium collection to obtain pure specific RNA, Biochem. Biophys. Res. Commun. 376: 611–614.
Yoon K. G., Rutledge S. J., Buenaga R. F et al (1988) Characterization of the rat osteocalcin gene: Stimu-lation of promoter activity by 1,25-dihydroxyvitamin D3, Biochemistry 27: 8521–8526.
Nishida W., Kitami Y., Hiwada H (1993) cDNA cloning and mRNA expression of calponin and SM22 in rat aorta smooth muscle cells, Gene 130: 297–302.
Gamba G., Miyanoshita A., Lombardi M et al (1994) Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney, J. Biol. Chem. 269: 17713–17722.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research (#13671783 and #16591711 to N.M., #16791003 and #791208 to T.M.).
An outline of this work was published in ref. 9 and is reproduced with permission from Elsevier Science.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media, LLC
About this protocol
Cite this protocol
Akiyama, K., Miyashita, T., Matsubara, A., Mori, N. (2011). Specific RNA Collection from the Rat Endolymphatic Sac by Laser-Capture Microdissection (LCM): LCM of a Very Small Organ Surrounded by Bony Tissues. In: Murray, G. (eds) Laser Capture Microdissection. Methods in Molecular Biology, vol 755. Humana Press. https://doi.org/10.1007/978-1-61779-163-5_37
Download citation
DOI: https://doi.org/10.1007/978-1-61779-163-5_37
Published:
Publisher Name: Humana Press
Print ISBN: 978-1-61779-162-8
Online ISBN: 978-1-61779-163-5
eBook Packages: Springer Protocols