Abstract
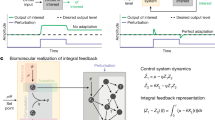
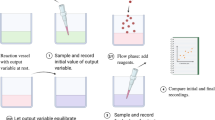
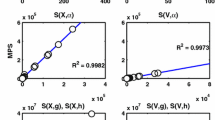
Organisms have the ability to counteract environmental perturbations and keep certain components within a cell homeostatically regulated. Closely related to homeostasis is the behavior of perfect adaptation where an organism responds to a step-wise perturbation by regulating some of its components, after a transient period, to their original pre-perturbation values. A particular interesting type of model relates to the so-called robust behavior where the homeostatic or perfect adaptation property is independent of the magnitude of the applied step-wise perturbation. It has been shown that this type of behavior is related to the control-theoretic concept of integral feedback (or integral control). Using downloadable MATLAB examples, we demonstrate how robust perfect adaptation sites can be identified in reaction kinetic networks by linearizing the system, applying the Laplace transform and inspecting the transfer function. We also show how the homeostatic set point in perfect adaptation is related to the presence of zero-order fluxes.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Grylls, F. S., and J. S. Harrison, 1956. Adaptation of yeast to maltose fermentation. Nature 178, 1471–2.
Berg, H. C., and P. M. Tedesco, 1975. Transient response to chemotactic stimuli in Escherichia coli. Proc Natl Acad Sci U S A 72, 3235–9.
Alon, U., M. G. Surette, N. Barkai, and S. Leibler, 1999. Robustness in bacterial chemotaxis. Nature 397, 168–71.
Bray, D., 2002. Bacterial chemotaxis and the question of gain. Proc Natl Acad Sci U S A 99, 7–9.
Mello, B. A., and Y. Tu, 2003. Perfect and near-perfect adaptation in a model of bacterial chemotaxis. Biophys J 84, 2943–56.
Berg, H. C., 2004. E. coli in Motion. Springer-Verlag, New York.
Mello, B. A., and Y. Tu, 2007. Effects of adaptation in maintaining high sensitivity over a wide range of backgrounds for Escherichia coli chemotaxis. Biophys J 92, 2329–37.
Hansen, C. H., R. G. Endres, and N. S. Wingreen, 2008. Chemotaxis in Escherichia coli : A Molecular Model for Robust Precise Adaptation. PLoS Computational Biology 4, 0014–0027.
Levchenko, A., and P. A. Iglesias, 2002. Models of eukaryotic gradient sensing: application to chemotaxis of amoebae and neutrophils. Biophys J 82, 50–63.
Ratliff, F., H. K. Hartline, and W. H. Miller, 1963. Spatial and temporal aspects of retinal inhibitory interaction. J Opt Soc Am 53, 110–20.
He, Q., and Y. Liu, 2005. Molecular mechanism of light responses in Neurospora: from light-induced transcription to photoadaptation. Genes Dev 19, 2888–99.
Walters, R. G., 2005. Towards an understanding of photosynthetic acclimation. J Exp Bot 56, 435–47.
Arthur, H., and K. Watson, 1976. Thermal adaptation in yeast: growth temperatures, membrane lipid, and cytochrome composition of psychrophilic, mesophilic, and thermophilic yeasts. J Bacteriol 128, 56–68.
Margesin, R., 2009. Effect of temperature on growth parameters of psychrophilic bacteria and yeasts. Extremophiles 13, 257–62.
Asthagiri, A. R., and D. A. Lauffenburger, 2000. Bioengineering models of cell signaling. Annu Rev Biomed Eng 2, 31–53.
Koshland, J., D. E., A. Goldbeter, and J. B. Stock, 1982. Amplification and adaptation in regulatory and sensory systems. Science 217, 220–5.
Muzzey, D., C. A. Gomez-Uribe, J. T. Mettetal, and A. van Oudenaarden, 2009. A systems-level analysis of perfect adaptation in yeast osmoregulation. Cell 138, 160–71.
Asthagiri, A. R., C. M. Nelson, A. F. Horwitz, and D. A. Lauffenburger, 1999. Quantitative relationship among integrin-ligand binding,adhesion, and signaling via focal adhesion kinase and extracellularsignal-regulated kinase 2. J Biol Chem 274, 27119–27.
Hao, N., M. Behar, T. C. Elston, and H. G. Dohlman, 2007. Systems biology analysis of G protein and MAP kinase signaling in yeast. Oncogene 26, 3254–66.
Mettetal, J. T., D. Muzzey, C. Gomez-Uribe, and A. van Oudenaarden, 2008. The Frequency Dependence of Osmo-Adaptation in Saccha- romyces cerevisiae. Science 319, 482–4.
Hong, C. I., E. D. Conrad, and J. J. Tyson, 2007. A proposal for robust temperature compensation of circadian rhythms. Proc Natl Acad Sci U S A 104, 1195–200.
Yi, T. M., Y. Huang, M. I. Simon, and J. Doyle, 2000. Robust perfect adaptation in bacterial chemotaxis through integral feedback control. Proc Natl Acad Sci U S A 97, 4649–53.
Wilkie, J., M. Johnson, and K. Reza, 2002. Control Engineering. An Introductory Course. Palgrave, New York.
Drengstig, T., H. R. Ueda, and P. Ruoff, 2008. Predicting Perfect Adaptation Motifs in Reaction Kinetic Networks. J Phys Chem B 112, 16752–16758.
Ni, X. Y., T. Drengstig, and P. Ruoff, 2009. The control of the controller: molecular mechanisms for robust perfect adaptation and temperature compensation. Biophys J 97, 1244–53.
Kacser, H., and J. A. Burns, 1979. Molecular democracy: who shares the controls? Biochem Soc Trans 7, 1149–60.
Burns, J. A., A. Cornish-Bowden, A. K. Groen, R. Heinrich, H. Kacser, J. W. Porteous, S. M. Rapoport, T. A. Rapoport, J. W. Stucki, J. M. Tager, R. J. A. Wanders, and H. V. Westerhoff, 1985. Control analysis of metabolic systems. Trends Biochem Sci 19, 16.
Heinrich, R., and S. Schuster, 1996. The Regulation of Cellular Systems. Chapman and Hall, New York.
Fell, D., 1997. Understanding the Control of Metabolism. Portland Press, London and Miami.
Drengstig, T., T. Kjosmoen, and P. Ruoff. On the Relationships between Sensitivity Coefficients and Transfer Functions in Reaction Kinetic Networks. To be published.
Lutkepohl, H., 1996. Handbook of matrices. John Wiley & Sons.
Ingalls, B. P., 2004. A Frequency Domain Approach to Sensitivity Analysis of Biochemical Networks. J. Phys. Chem. B 108, 1143–1152.
Beckskei, A., and L. Serrano, 2000. Engineering stability in gene networks by autoregulation. Nature 405, 261–74.
Lahav, G., N. Rosenfeld, A. Sigal, N. Geva-Zatorsky, A. J. Levine, M. B. Elowitz, and U. Alon, 2004. Dynamics of the p53-Mdm2 feedback loop in individual cells. Nat Genet 36, 147–50.
Geva-Zatorsky, N., N. Rosenfeld, S. Itzkovitz, R. Milo, A. Sigal, E. Dekel, T. Yarnitzky, Y. Liron, P. Polak, G. Lahav, and U. Alon, 2006. Oscillations and variability in the p53 system. Mol Syst Biol 2, 2006 0033.
Jolma, I. W., X. Y. Ni, L. Rensing, and P. Ruoff, 2010. Harmonic Oscillations in Homeostatic Controllers: Dynamics of the p53 Regulatory System. Biophys J 98, 743–752.
Toettcher, J. E., C. Mock, E. Batchelor, A. Loewer, and G. Lahav, 2010. A synthetic-natural hybrid oscillator in human cells. Proc Natl Acad Sci U S A 107, 17047–52.
Chadwick, D. J., and J. A. Goode, 2000. Mechanisms and Biological Significance of Pulsatile Hormone Secretion. Wiley, New York.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media, LLC
About this protocol
Cite this protocol
Drengstig, T., Kjosmoen, T., Ruoff, P. (2011). Studying Adaptation and Homeostatic Behaviors of Kinetic Networks by Using MATLAB. In: Becskei, A. (eds) Yeast Genetic Networks. Methods in Molecular Biology, vol 734. Humana Press. https://doi.org/10.1007/978-1-61779-086-7_8
Download citation
DOI: https://doi.org/10.1007/978-1-61779-086-7_8
Published:
Publisher Name: Humana Press
Print ISBN: 978-1-61779-085-0
Online ISBN: 978-1-61779-086-7
eBook Packages: Springer Protocols