Summary
Rhesus D (RhD) blood group incompatibility between mother and fetus can occasionally result in maternal alloimmunization where the resultant anti-D can cross the placenta and attack the fetal red cells, which in worse case scenarios can cause fetal anemia and ultimately death. Fetal RHD genotyping was introduced in the mid-1990s after the molecular characterization of the RH genes as an aid to the clinical management of these cases. Initially, these tests used fetal DNA extracted invasively from chorionic villus and amniocyte samples.
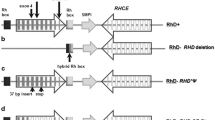
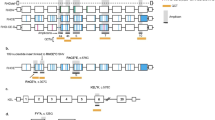
RHD genotyping of fetuses carried by RhD-negative women has become the first large-scale application of noninvasive prenatal diagnosis (NIPD). Initially the real-time polymerase chain reaction (PCR)-based tests were devised to characterize free fetal DNA in maternal plasma and serum, and RHD genotyping was a convenient assay to develop this exciting new technology, because the accuracy of tests could easily be confirmed after the simple RhD phenotyping of fetal cord blood cells after birth. “First generation” RHD genotyping tests were based on the incorrect concept that all D-negative phenotypes were caused by a complete RHD gene deletion. Thus, it was a relatively simple task to develop diagnostic PCR strategies based on the detection of RHD where D-negative genomes will completely lack RHD. Subsequent research into the molecular basis of D-negative phenotypes revealed that a significant number of D-negative genomes possess fragments of, or mutated RHD genes, the most notable of which is the RHD pseudogene found in Africans. Thus, more comprehensive RHD genotyping tests have evolved to differentiate these alleles, and are more appropriate in the diagnosis of multi-ethnic population groups such as those found in Europe and North America.
Many European Union countries have suggested the mass application of RHD NIPD for all fetuses carried by D-negative women. This is of clear benefit, because most RhD prophylaxis programs have switched to antenatal administration. This will help conserve anti-D stocks and it will prevent unnecessary administration of a human-derived blood product to a vulnerable patient group. Although anti-D stocks are inherently safe, there is a moral obligation to eliminate unnecessary administration of it because there have been instances of hepatitis C infection due to contamination. Furthermore, as yet undescribed viruses may be contaminants of blood products. Mass-scale RHD NIPD will shortly be implemented in several countries in the European Community as a consequence.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Reference
Urbaniak, S. J. and Greiss, M. A. (2000) RhD haemolytic disease of the fetus and the newborn. Blood Rev. 14, 44–61.
Hadley, A. G., Poole, G. D., Poole, J., Anderson, N. A., and Robson, M. (1996) Haemolytic disease of the newborn due to anti-G. Vox Sang. 71, 108–112.
Avent, N. D., Madgett, T. E., Lee, Z. E., Head, D. J., Maddocks, D. G., and Skinner, L. H. (2006) Molecular biology of Rh proteins and relevance to molecular medicine. Expert Rev. Mol. Med. 8, 1–20.
Avent, N. D. and Reid, M. E. (2000) The Rh blood group system: a review. Blood 95, 375–387.
Van Kim, C. L., Colin, Y., and Cartron, J. P. (2005) Rh proteins: key structural and functional components of the red cell membrane. Blood Rev. 20, 93–110.
Huang, C. H. and Liu, P. Z. (2001) New insights into the Rh superfamily of genes and proteins in erythroid cells and nonerythroid tissues. Blood Cells Mol. Dis. 27, 90–101.
Wagner, F. F. and Flegel, W. A. (2004) Review: the molecular basis of the Rh blood group phenotypes. Immunohematology 20, 23–36.
Westhoff, C. M. (2004) The Rh blood group system in review: a new face for the next decade. Transfusion 44, 1663–1673.
Conroy, M. J., Bullough, P. A., Merrick, M., and Avent, N. D. (2005) Modelling the human rhesus proteins: implications for structure and function. Br. J. Haematol. 131, 543–551.
Stott, L. M., Barker, R. N., and Urbaniak, S. J. (2000) Identification of alloreactive T-cell epitopes on the Rhesus D protein. Blood 96, 4011–4019.
Hall, A. M., Cairns, L. S., Altmann, D. M., Barker, R. N., and Urbaniak, S. J. (2005) Immune responses and tolerance to the RhD blood group protein in HLA-transgenic mice. Blood 105, 2175–2179.
Cherif-Zahar, B., Bloy, C., Van Kim, C. L., et al. (1990) Molecular cloning and protein structure of a human blood group Rh polypeptide. Proc. Natl. Acad. Sci. USA 87, 6243–6247.
Avent, N. D., Finning, K. M., Martin, P. G., and Soothill, P. W. (1990) cDNA cloning of a 30 kDa erythrocyte membrane protein associated with Rh (Rhesus)-blood-group-antigen expression. Biochem. J. 271, 821–825.
Arce, M. A., Thompson, E. S., Wagner, S., Coyne, K. E., Ferdmann, B. A., and Lublin, D. M. (1993) Molecular cloning of RhD cDNA derived derived from a gene present in RhD-positive, but not RhD-negative individuals. Blood 82,651–655.
Van Kim, C. L., Mouro. I., Cherif-Zahar, B., et al. (1992) Molecular cloning and primary structure of the human blood group RhD polypeptide. Proc. Natl. Acad. Sci. USA 89, 10925–10929.
Avent, N. D. (2002) Fetal genotyping. In: Hadley, A. G. and Soothill, P. W. (eds.). Alloimmune disorders of pregnancy, pp 121–139. Cambridge University Press, Cambridge, UK.
Avent, N. D., Finning, K. M., Martin, P. G., and Soothill, P. W. (2000) Prenatal determination of fetal blood group status. Vox Sang. 78, 155–162.
Avent, N. D. (1998) Antenatal genotyping of the blood groups of the fetus. Vox Sang. 74, 365–374.
Bloy, C., Blanchard, D., Dahr, W., Beyreuther, K., Salmon, C., and Cartron, J. P. (1988) Determination of the N-terminal sequence of human red cell Rh(D) polypeptide and demonstration that the Rh(D), (c), and (E) antigens are carried by distinct polypeptide chains. Blood 72, 661–666.
Avent, N. D., Ridgwell, K., Wanner, M. J., and Anstee, D. J. (1988) Protein-sequence studies on Rh-related polypeptides suggest the presence of at least two groups of proteins which associate in the human red-cell membrane. Biochem. J. 256, 1043–1046.
Saboori, A. M., Smith, B. L., and Agre, P. (1988) Polymorphism in the Mr 32,000 Rh protein purified from Rh(D)-positive and -negative erythrocytes. Proc. Natl. Acad. Sci. USA 85, 4042–4045.
Bennett, P. R., Le Van Kim, C., Colin, Y., Warwick, R. M., Cherif-Zahar, R., Fisk, N. and Cartron, J. P. (1993) Prenatal determination of fetal RhD type by DNA amplification. N. Engl. J. Med. 329, 607–610.
Colin, Y., Cherif-Zahar, F., Le Van Kim, C., Raynal, V., Van Huffel, V., and Cartron, J. P. (1991) Genetic basis of the RhD-positive and RhD-positive and RhD-negative blood group polymorphism as analysis. Blood 78, 2747–2752.
Ridgwell, K., Spurr, N. K., Laguda, B., MacGeoch, C., Avent, N. D. and Tanner, M. J. (1992) Isolation of cDNA clones for a 50 kDa glycoprotein of the human erythrocyte membrane associated with Rh (rhesus) blood-group antigen expression. Biochem. J. 287, 223–338.
Wagner, F. F. and Flegel, W. A. (2000) RHD gene deletion occurred in the Rhesus box. Blood 95, 3662–3668.
Wagner, F. F., Moulds, J. M., and Flegel, W. A. (2005) Genetic mechanisms of Rhesus box variation. Transfusion 45, 338–344.
Matheson, K. A. and Denomme, G. A. (2002) Novel 3′ Rhesus box sequences confound RHD zygosity assignment. Transfusion 42, 645–650.
Engelfriet, C. P., Reesink, H. W., Judd, W. J., et al. (2003) Current status of immunoprophylaxis with anti-D immunoglobin. Vox Sang. 85, 328–337.
Urbaniak, S. J. (1998) Consensus conference on anti-D prophylaxis, April 7 & 8, 1997: final consensus statement. Royal College of Physicians of Edinburgh/Royal College of Obstetricians and Gynaecologists. Transfusion. 38, 97–99.
Van der Schoot, C. E., Soussan, A. A., Koelewijn, J., Bonsel, G., Paget-Christiaens, L. G., and de Haas, M. (2006) Non-invasive antenatal RHD typing. Transfus. Clin. Biol. 13, 53–57.
Finning, K., Martin, P. and Daniels, G. (2004) A clinical service in the UK to predict fetal Rh (Rhesus) D blood group using free fetal DNA in maternal plasma. Ann. N Y Acad. Sci. 1022, 119–123.
Rouillac-Le Sciellour, C., Puillandre, P., Gillot, R., et al. (2004) Large-scale pre-diagnosis study of fetal RHD genotyping by PCR on plasma DNA from RhD-negative pregnant women. Mol. Diagn. 8, 23–31.
Gautier, E., Benachi, A., Giovangrand, Y., et al., (2005) Fetal RhD genotyping by maternal serum analysis: a two-year experience. Am. J. Obstet. Gynecol. 192, 666–669.
Lubenko, A., Contreras, M., and Habash, J. (1989) Should anti-Rh immunoglobulin be given D variant women? Br. J. Hematol. 72, 429–433.
Lacey, P. A., Caskey, C. R., Werner, D. J., and Moulds, J. J. (1983) Fatal hemolytic disease of a newborn due to anti-D in an Rh-positive Du variant mother. Transfusion 23, 91–94.
Flegel, W. A. (2006) Molecular genetics of RH and its clinical application Genetique moleculaire sur systeme RH et ses applications cliniques. Transfus. Clin. Biol. 13, 4–12.
Avent, N. D. (2001) Molecular biology of the Rh blood group system. J. Pediatr. Hematol. Oncol. 23, 394–402.
Avent, N. D. (1999) The rhesus blood group system: insights from recent advances in molecular biology. Transfus. Med. Rev. 13, 245–266.
Denomme, G. A. (2004) The structure and function of the molecules that carry human red blood cell and platelet antigens. Transfus. Med. Rev. 18,203–231.
Simsek, S., Bleeker, P. M. and von dem Borne, A. E. (1994) Prenatal determination of fetal RhD type. N. Engl. J. Med. 330, 795–796.
Carritt, B., Steers, F. J., and Avent, N. D. (1994) Prenatal determination of fetal RhD type. Lancet 344, 205–206.
Avent, N. D., Martin, P. G., Armstrong-Fisher, S. S., et al. (1997) Evidence of genetic diversity underlying Rh D-, weak D (Du), and partial D phenotypes as determined by multiplex polymerase chain reaction analysis of the RHD gene. Blood 89, 2568–2577.
Andrews, K. T., Wolter, L. C., Saul, A., and Hyland, C. A. (1998) The RhD– trait in a white patient with the RhCCee phenotype attributed to a four-nucleotide deletion in the RHD gene. Blood 92, 1839–1840.
Huang, C. H. (1996) Alteration of RH gene structure and expression in human dCCee and DCW-red blood cells: phenotypic homozygosity versus genotypic heterozygosity. Blood 88, 2326–2333.
Wagner, F. F., Frohmajer, A., and Flegel, W. A. (2001) RHD positive haplotypes in D negative Europeans. BMC Genet. 2, 10.
Singleton, B. K., Green, C. A., Avent, N. D., et al. (2000) The presence of an RHD pseudogene containing a 37 base pair duplication and a nonsense mutation in Africans with the Rh D-negative blood group phenotype. Blood 95,12–18.
Daniels, G. L., Faas, B. H., Green, C. A., et al. (1998) The VS and V blood group polymorphisms in Africans: a serologic and molecular analysis. Transfusion. 38, 951–958.
Faas, B. H., et al. (1997) Molecular background of VS and weak C expression in blacks. Transfusion 37, 38–44.
Finning, K. M., Martin, P. G., Soothill, P. W., and Avent, N. D. (2002) Prediction of fetal D status from maternal plasma: introduction of a new noninvasive fetal RHD genotyping service. Transfusion 42, 1079–1085.
Jones, J. W., Lloyd-Evans, P., and Kumpel, B. M. (1996) Quantitation of Rh D antigen sites on weak D and D variant red cells by flow cytometry. Vox Sang. 71, 176–183.
Wagner, F. F., Gassner, C., Muller, T. H., Schonitzer, D., Schunter, F., and Flegel, W. A. (1998) Three molecular structures cause rhesus D category VI phenotypes with distinct immunohematologic features. Blood 91, 2157–2168.
Mayne, K., Bowell, P., Woodward, T., Sibley, C., Lomas, C., and Tippett, P. (1990) Rh immunization by the partial D antigen of category DVa. Br. J. Haematol. 76, 537–539.
Leschek, E., Pearlman, S. A., Boudreaux, I., and Meek, R. (1993) Severe hemolytic disease of the newborn caused by anti-Gonzales antibody. Am. J. Perinatol. 10, 362–364.
Levene, C., Sela, R., Grunberg, L., Gale, R., Lomas, C., and Tippett, P. (1983) The Rh antigen Tar (Rh40) causing haemolytic disease of the newborn. Clin. Lab. Haematol. 5, 303–305.
Gabra, G. S., Bruce, M., Watt, A., and Mitchell, R. (1987) Anti-Rh 29 in a primigravida with rhesus null syndrome resulting in haemolytic disease of the newborn. Vox Sang. 53, 143–146.
Lenkiewicz, B. and Zupanska, B. (2000) Moderate hemolytic disease of the newborn due to anti-Hr0 in a mother with the D–/D– phenotype. Immunohematology 16, 109–111.
Lubenko, A., Contreras, M., Portugal, C. L., et al. (1992) Severe haemolytic disease in an infant born to an Rh(null) proposita. Vox Sang. 63, 43–47.
Zimmermann, B., El-Sheikhah, A., Nicolaides, K., Holzgreve, W., and Hahn, S. (2005) Optimized real-time quantitative PCR measurement of male fetal DNA in maternal plasma. Clin. Chem. 51, 1598–1604.
Daniels, G., van der Schoot, C. E., and Olsson, M. L. (2005) Report of the First International Workshop on molecular blood group genotyping. Vox Sang. 88,136–142.
Alizadeh, M., Bernard, M., Danic, B., et al. (2002) Quantitative assessment of hematopoietic chimerism after bone marrow transplantation by real-time quantitative polymerase chain reaction. Blood 99, 4618–4625.
Chim, S. S., Tong, Y. K., Chiu, R. W., et al. (2005) Detection of the placental epigenetic signature of the maspin gene in maternal plasma. Proc. Natl. Acad. Sci. USA 102, 14753–14758.
Chang, et al., (2006) Hypermethylated RASSF1A in maternal plasma: A universal fetal DNA marker that improves the reliability of noninvasive prenatal diagnosis. Clin Chem. 52, 2211–2218.
Lo, Y. M., Corbetta, N., Chamberlain, P. F., et al. (1997) Presence of fetal DNA in maternal plasma and serum. Lancet 350, 485–487.
Legler, et al. (2007) Workshop report on the extraction of foetal DNA from maternal plasma. Prenatal Diagnosis 27, 824–829.
Madgett, T. E. (2005) Rapid molecular genotyping for RHD using multiplex ligation-dependent probe amplification technology. Transfusion 45, 129A.
Chan, K. C., Zhang, J., Hui, A. B., et al. (2004) Size distributions of maternal and fetal DNA in maternal plasma. Clin. Chem. 50, 88–92.\vvsp{-2}
Li, Y., Di Naro, E., Vitucci, A., Zimmermann, B., Holzgreve, W., and Hahn, S. (2005) Detection of paternally inherited fetal point mutations for beta-thalassemia using size-fractionated cell-free DNA in maternal plasma. J. Am. Med. Assoc. 293, 843–849.
Gassner, C., Doescher, A., Drnovsek, T. D., et al. (2005) Presence of RHD in serologically D–, C/E+ individuals: a European multicenter study. Transfusion. 45, 527–538.
Shao, C. P. and Xiong, W. (2004) A new hybrid RHD-positive, D antigen-negative allele. Transfus. Med. 14, 185–186.
Blunt, T., Daniels, G., and Carritt, B. (1994) Serotype switching in a partially deleted RHD gene. Vox Sang. 67, 397–401.
Faas, B. H., Beckers, E. A., Simsek, S., et al. (1996) Involvement of Ser103 of the Rh polypeptides in G epitope formation. Transfusion 36, 506–11.
Gassner, C., Schmarda A., Kilga-Nogler, S., et al. (1997) RHD/CE typing by polymerase chain reaction using sequence-specific primers. Transfusion 37, 1020–1026.
Shao, C. P., Maas, J. H., Su, Y. Q., Kohler, M., and Legler, T. J. (2002) Molecular background of Rh D-positive, D-negative, D(el), and weak D phenotypes in Chinese. Vox Sang. 83, 156–161.
Cherif-Zahar, B., Bony, V., Steffensen, R., et al. (1998) Shift from Rh-positive to Rh-negative phenotype caused by a somatic mutation within the RHD gene in a patient with chronic myelocytic leukaemia. Br. J. Haematol. 102, 1263–1270.
Okuda, H., Kawano, M., Iwamoto, S., et al. (1997) The RHD gene is highly detectable in RhD-negative Japanese donors. J. Clin. Invest. 100, 373–379.
Qun, X., Grootkerk-Tax, M. G., Maaskant-van Wijk, P. A., and van der Schoot, C. E. (2005) Systemic analysis and zygosity determination of the RHD gene in a D-negative Chinese Han population reveals a novel D-negative RHD gene. Vox Sang. 88, 35–40.
Kim, J. Y., Kim, S. Y., Kim, C. A., Yon, G. S., and Park, S. S. (2005) Molecular characterization of D– Korean persons: development of a diagnostic strategy. Transfusion 45, 345–352.
Faas, B. H., Beuling, E. A., Christiaens, G. C., von dem Borne, A. E., and van der Schoot, C. E. (1998) Detection of fetal RHD-specific sequences in maternal plasma. Lancet 352, 1196.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2008 Humana Press, a part of Springer Science+Business Media, LLC
About this protocol
Cite this protocol
Avent, N.D. (2008). RHD Genotyping from Maternal Plasma: Guidelines and Technical Challenges. In: Hahn, S., Jackson, L.G. (eds) Prenatal Diagnosis. Methods in Molecular Biology™, vol 444. Humana Press. https://doi.org/10.1007/978-1-59745-066-9_14
Download citation
DOI: https://doi.org/10.1007/978-1-59745-066-9_14
Publisher Name: Humana Press
Print ISBN: 978-1-58829-803-4
Online ISBN: 978-1-59745-066-9
eBook Packages: Springer Protocols