Abstract
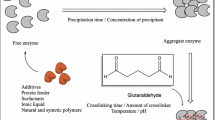
The economic viability of biocatalytic conversions is often dependent on finding an effective method for immobilization of the enzyme involved. This provides for its improved operational stability and facile recovery and re-use. Cross-linked enzyme aggregates (CLEAs®) constitute an effective methodology for enzyme immobilization with broad scope. The technique is exquisitely simple, involving precipitation from aqueous buffer and subsequent cross-linking of the resulting physical aggregates of enzyme molecules, and amenable to rapid optimization. The resulting CLEAs are stable, recyclable biocatalysts exhibiting high activity retention, in some cases higher than that of the free enzyme they were derived from. The enzyme does not need to be of high purity since the methodology essentially combines purification and immobilization into a single operation. The technique can also be applied to the preparation of combi-CLEAs containing two or more enzymes. For example, an oxynitrilase/nitrilase CLEA for the one-pot synthesis of (S) mandelic acid from benzaldehyde in high yield and enantioselectivity.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
D’Souza S. F. (1999) Immobilized enzymes in bioprocess. Curr. Sci. 77, 69–79.
Kragl U. (1996) Enzyme membrane reactors. In: Industrial Enzymology, 2nd edition (Godfrey T., and West S., eds.), MacMillan, Basingstoke, pp. 274–283.
Fernández-Lorente G., Terreni M., Mateo C., et al. (2001) Modulation of lipase properties in macro-aqueous systems by controlled enzyme immobilization: enantioselective hydrolysis of a chiral ester by immobilized Pseudomonas lipase. Enzyme Microb. Technol. 28, 389–396.
Cao L., van Langen L. M., and Sheldon R. A. (2003) Immobilised enzymes: carrier-bound or carrier-free? Curr. Opin. Biotechnol. 14, 387–394.
Doscher M. S. and Richards F. M. (1963) The actvitiy of an enzyme in the crystalline state: Ribonuclease S. J. Biol. Chem. 238, 2399–2406.
Quiocho F. A. and Richards F. M. (1964) Intermolecular cross-linking of a protein in the crystalline state: carboxypeptidase A. Proc. Natl. Acad. Sci. USA 114, 7314–7316.
St. Clair N. L. and Navia M. A. (1992) Cross-linked enzyme crystals as robust biocatalysts. J. Am. Chem. Soc. 114, 7314–7316.
Margolin A. L. and Navia M. A. (2001) Protein crystals as novel catalytic materials. Angew. Chem. Int. Ed. Engl. 40, 2204–2222.
Lalonde J. (1997) Practical catalysis with enzyme crystals. Chemtech 27(2), 38–45.
Margolin A. L. (1996) Novel crystalline catalysts. Tibtech 14, 223–230.
Häring D. and Schreier P. (1999) Cross-linked enzyme crystals. Curr. Opin. Biotechnol. 3, 35–38.
Brown D. L. and Glatz C. E. (1987) Aggregate breakage in protein precipitation. Chem. Eng. Sci. 42, 1831–1839.
Cao L., van Rantwijk F., and Sheldon R. A. (2000) Cross-linked enzyme aggregates: a simple and effective method for the immobilization of penicillin acylase. Org. Lett. 2, 1361–1364.
Wegman M. A., Janssen M. H. A., van Rantwijk F., and Sheldon R. A. (2001) Towards biocatalytic synthesis of β-lactam antibiotics. Adv. Synth. Catal. 343, 559–576.
van Langen L. M., Oosthoek N. H. P., van Rantwijk F., and Sheldon R. A. (2003) Pencillin acylase catalysed synthesis of ampicillin in hydrophilic organic solvents. Adv. Synth. Catal. 345, 797–801.
Lopez-Serrano P., Cao L., van Rantwijk F., and Sheldon R. A. (2002) NL 1017258, to Delft University of Technology.
Theil F. (2000) Enhancement of selectivity and reactivity of lipases by additives. Tetrahedron 56, 2905–2919.
López-Serrano P., Cao L., van Rantwijk F., and Sheldon R. A. (2002) Crosslinked enzyme aggregates with enhanced activity: application to lipases. Biotechnol. Lett. 24, 1379–1383.
Mateo C., Chmura A., Rustler S., van Rantwijk F., Stolz A., and Sheldon R. A., manuscript in preparation.
Bourbonnais R. and Paice M. G. (1990) Oxidation of nonphenolic substrates-an expanded role for laccase in lignin biodegradation. FEBS Lett 267, 99–102.
Dvorakova J., Volfova O., and Kopecky J. (1997) Characterization of phytase produced by Aspergillus niger. Folia Microbiologica 42, 349–352.
Avigad G., Asensio C., Horecker B. L., and Amaral D. (1962) d-Galactose oxidase of Polyporys circinatus. J. Biol. Chem. 237, 2736–2740.
Asaad N. and Engberts J. F. B. N. (2003) Cytosol-mimetic chemistry: Kinetics of the trypsin-catalyzed hydrolysis of p-nitrophenyl acetate upon addition of polyethylene glycol and N-tert-butyl acetoacetamide. J. Am. Chem. Soc. 125, 6874–6875.
Kim C. S., Ji E. S., and Oh D. K. (2003) Expression and characterization of Kluyveromyces lactis β-galactosidase in Escherichia coli. Biotechnol. Lett. 25, 1769–1774.
Rella R., Raia C. A., Pensa M., et al. (1987) A novel archaebacterial NAD+-dependent alcohol dehydrogenase. Purification and properties. Eur. J. Biochem. 167, 475–479.
Gröger H., Hummel W., Rollmann C., et al. (2004) Preparative asymmetric reduction of ketones in a biphasic medium with an (S)-alcohol dehydrogenase under in situ-cofactor-recycling with a formate dehydrogenase. Tetrahedron 60, 633–640.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2006 Humana Press Inc.
About this protocol
Cite this protocol
Sheldon, R.A., Schoevaart, R., van Langen, L.M. (2006). Cross-Linked Enzyme Aggregates. In: Guisan, J.M. (eds) Immobilization of Enzymes and Cells. Methods in Biotechnology™, vol 22. Humana Press. https://doi.org/10.1007/978-1-59745-053-9_3
Download citation
DOI: https://doi.org/10.1007/978-1-59745-053-9_3
Publisher Name: Humana Press
Print ISBN: 978-1-58829-290-2
Online ISBN: 978-1-59745-053-9
eBook Packages: Springer Protocols