Abstract
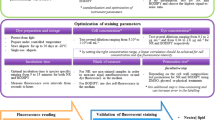
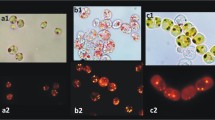
Over the last decade, finding bacterial strains with ability to accumulate high concentrations of lipids has gained increasing interest, since these lipids may be used in different industries. Here we describe two methods for evaluation of lipid accumulation in cyanobacteria, following by our personal reflection on issues surrounding the use of these methods. First, we present the Bligh and Dyer protocol as a traditional extraction method using organic solvents for quantitative determination of lipids and next Nile red, a selective fluorescent stain, that has been used as a rapid approach for both qualitative and quantitative measurement of lipids.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Thomson N, Summers D, Sivaniah E, Abe H, Doi Y, Taguchi S et al (2010) Synthesis, properties and uses of bacterial storage lipid granules as naturally occurring nanoparticles. Soft Matter 6:4045
Liu X, Sheng J, Curtiss R (2011) Fatty acid production in genetically modified cyanobacteria. Proc Natl Acad Sci 108:6899–6904
Modiri S, Hajfarajollah H, Zahiri HS, Sharafi H, Noghabi KA, Zamanzadeh Z et al (2015) Lipid production and mixotrophic growth features of cyanobacterial strains isolated from various aquatic sites. Microbiology 161:662–673
Halim R, Danquah MK, Webley PA (2012) Extraction of oil from microalgae for biodiesel production: a review. Biotechnol Adv 30:709–732
Satpati GG, Pal R (2014) Rapid detection of neutral lipid in green microalgae by flow cytometry in combination with Nile red staining-an improved technique. Ann Microbiol 65:937–949
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Iverson SJ, Lang SL, Cooper MH (2001) Comparison of the Bligh and Dyer and Folch methods for total lipid determination in a broad range of marine tissue. Lipids 36:1283–1287
Abdo SM, Ahmed E, El-enin SA, El Din RS, El Diwani G, Ali G (2014) Qualitative and quantitative determination of lipid content in microalgae for biofuel production. J Algal Biomass Util 5:23–28
Gikonyo B (2013) Advances in biofuel production: algae and aquatic plants. CRC Press (Taylor & Francis Group), Boca Raton FL
Chen W, Zhang C, Song L, Sommerfeld M, Hu Q (2009) A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J Microbiol Methods 77:41–47
Genicot G, Leroy JLMR, Van Soom A, Donnay I (2005) The use of a fluorescent dye, Nile red, to evaluate the lipid content of single mammalian oocytes. Theriogenology 63:1181–1194
Evans CT, Ratledge C, Gilbert SC (1985) A rapid screening method for lipid-accumulating yeast using a replica-printing technique. J Microbiol Methods 4:203–210
McGinnis KM, Dempster TA, Sommerfeld MR (1997) Characterization of the growth and lipid content of the diatom Chaetoceros muelleri. J Appl Phycol 9:19–24
Castro GR, Larson BK, Panilaitis B, Kaplan DL (2005) Emulsan quantitation by Nile red quenching fluorescence assay. Appl Microbiol Biotechnol 67:767–770
Lv JM, Cheng LH, Xu XH, Zhang L, Chen HL (2010) Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresour Technol 101:6797–6804
Alemán-Nava GS, Cuellar-Bermudez SP, Cuaresma M, Bosma R, Muylaert K, Ritmann BE et al (2016) How to us Nile red, a selective fluorescent stain for microalgal neutral lipids. J Microbiol Methods 128:74–79
Castell LL, Mann R (1994) Optimal staining of lipids in bivalve larvae with Nile red. Aquaculture 119:89–100
Rumin J, Bonnefond H, Saint-Jean B, Rouxel C, Sciandra A, Bernard O et al (2015) The use of fluorescent Nile red and BODIPY for lipid measurement in microalgae. Biotechnol Biofuels 8:42
Pick U, Rachutin-Zalogin T (2012) Kinetic anomalies in the interactions of Nile red with microalgae. J Microbiol Methods 88:189–196
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Modiri, S., Zahiri, H.S., Noghabi, K.A. (2019). Evaluation of Bacterial Lipid Production: Quantitative and Qualitative Measurements: Tips and Guidelines. In: Balan, V. (eds) Microbial Lipid Production. Methods in Molecular Biology, vol 1995. Humana, New York, NY. https://doi.org/10.1007/978-1-4939-9484-7_23
Download citation
DOI: https://doi.org/10.1007/978-1-4939-9484-7_23
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-4939-9483-0
Online ISBN: 978-1-4939-9484-7
eBook Packages: Springer Protocols