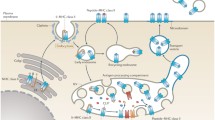
Abstract
Major histocompatibility complex (MHC) class I molecules function to present pathogen derived peptides to cytotoxic T cells and act as ligands for Natural Killer cells, thus alerting the immune system to the presence of invading pathogens. However, some MHC class I molecules, most notably HLA-B27, can be strongly associated with autoimmune diseases. In addition, the MHC class I pathway is a target for numerous viral evasion strategies Understanding not only the antigen presenting functions, but also the biosynthesis and the degradation pathways of MHC class I molecules has therefore become important in determining their role in pathogen and autoimmune related diseases. Here, we describe how using epitope tagged MHC class I molecules can aid in the analysis of MHC class I molecule biosynthesis and degradation as well as complementary studies using conventional conformationally specific antibodies. Coupled together with pharmacological manipulation which can target both biosynthetic and degradative pathways, this offers a powerful tool in analyzing MHC class I molecules.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC (1987) Structure of the human class I histocompatibility antigen, HLA-A2. Nature 329:506–512
Bjorkman PJ, Parham P (1990) Structure, function, and diversity of class I major histocompatibility complex molecules. Annu Rev Biochem 59:253–288
Falk K, Rotzschke O, Rammensee HG (1990) Cellular peptide composition governed by major histocompatibility complex class I molecules. Nature 348:248–251
Rotzschke O, Falk K, Deres K, Schild H, Norda M, Metzger J, Jung G, Rammensee HG (1990) Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature 348:252–254
Lewis JW, Elliott T (1998) Evidence for successive peptide binding and quality control stages during MHC class I assembly. Curr Biol 8:717–720
Williams AP, Peh CA, Purcell AW, McCluskey J, Elliott T (2002) Optimization of the MHC class I peptide cargo is dependent on tapasin. Immunity 16:509–520
Cresswell P (2005) Antigen processing and presentation. Immunol Rev 207:5–7
Antoniou AN, Powis SJ, Elliott T (2003) Assembly and export of MHC class I peptide ligands. Curr Opin Immunol 15:75–81
Boyle LH, Hermann C, Boname JM, Porter KM, Patel PA, Burr ML, Duncan LM, Harbour ME, Rhodes DA, Skjodt K, Lehner PJ, Trowsdale J (2013) Tapasin-related protein TAPBPR is an additional component of the MHC class I presentation pathway. Proc Natl Acad Sci U S A 110:3465–3470
Neerincx A, Hermann C, Antrobus R, van Hateren A, Cao H, Trautwein N, Stevanovic S, Elliott T, Deane JE, Boyle LH (2017) TAPBPR bridges UDP-glucose:glycoprotein glucosyltransferase 1 onto MHC class I to provide quality control in the antigen presentation pathway. eLife 6
Chang SC, Momburg F, Bhutani N, Goldberg AL (2005) The ER aminopeptidase, ERAP1, trims precursors to lengths of MHC class I peptides by a "molecular ruler" mechanism. Proc Natl Acad Sci U S A 102:17107–17112
Saveanu L, Carroll O, Lindo V, Del Val M, Lopez D, Lepelletier Y, Greer F, Schomburg L, Fruci D, Niedermann G, van Endert PM (2005) Concerted peptide trimming by human ERAP1 and ERAP2 aminopeptidase complexes in the endoplasmic reticulum. Nat Immunol 6:689–697
Harris MR, Yu YY, Kindle CS, Hansen TH, Solheim JC (1998) Calreticulin and calnexin interact with different protein and glycan determinants during the assembly of MHC class I. J Immunol 160:5404–5409
Ford S, Antoniou A, Butcher GW, Powis SJ (2004) Competition for access to the rat major histocompatibility complex class I peptide-loading complex reveals optimization of peptide cargo in the absence of transporter associated with antigen processing (TAP) association. J Biol Chem 279:16077–16082
Hughes EA, Hammond C, Cresswell P (1997) Misfolded major histocompatibility complex class I heavy chains are translocated into the cytoplasm and degraded by the proteasome. Proc Natl Acad Sci U S A 94:1896–1901
Stagg HR, Thomas M, van den Boomen D, Wiertz EJ, Drabkin HA, Gemmill RM, Lehner PJ (2009) The TRC8 E3 ligase ubiquitinates MHC class I molecules before dislocation from the ER. J Cell Biol 186:685–692
Burr ML, Cano F, Svobodova S, Boyle LH, Boname JM, Lehner PJ (2011) HRD1 and UBE2J1 target misfolded MHC class I heavy chains for endoplasmic reticulum-associated degradation. Proc Natl Acad Sci U S A 108:2034–2039
Cormier JH, Tamura T, Sunryd JC, Hebert DN (2009) EDEM1 recognition and delivery of misfolded proteins to the SEL1L-containing ERAD complex. Mol Cell 34:627–633
Guiliano DB, Fussell H, Lenart I, Tsao E, Nesbeth D, Fletcher AJ, Campbell EC, Yousaf N, Williams S, Santos S, Cameron A, Towers GJ, Kellam P, Hebert DN, Gould KG, Powis SJ, Antoniou AN (2014) Endoplasmic reticulum degradation-enhancing alpha-mannosidase-like protein 1 targets misfolded HLA-B27 dimers for endoplasmic reticulum-associated degradation. Arthritis Rheumatol 66:2976–2988
Kincaid MM, Cooper AA (2007) ERADicate ER stress or die trying. Antioxid Redox Signal 9:2373–2387
Li J, Ni M, Lee B, Barron E, Hinton DR, Lee AS (2008) The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ 15:1460–1471
Park B, Lee S, Kim E, Chang S, Jin M, Ahn K (2001) The truncated cytoplasmic tail of HLA-G serves a quality-control function in post-ER compartments. Immunity 15:213–224
Molinari M, Galli C, Piccaluga V, Pieren M, Paganetti P (2002) Sequential assistance of molecular chaperones and transient formation of covalent complexes during protein degradation from the ER. J Cell Biol 158:247–257
Antoniou AN, Lenart I, Guiliano DB (2011) Pathogenicity of misfolded and dimeric HLA-B27 molecules. Int J Rheumatol 2011:486856
Welihinda AA, Tirasophon W, Kaufman RJ (1999) The cellular response to protein misfolding in the endoplasmic reticulum. Gene Expr 7:293–300
Barnstable CJ, Bodmer WF, Brown G, Galfre G, Milstein C, Williams AF, Ziegler A (1978) Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell 14:9–20
Sakaguchi K, Ono R, Tsujisaki M, Richiardi P, Carbonara A, Park MS, Tonai R, Terasaki PI, Ferrone S (1988) Anti-HLA-B7,B27,Bw42,Bw54,Bw55,Bw56, Bw67,Bw73 monoclonal antibodies: specificity, idiotypes, and application for a double determinant immunoassay. Hum Immunol 21:193–207
Parham P, Bodmer WF (1978) Monoclonal antibody to a human histocompatibility alloantigen, HLA-A2. Nature 276:397–399
Stam NJ, Spits H, Ploegh HL (1986) Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol 137:2299–2306
Antoniou AN, Ford S, Taurog JD, Butcher GW, Powis SJ (2004) Formation of HLA-B27 homodimers and their relationship to assembly kinetics. J Biol Chem 279:8895–8902
Santos SG, Antoniou AN, Sampaio P, Powis SJ, Arosa FA (2006) Lack of tyrosine 320 impairs spontaneous endocytosis and enhances release of HLA-B27 molecules. J Immunol 176:2942–2949
Schaefer MR, Williams M, Kulpa DA, Blakely PK, Yaffee AQ, Collins KL (2008) A novel trafficking signal within the HLA-C cytoplasmic tail allows regulated expression upon differentiation of macrophages. J Immunol 180:7804–7817
Gruda R, Achdout H, Stern-Ginossar N, Gazit R, Betser-Cohen G, Manaster I, Katz G, Gonen-Gross T, Tirosh B, Mandelboim O (2007) Intracellular cysteine residues in the tail of MHC class I proteins are crucial for extracellular recognition by leukocyte Ig-like receptor 1. J Immunol 179:3655–3661
Acknowledgments
The work was in part supported by Arthritis Research UK (grant 21261; to A.N.A. and S.J.P.).
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Powis, S.J., Antoniou, A.N. (2019). Measuring Synthesis and Degradation of MHC Class I Molecules. In: van Endert, P. (eds) Antigen Processing. Methods in Molecular Biology, vol 1988. Humana, New York, NY. https://doi.org/10.1007/978-1-4939-9450-2_7
Download citation
DOI: https://doi.org/10.1007/978-1-4939-9450-2_7
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-4939-9449-6
Online ISBN: 978-1-4939-9450-2
eBook Packages: Springer Protocols