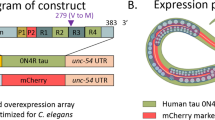
Abstract
Caenorhabditis elegans is widely used to investigate biological processes related to health and disease. Multiple C. elegans models for human neurodegenerative diseases do exist, including those expressing human α-synuclein. Even though these models do not feature all pathological and molecular hallmarks of the disease they mimic, they allow for the identification and dissection of molecular pathways that are involved. In line with this, genetic screens have yielded multiple modifiers of proteotoxicity in C. elegans models for neurodegenerative diseases. Here, we describe a set of common screening approaches and tools that can be used to study synucleinopathies and other neurodegenerative diseases in C. elegans. RNA interference and mutagenesis screens can be used to find genes that affect proteotoxicity, while relatively simple molecular, cellular (fractionation studies), metabolic (respiration studies), and behavioral (thrashing and crawling) readouts can be used to study the effects of disease proteins and modifiers more closely.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Morley JF, Brignull HR, Weyers JJ et al (2002) The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci U S A 99:10417–10422
Dillin A, Hsu AL, Arantes-Oliveira N et al (2002) Rates of behavior and aging specified by mitochondrial function during development. Science 298:2398–2401
Lee SS, Kennedy S, Tolonen AC et al (2003) DAF-16 target genes that control C. elegans life-span and metabolism. Science 300:644–647
Nollen EAA, Garcia SM, van Haaften G et al (2004) Genome-wide RNA interference screen identifies previously undescribed regulators of polyglutamine aggregation. Proc Natl Acad Sci U S A 101:6403–6408
Kim Y, Sun H (2007) Functional genomic approach to identify novel genes involved in the regulation of oxidative stress resistance and animal lifespan. Aging Cell 6:489–503
Van Ham TJ, Thijssen KL, Breitling R et al (2008) C. elegans model identifies genetic modifiers of α-synuclein inclusion formation during aging. PLoS Genet 4:e1000027–e1000011
Habchi J, Arosio P, Perni M et al (2016) An anticancer drug suppresses the primary nucleation reaction that initiates the production of the toxic A 42 aggregates linked with Alzheimer’s disease. Sci Adv 2:e1501244–e1501244
Arvanitis M, Li DD, Lee K et al (2013) Apoptosis in C. elegans: lessons for cancer and immunity. Front Cell Infect Microbiol 3:67
Jorgensen EM, Mango SE (2002) The art and design of genetic screens: caenorhabditis elegans. Nat Rev Genet 3:356–369
Hamilton B, Dong Y, Shindo M et al (2005) A systematic RNAi screen for longevity genes in C. elegans. Genes Dev 19:1544–1555
Van der Goot AT, Zhu W, Vázquez-Manrique RP et al (2012) Delaying aging and the aging-associated decline in protein homeostasis by inhibition of tryptophan degradation. Proc Natl Acad Sci U S A 109:14912–14917
Van Ham TJ, Holmberg MA, van der Goot AT et al (2010) Identification of MOAG-4/SERF as a regulator of age-related proteotoxicity. Cell 142:601–612
Lai CH, Chou CY, Chang LY et al (2000) Identification of novel human genes evolutionarily conserved in Caenorhabditis elegans by comparative proteomics. Genome Res 10:703–713
Shaye DD, Greenwald I (2011) OrthoList: a compendium of C. elegans genes with human orthologs. PLoS One 6(5):e20085
Faber PW, Alter JR, Macdonald ME et al (1999) Polyglutamine-mediated dysfunction and apoptotic death of a Caenorhabditis elegans sensory neuron. Proc Natl Acad Sci U S A 96:179–184
Lasko M, Vartianen S, Moilanen AM et al (2003) Dopaminergic neuronal loss and motor deficits in Caenorhabditis elegans overexpressing human alpha-synuclein. J Neurosci 86:165–172
Wang J, Farr GW, Hall DH et al (2009) An ALS-linked mutant SOD1 produces a locomotor defect associated with aggregation and synaptic dysfunction when expressed in neurons of Caenorhabditis elegans. PLoS Genet 5:e1000350
Ash PE, Zhang YJ, Roberts CM et al (2010) Neurotoxic effects of TDP-43 overexpression in C. elegans. Hum Mol Genet 19:3206–3218
Treusch S, Hamamichi S, Goodman JL (2011) Functional links between Aβ toxicity, endocytic trafficking and Alzheimer’s disease risk factors in yeast. Science 334(6060):1241–1245
Martinez BA, Petersen DA, Gaeta AL et al (2017) Dysregulation of the mitochondrial unfolded protein response induces non-apoptotic dopaminergic neurodegeneration in C. elegans models of Parkinson's disease. J Neurosci 37(46):11085–11100
Link CD (1995) Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc Natl Acad Sci U S A 92:9368–9372
Ben-Zvi A, Miller EA, Morimoto RI (2009) Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc Natl Acad Sci U S A 106:14914–14919
Yano H, Baranov SV, Baranova OV et al (2014) Inhibition of mitochondrial protein important by mutant huntingtin. Nat Neurosci 17(6):822–831
Wang W, Wang L, Lu J et al (2016) The inhibition of TDP-43 mitochondrial localization blocks its neuronal toxicity. Nat Med 22:869–878
Ryan T, Bamm VV, Stykel MG et al (2018) Cardiolipin exposure on the outer mitochondrial membrane modulates α-synuclein. Nat Commun 9:817
Ferreira IL, Resende R, Ferreiro E et al (2010) Multiple defects in energy metabolism in Alzheimer’s disease. Curr Drug Targets 11:1193–1206
Jarrett SF, Lewin AS, Boulton ME (2010) The importance of mitochondria in age-related and inherited eye disorders. Ophthalmic Res 44:179–190
Kawamata H, Manfredi G (2010) Mitochondrial dysfunction and intracellular calcium dysregulation in ALS. Mech Ageing Dev 131:517–526
Ren J, Pulakat L, Whaley-Connell A et al (2010) Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med 88:993–1001
Koopman M, Michels H, Dancy BM et al (2016) A screening-based platform for the assessment of cellular respiration in Caenorhabditis elegans. Nat Protoc 11(10):1798–1816
Kamath RS, Ahringer J (2003) Genome-wide RNAi screening in Caenorhabditis elegans. Methods 30(4):313–321
Rual JF, Ceron J, Koreth J et al (2004) Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res 14(10b):2162–2168
Perni M, Challa PK, Kirkegaard JB et al (2018) Massively parallel C. elegans tracking provides multi-dimensional fingerprints for phenotypic discovery. J Neurosci Methods 306:57–67. https://doi.org/10.1016/j.jneumeth.2018.02.005
Stiernagle T (2006) Maintenance of C. elegans. In: Wormbook (ed) The C. elegans Research Community
Corsi AK, Wightman B, Chalfie M (2015) A Transparent window into biology: a primer on Caenorhabditis elegans. In: Wormbook (ed) The C. elegans Research Community
Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77:71–94
Wicks SR, Yeh RT, Gish WR et al (2001) Rapig gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat Genet 28(2):160–164
Swan KA, Curtis DE, McKusick KB et al (2002) High-throughput gene mapping in Caenorhabditis elegans. Genome Res 12(7):1100–1105
Sin O, Michels H, Nollen EA (2014) Genetic screens in Caenorhabditis elegans models for neurodegenerative diseases. Biochim Biophys Acta 1842(10):1951–1959
Herndon LA, Schmeissner PJ, Dudaronek JM et al (2002) Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 419:808–814
Augustin H, Partridge L (2009) Invertebrate models of age-related muscle degeneration. Biochim Biophys Acta 1790:1084–1094
Pierce-Shimomura JT, Chen BL, Mun JJ (2008) Genetic analysis of crawling and swimming locomotory patterns in C. elegans. Proc Natl Acad Sci U S A 105(52):20982–20987
Kontopoulos E, Parvin JD, Feany MB (2006) Alpha-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum Mol Genet 15(20):3012–3023
Barmada SJ, Skibinski G, Korb E et al (2010) Cytoplasmic mislocalization of TDP-43 is toxic to neurons and enhanced by a mutation associated with familial ALS. J Neurosci 30(2):639
Chen F, Hersh BM, Conradt B et al (2000) Translocation of C. elegans CED-4 to nuclear membranes during programmed cell death. Science 287(5457):1485–1489
La Rocca G, Burgio G, Corona DF (2007) A protein nuclear extract from D. melanogaster larval tissues. Fly (Austin) 1(6):343–345
Mitchell DH, Stiles JW, Santelli J et al (1979) Synchronous growth and aging of Caenorhabditis elegans in the presence of fluorodeoxyuridine. J Gerontol 34:28–36
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Koopman, M., Seinstra, R.I., Nollen, E.A.A. (2019). C. elegans as a Model for Synucleinopathies and Other Neurodegenerative Diseases: Tools and Techniques. In: Bartels, T. (eds) Alpha-Synuclein. Methods in Molecular Biology, vol 1948. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-9124-2_9
Download citation
DOI: https://doi.org/10.1007/978-1-4939-9124-2_9
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-9123-5
Online ISBN: 978-1-4939-9124-2
eBook Packages: Springer Protocols