Abstract
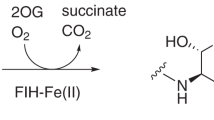
Kinetic analyses of HIF prolyl 4-hydroxylases (HIF-P4Hs) allow determination of substrate, cosubstrate and cofactor requirements, analysis of the reaction rate, and inhibitory properties of the isoenzymes in vitro. Here we describe an assay measuring the substrate hydroxylation-coupled decarboxylation of radioactive 2-oxoglutarate to radioactive carbon dioxide as a fast, efficient, and diverse method to analyze the enzyme kinetics of HIF-P4Hs.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Epstein AC, Gleadle JM, McNeill LA et al (2001) C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107(1):43–54
Bruick RK, McKnight SL (2001) A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294(5545):1337–1340
Ivan M, Haberberger T, Gervasi DC et al (2002) Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc Natl Acad Sci U S A 99(21):13459–13464
Hirsilä M, Koivunen P, Gunzler V et al (2003) Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J Biol Chem 278(33):30772–30780
Flashman E, Davies SL, Yeoh KK et al (2010) Investigating the dependence of the hypoxia-inducible factor hydroxylases (factor inhibiting HIF and prolyl hydroxylase domain 2) on ascorbate and other reducing agents. Biochem J 427(1):135–142
Briggs KJ, Koivunen P, Cao S et al (2016) Paracrine induction of HIF by glutamate in breast cancer: EglN1 senses cysteine. Cell 166(1):126–139
Metzen E, Berchner-Pfannschmidt U, Stengel P et al (2003) Intracellular localisation of human HIF-1 alpha hydroxylases: implications for oxygen sensing. J Cell Sci 116(Pt 7):1319–1326
Koivunen P, Hirsilä M, Kivirikko KI et al (2006) The length of peptide substrates has a marked effect on hydroxylation by the hypoxia-inducible factor prolyl 4-hydroxylases. J Biol Chem 281(39):28712–28720
Li D, Hirsilä M, Koivunen P et al (2004) Many amino acid substitutions in a hypoxia-inducible transcription factor (HIF)-1alpha-like peptide cause only minor changes in its hydroxylation by the HIF prolyl 4-hydroxylases: substitution of 3,4-dehydroproline or azetidine-2-carboxylic acid for the proline leads to a high rate of uncoupled 2-oxoglutarate decarboxylation. J Biol Chem 279(53):55051–55059
Huang LE, Pete EA, Schau M et al (2002) Leu-574 of HIF-1alpha is essential for the von Hippel-Lindau (VHL)-mediated degradation pathway. J Biol Chem 277(44):41750–41755
Hirsilä M, Koivunen P, Xu L et al (2005) Effect of desferrioxamine and metals on the hydroxylases in the oxygen sensing pathway. FASEB J 19(10):1308–1310
Koivunen P, Hirsilä M, Remes AM et al (2007) Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J Biol Chem 282(7):4524–4532
Isaacs JS, Jung YJ, Mole DR et al (2005) HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell 8(2):143–153
Selak MA, Armour SM, MacKenzie ED et al (2005) Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 7(1):77–85
McNeill LA, Flashman E, Buck MR et al (2005) Hypoxia-inducible factor prolyl hydroxylase 2 has a high affinity for ferrous iron and 2-oxoglutarate. Mol Biosyst 1(4):321–324
Myllyharju J (2013) Prolyl 4-hydroxylases, master regulators of the hypoxia response. Acta Physiol (Oxf) 208(2):148–165
Kivirikko KI, Myllylä R (1982) Posttranslational enzymes in the biosynthesis of collagen: intracellular enzymes. Methods Enzymol 82(Pt A):245–304
Koivunen P, Hirsilä M, Gunzler V et al (2004) Catalytic properties of the asparaginyl hydroxylase (FIH) in the oxygen sensing pathway are distinct from those of its prolyl 4-hydroxylases. J Biol Chem 279(11):9899–9904
Laukka T, Mariani CJ, Ihantola T et al (2016) Fumarate and succinate regulate expression of hypoxia-inducible genes via TET enzymes. J Biol Chem 291(8):4256–4265
Tiainen P, Pasanen A, Sormunen R et al (2008) Characterization of recombinant human prolyl 3-hydroxylase isoenzyme 2, an enzyme modifying the basement membrane collagen IV. J Biol Chem 283(28):19432–19439
Juva K, Prockop DJ (1966) Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem 15(1):77–83
Jaakkola P, Mole DR, Tian YM et al (2001) Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292(5516):468–472
Ivan M, Kondo K, Yang H et al (2001) HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292(5516):464–468
Hewitson KS, Schofield CJ, Ratcliffe PJ (2007) Hypoxia-inducible factor prolyl-hydroxylase: purification and assays of PHD2. Methods Enzymol 435:25–42
Myllylä R, Tuderman L, Kivirikko KI (1977) Mechanism of the prolyl hydroxylase reaction. 2. Kinetic analysis of the reaction sequence. Eur J Biochem 80(2):349–357
Acknowledgments
This work was supported by Academy of Finland through Grants 120156, 140765 and 218129 (P.K.), 200471, 202469, and 296498 and Center of Excellence 2012–2017 Grant 251314 (J.M.) and by the S. Jusélius Foundation (P.K., J.M.), the Emil Aaltonen Foundation (P.K.), the Jane and Aatos Erkko Foundation (P.K., J.M.), and FibroGen, Inc. (J.M.).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Science+Business Media, LLC
About this protocol
Cite this protocol
Koivunen, P., Myllyharju, J. (2018). Kinetic Analysis of HIF Prolyl Hydroxylases. In: Huang, L. (eds) Hypoxia. Methods in Molecular Biology, vol 1742. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-7665-2_2
Download citation
DOI: https://doi.org/10.1007/978-1-4939-7665-2_2
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-7664-5
Online ISBN: 978-1-4939-7665-2
eBook Packages: Springer Protocols