Abstract
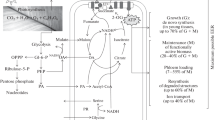
Carbohydrates are the dominant respiratory substrate in many plant cells. However, the route of carbohydrate oxidation varies depending on the relative cellular demands for energy, reductant, and precursors for biosynthesis. During these processes individual substrate carbon atoms are differentially released as carbon dioxide by specific reactions in the network, and this can be measured by monitoring the release of 14CO2 from a range of positionally labeled forms of [14C]glucose. Although the relative amounts of carbon dioxide produced from different carbon positions do not allow precise determination of fluxes, they are indicative of the route of carbohydrate utilization. Such information can be used to determine whether a comprehensive metabolic flux analysis is merited, and also to facilitate independent verification of flux maps generated by other techniques. This chapter describes an approach to determine and interpret the pattern of oxidation of carbohydrates by monitoring 14CO2 release during metabolism of exogenously supplied [1-14C]-, [2-14C]-, [3,4-14C]-, and [6-14C]glucose. The method is exemplified by studies on Arabidopsis cell suspension cultures, but the protocol can be easily adapted for the investigation of other plant materials.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Wang CH (1963) Metabolism studies by radiorespirometry. Adv Tracer Methodol 1:274–290
Zeeman SC (2015) Carbohydrate metabolism. In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology of plants, 2nd edn. Wiley, Chichester, pp 567–609
Bloom B, Stetten D Jr (1953) Pathways of glucose catabolism. J Am Chem Soc 75:5446–5446
Katz J, Wood HG (1963) The use of C14O2 yields from glucose-1- and -6-C14 for the evaluation of the pathways of glucose metabolism. J Biol Chem 238:517–523
Katz J, Rognstad R (1967) The labelling of pentose phosphate from glucose-14C and estimation of the rates of transaldolase, transketolase, the contribution of the pentose cycle, and ribose phosphate synthesis. Biochemistry 6:2227–2247
Wood HG, Katz J, Landau BR (1963) Estimation of pathways of carbohydrate metabolism. Biochem Z 338:809–847
ap Rees T (1980) Contributions of metabolic pathways to respiration. In: Davies DD (ed) The biochemistry of plants, Metabolism and respiration, vol 2. Academic Press, London, pp 1–29
Landau BR (1985) Use of radioisotopes in elucidating the nature of and quantitating the pentose pathway. In: Wood T (ed) The pentose phosphate pathway. Academic Press, London, pp 153–178
Larrabee MG (1989) The pentose cycle (hexose monophosphate shunt). J Biol Chem 264:15875–15879
Larrabee MG (1990) Evaluation of the pentose phosphate pathway from 14CO2 data. Biochem J 272:127–132
Katz J, Wood HG (1960) The use of glucose-C14 for the evaluation of the pathways of glucose metabolism. J Biol Chem 235:2165–2177
Masakapalli SK, Le Lay P, Huddleston JE, Pollock NL, Kruger NJ, Ratcliffe RG (2010) Subcellular flux analysis of central metabolism in a heterotrophic Arabidopsis thaliana cell suspension using steady-state stable isotope labeling. Plant Physiol 152:602–619
Masakapalli SK, Kruger NJ, Ratcliffe RG (2013) The metabolic flux phenotype of heterotrophic Arabidopsis cells reveals a complex response to changes in nitrogen supply. Plant J 74:569–582
Garlick AP, Moore C, Kruger NJ (2002) Monitoring flux through the oxidative pentose phosphate pathway using [1-14C]gluconate. Planta 216:265–272
Fowler MW (1971) Studies on the growth in culture of plant cells XIV. Carbohydrate oxidation during the growth of Acer pseudoplatanus L. cells in suspension culture. J Exp Bot 72:715–724
Malone JG, Mittova V, Ratcliffe RG, Kruger NJ (2006) The response of carbohydrate metabolism in potato tubers to low temperature. Plant Cell Physiol 47:1309–1322
Gupta KJ, Hebelstrup KH, Kruger NJ, Ratcliffe RG (2014) Nitric oxide is required for homeostasis of oxygen and reactive oxygen species in barley roots under anaerobic conditions. Mol Plant 7:747–750
Stitt M, ap Rees T (1978) Pathways of carbohydrate oxidation in leaves of Pisum sativum and Triticum aestivum. Phytochemistry 17:1251–1256
ap Rees T, Cerasi E, Wright BW (1976) Pathways of carbohydrate oxidation during thermogenesis by the spadix of Arum maculatum. Biochim Biophys Acta 437:22–35
Harrison PW, Kruger NJ (2008) Validation of the design of feeding experiments involving [14C]substrates used to monitor metabolic flux in higher plants. Phytochemistry 69:2920–2927
Sweetlove LJ, Williams TCR, Cheung CYM, Ratcliffe RG (2013) Modelling metabolic CO2 evolution – a fresh perspective on respiration. Plant Cell Environ 36:1631–1640
Yang TH, Heinzle E, Wittmann C (2005) Theoretical aspects of 13C metabolic flux analysis with sole quantification of carbon dioxide labelling. Comput Biol Chem 29:121–133
Bonarius HPJ, Schmid G, Tramper J (1997) Flux analysis of underdetermined metabolic networks: the quest for the missing constraints. Trends Biotechnol 15:308–314
Duncombe WG, Rising TJ (1969) Scintillation counting of 14CO2 from in vitro systems. Anal Biochem 30:275–278
Brouquisse R, James F, Raymond P, Pradet A (1991) Study of glucose starvation in excised maize root tips. Plant Physiol 96:619–626
ap Rees T, Beevers H (1960) Pentose phosphate pathway as a major component of induced respiration of carrot and potato slices. Plant Physiol 35:839–847
Field A, Miles J, Field Z (2012) Discovering statistics using R. Sage, London, pp 696–748
Acknowledgments
SKM acknowledges financial support from the University of Oxford (Clarendon Scholarship), Exeter College (Mr Krishna Pathak Scholarship), and a UK Overseas Research Student award.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media LLC
About this protocol
Cite this protocol
Kruger, N.J., Masakapalli, S.K., Ratcliffe, R.G. (2017). Assessing Metabolic Flux in Plants with Radiorespirometry. In: Jagadis Gupta, K. (eds) Plant Respiration and Internal Oxygen. Methods in Molecular Biology, vol 1670. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-7292-0_1
Download citation
DOI: https://doi.org/10.1007/978-1-4939-7292-0_1
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-7291-3
Online ISBN: 978-1-4939-7292-0
eBook Packages: Springer Protocols