Abstract
Nestin, a neural stem cell marker protein, is expressed in hair follicle cells above the bulge area. These nestin-positive hair follicle-associated-pluripotent (HAP) stem cells are negative for the keratinocyte marker K15 and can differentiate into neurons, glia, keratinocytes, smooth muscle cells, cardiac muscle cells, and melanocytes in vitro. HAP stem cells are positive for the stem cell marker CD34, as well as K15-negative, suggesting their relatively undifferentiated state. HAP stem cells promoted the functional recovery of injured peripheral nerves and the spinal cord. HAP stem cells differentiated into glial fibrillary acidic protein (GFAP)-positive Schwann cells when implanted in severed sciatic nerves and spinal cords in mice. These results suggest that HAP stem cells provide an important accessible, autologous source of adult stem cells for regenerative medicine, that have critical advantages over ES and iPS stem cells.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
The stem cell marker, nestin , is expressed in hair follicles in cells located above the bulge area (BA), below the sebaceous gland. The nestin-expressing hair follicle cells were discovered in transgenic mice with ND-GTP-driven green fluorescent protein (ND-GFP) [1–4]. The nestin-expressing cells of the hair follicle can differentiate into neurons , glial cells, smooth muscle cells, keratinocytes , and other cell types [3]. Nestin-expressing hair follicle stem cells can also differentiate to beating cardiac muscle cells [5]. We have termed these cells as hair-follicle-associated pluripotent (HAP) stem cells [6]. HAP stem cells were shown to effect functional-nerve and spinal-cord repair [7–11].
HAP stem cells originate in the BA and migrate to the dermal papilla (DP) (Fig. 1). HAP stem cells from the DP and BA differentiated into neuronal and glial cells after transplantation to the injured nerve or spinal cord and enhanced injury repair and locomotor recovery within 4 weeks [2–4, 11].
Nestin-positive, K15-negative HAP stem cells and nestin-negative, K-15 positive keratinocyte progenitor cells are in seprate locations in the hair follicles [9]
In Gelfoam® histoculture , HAP stem cells from mouse whisker follicles formed nerve-like structures containing β-III tubulin-positive fibers [12]. The growing fibers had growth cones on their tips expressing F-actin, indicating they were growing axons. These results suggest a major function of HAP stem cells is for growth of the follicle sensory nerve [12]. HAP stem cells can be cryopreserved with full function [13].
2 Materials
2.1 Reagents
-
1.
Transgenic mice with nestin-regulatory-element-driven green fluorescent protein (ND-GFP mice) (AntiCancer Inc., San Diego, CA).
-
2.
GFP-expressing transgenic mice (GFP mice) (AntiCancer Inc.).
-
3.
Non-transgenic nude mice (AntiCancer Inc.).
-
4.
Human scalp skin samples. (The human scalp skin samples were obtained from surgical specimens of normal human scalp skin . All experiments were performed according to Helsinki guidelines, in compliance with national regulations for the experimental use of human material.)
-
5.
Immuno-competent and immuno-deficient mice (AntiCancer Inc.).
-
6.
DMEM-F12 medium (GIBCO-BRL); (Life Technologies, Inc., Gaithersburg, MD).
-
7.
B-27 (GIBCO-BRL) 1 % penicillin-streptomycin (GIBCO-BRL).
-
8.
1 % methylcellulose (Sigma–Aldrich).
-
9.
Basic FGF at 20 ng/ml (Chemicon).
-
10.
RPMI medium 1640 (Cellgro) containing 10 % FBS.
-
11.
96-well uncoated tissue-culture dishes (BD Biosciences).
-
12.
SonicSeal four-well chamber slides (Nunc Inc.).
-
13.
Anti-β3-tubulin mAb (1:500, Tuj1 clone; Covance Research Products).
-
14.
Anti-neurofilament 200 polyclonal Ab (1:80; Sigma–Aldrich).
-
15.
Anti-GABA polyclonal Ab (1:200; Chemicon).
-
16.
Anti-neuronal-specific enolase mAb (1:800; Lab Vision).
-
17.
Anti-tyrosine hydroxylase polyclonal Ab (1:100; Chemicon).
-
18.
Anti-glial fibrillary acidic protein (GFAP) mAb (1:100; Molecular Probes).
-
19.
Anti-2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) mAb (1:50; Lab Vision).
-
20.
Anti-keratin 5/8 (K5/8) mAb (1:250; Chemicon).
-
21.
Anti-keratin 15 (K15) mAb (1:100; Lab Vision).
-
22.
Anti-smooth muscle actin mAb (1:200; Lab Vision).
-
23.
Anti-BrdUrd mAb (1:10; BD PharMingen).
-
24.
Anti-CD31 mAb (1:50; Chemicon).
-
25.
Anti-CD34 mAb (1:10; BD PharMingen).
-
26.
Secondary Abs: Alexa Fluor 568-conjugated goat anti-mouse (1:200; Molecular Probes); Alexa Fluor 568-conjugated goat anti-rabbit (1:200; Molecular Probes); and Alexa Fluor 647-conjugated chicken anti-rat (1:200; Molecular Probes).
-
27.
Mouse-on-mouse (MOM) immunodetection kit (Vector Laboratories).
-
28.
Ig horseradish peroxidase detection kit (BD PharMingen).
2.2 Equipment
-
1.
OV100 Small Animal Imaging System (Olympus).
-
2.
IMT-2 inverted microscope equipped with a mercury lamp power supply (Olympus).
-
3.
Hamamatsu C5810 3-chip cooled color CCD camera (Hamamatsu Photonics Systems).
-
4.
Lightools Fluorescence Imaging System (Lightools Research).
-
5.
Sony VCR model SLV-R1000 (Sony).
-
6.
Image Pro Plus 3.1 software (Media Cybernetics).
-
7.
1 ml 27G2 latex-free syringe (Becton Dickinson).
-
8.
25-ml Hamilton syringe (Fisher Scientific).
-
9.
D470/40 excitation filter (Chroma Technology).
-
10.
GG475 emission filter (Chroma Technology).
-
11.
Cloning cylinders (Bel-Art Products).
-
12.
Hemocytometer (Reichert Scientific Instruments).
-
13.
Blunt-end hook (Fine Science Tools).
-
14.
33-G needle (Fine Science Tools).
-
15.
Electric stimulator (FGK-1S, Medical Access).
-
16.
Higgins7 black waterproof ink (Sanford).
-
17.
Leica CM1850 cryostat (Leica Biosystems).
2.3 Equipment Setup
2.3.1 Whole-Body Imaging Equipment
The Olympus OV100 Small Animal Imaging System, containing an MT-20 light source and a DP70 CCD camera, can be used for whole-body and skin-flap imaging in live mice at variable magnification. The optics of the OV100 fluorescence imaging system have been specially developed for macroimaging as well as microimaging with high light-gathering capacity [14]. Many other fluorescence imaging systems can also be used to acquire subcellular images of HAP stem cells.
3 Methods
3.1 Isolation and Culture of Nestin-Positive HAP Stem Cells and Spheres
-
1.
Isolate vibrissa follicles by exposing the upper lip containing the vibrissa pad of ND-GFP mice. Dissect the vibrissa follicles under a binocular microscope. Pluck the vibrissa from the pad by pulling them gently by the neck with fine forceps. Wash the isolated vibrissae in DMEM-F12, containing B-27 and 1 % penicillin/streptomycin. Perform all surgical procedures under a sterile environment. Isolate ND-GFP HAP stem cells under fluorescence microscopy. Suspend the isolated cells in 1 ml DMEM-F12 containing B-27 with 1 % methylcellulose, and 20 ng/ml basic-FGF (bFGF) [1, 15]. Culture cells in 24-well tissue-culture dishes at 37 °C in a 5 % CO2 95 % air tissue-culture incubator. After 4 weeks, the ND-GFP-expressing hair follicle stem cells form spheres.
-
2.
For differentiation , centrifuge the spheres and remove the growth factor-containing DMEM-F12 medium. Resuspend the spheres in fresh RPMI 1640 medium containing 10 % FBS. Culture the spheres in SonicSeal four-well chamber slides.
-
3.
For cloning experiments, trypsinize ND-GFP spheres that had been cultured for 2 months and serially dilute them into DMEM-F12 containing B-27 in 96-well uncoated tissue-culture dishes. Supplement the medium with 1 % methylcellulose and 20 ng/ml bFGF. Change the medium every 2 days. After 4 weeks of clonal expansion, switch the ND-GFP spheres to RPMI 1640 medium containing 10 % FBS in SonicSeal four-well chamber slides. Label ND-GFP cells with BrdUrd for 7 days. Immuno-stain the cells for anti-βIII-tubulin and BrdU.
-
4.
Detect the immuno-cytochemical staining of βIII-tubulin and K15 in the ND-GFP cells with the Mouse-on-mouse (MOM) immuno-detection kit. Detect CD31 and CD34 with the Ig horseradish peroxidase detection kit, using the antibodies listed in Materials. For quantification of the percentage of cells producing a given marker protein, photograph at least three microscopic fields in any given experiment and determine the number of positive cells relative to the total number of cells.
3.2 Sciatic Nerve Regeneration with HAP Stem Cells
-
1.
Culture HAP stem cells and spheres from vibrissa follicles of GFP transgenic mice using the techniques described above (see Note 1 ).
-
2.
For differentiation , centrifuge GFP-expressing spheres and remove the growth factor-containing supernatant and resuspend the spheres in fresh RPMI 1640 medium containing 10 % FBS in Sonic-Seal four-well chamber slides. After 8 weeks of expansion, switch the GFP-expressing spheres to RPMI 1640 medium containing 10 % FBS in the SonicSeal four-well chamber slides (see Note 2 ).
-
3.
Transplant HAP stem cell spheres, isolated as described above, between the severed sciatic or tibial nerve fragments in immuno-competent C57BL6 mice under tribromoethanol anesthesia. Close the skin incision with nylon sutures (6–0). After 2 months, directly observe the sciatic nerve of the transplanted mouse by fluorescence microscopy under anesthesia (see Note 3 ).
-
4.
Embed sciatic nerve samples in tissue freezing-embedding medium and freeze at −80 °C overnight. Cut frozen sections 5 μm thick with a Leica CM1850 cryostat and air dry. Directly observe the sections under fluorescence microscopy.
-
5.
Use the frozen sections for immuno-fluorescence staining of β-III-tubulin, glial fibrillary acidic protein, K15, and smooth muscle actin as described above.
-
6.
Directly observe GFP fluorescence in the sciatic nerve in the live mouse with the OV100 and the excised sciatic nerve under an Olympus IMT-2 inverted microscope equipped with a mercury lamp power supply and a GFP filter set (see Note 4 ).
-
7.
Use an electric stimulator to deliver repetitious electric pulses of 0.05 mA at 10 Hz with pulse widths of 0.5 ms to stimulate control mice, mice with severed sciatic nerves, and mice that had HAP stem cells injected to join the severed nerve.
-
8.
Measure the difference of the gastrocnemius muscle length (from lateral epicondyle of femur to heel) before and after contraction by the electric stimulator in each case in 7 above.
-
9.
Obtain walking tracks by using a corridor open at one end to a darkened compartment. Soak the animal’s feet in Higgins 7 black waterproof ink and walk the animal multiple times to obtain measurable prints. Evaluate the tracks for print length and intermediate toe spread.
-
10.
For each experimental group, use at least seven mice, including control mice, mice with a severed tibial or sciatic nerve only, and mice with the tibial or sciatic nerve enjoined by injected HAP stem cells.
-
11.
Express the experimental data as the mean ± SD. Perform statistical analysis by using a two-tailed Student’s t-test.
3.3 Spinal-Cord Regeneration with HAP Stem Cells (Fig. 2)
Rejoining the severed thoracic region of the spinal cord with HAP stem cells . (a) Schematic of vibrissa follicle of GFP-transgenic mice shows the position of GFP-expressing HAP stem cells (red arrowheads). The HAP stem cells were cultured into DMEM-F12 containing B-27 supplemented with bFGF every 2 days, for 2 months. After 2 months, GFP-expressing HAP stem cells formed spheres. (b) (b1) Shows a sphere formed from GFP-expressing HAP stem cells. (b2, b3) GFP-expressing HAP stem cell spheres were transplanted to the severed thoracic region of the spinal cord in C57BL/6 immunocompetent mice (blue arrowheads). (c) Two months after transplantation of GFP-expressing HAP stem cell spheres between the severed thoracic region of the spinal cord, the GFP-expressing HAP stem cells joined the spinal cord (white arrowheads) [8]
-
1.
Using a binocular microscope, perform a laminectomy at the tenth thoracic spinal vertebra, followed by a transversal cut.
-
2.
Transplant GFP-expressing HAP stem cells between the severed thoracic region (spinal level T10) of the spinal cord in C57BL/6 immunocompetent mice.
-
3.
After 2 months, directly observe the spinal cord of the transplanted mice by fluorescence microscopy.
-
4.
Under anesthesia, excise spinal-cord samples of the transplanted mice.
-
5.
Freeze the spinal-cord sample sections and embed them in tissue freezing-embedding medium and store overnight at −80 °C.
-
6.
Cut frozen sections 5 μm thick with a Leica CM1850 cryostat, and air-dry.
-
7.
Directly observe the sections by fluorescence microscopy.
-
8.
Perform immuno-fluorescence staining of βIII-tubulin, GFAP, CNPase, K15, and SMA as described above.
-
9.
Conduct walking analyses for 12 weeks, as described above, using the Basso-Beattie-Bresnahan (BBB) locomotor rating scale.
-
10.
Express the experimental data as the mean ± SD. Perform statistical analysis using the two-tailed Student’s t-test (see Note 5 ).
3.4 Isolation of Human HAP Stem Cells (Figs. 3 and 4)
(a) Schema of a human hair follicle in the scalp. The human hair follicle was divided into three parts (upper, middle, and lower parts). (b1) HAP stem cells are located immediately below the sebaceous glands and above the hair-follicle bulge area in the upper part. The HAP stem cells were suspended in DMEM-F12 containing B-27 supplemented with bFGF every 2 days. (b2) The bulge area contained ND-GFP-expressing HAP stem cells (red arrowheads). Below the HAP stem cells in the hair follicle bulge area, nestin-negative, K15-positive cells (yellow arrowheads) are located (yellow arrowheads). (b3) Four weeks after culture in DMEM-F12, containing B-27 supplemented with bFGF every 2 days, HAP stem cells formed spheres
(a) HAP stem cells were switched into RPMI 1640 containing 10 % fetal bovine serum (FBS) from DMEM-F12 containing B-27 supplemented with bFGF every 2 days. Ten days after switching into RPMI 1640 medium containing 10 % FBS, the differentiating cells migrated away from the colonies. The nestin-positive, K15-negative HAP stem cells differentiated into β3-tubulin-positive neurons , S100- and GFAP-positive glial cells, K15-positive keratinocytes , and SMA-positive smooth muscle cells [16]
-
1.
Obtain surgical specimens of normal human scalp skin . Perform all experiments according to Helsinki guidelines, in compliance with national regulations for the experimental use of human material.
-
2.
Isolate whole hair follicles in the scalp skin by cutting the hair follicle pad and expose its inner surface.
-
3.
Dissect the scalp hair follicles under a binocular microscope. Pluck the follicles from the pad by pulling them gently by the neck with fine forceps. Wash in DMEM-F12 containing B-27 and 1 % penicillin/streptomycin.
-
4.
Perform all surgical procedures under a sterile environment.
-
5.
Isolate HAP stem cells under a binocular microscope, suspend in 1 ml DMEM-F12 containing B-27 with 1 % methylcellulose, 20 ng/ml basic FGF (bFGF).
-
6.
Culture cells in 24-well tissue culture dishes in a 37 °C, 5 % CO2/95 % air tissue-culture incubator for two months (see Note 6 ).
-
7.
Centrifuge HAP stem-cell spheres.
-
8.
For differentiation , resuspend the spheres in fresh RPMI 1640 medium containing 10 % fetal bovine serum in SonicSeal 4-well chamber slides (see Note 7 ).
3.5 Direct Transplantation of Upper Part of Hair Follicle-Containing HAP Stem Cells Promotes the Recovery of Peripheral-Nerve Injury (Fig. 5)
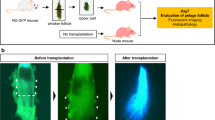
Rejoined severed sciatic nerve effected by transplantation of the upper part of separated hair follicle containing HAP stem cells to the severed sciatic nerve in C57BL/6 immunocompetent mice. (a1) Vibrissa hair follicle from GFP-transgenic mice were separated into three parts. (a2) Before transplantation. (a3) Right after transplantation of GFP-expressing upper parts of separated hair follicles. (b) Four weeks after transplantation, the GFP-expressing HAP stem cells joined the severed sciatic nerve [10]
-
1.
Isolate vibrissa follicles from transgenic C57/B6-GFP mice, as described above.
-
2.
Transplant the upper part of hair follicle containing HAP stem cells between the severed sciatic nerve in C57BL/6 immunocompetent mice as described above (see Note 8 ).
4 Notes
-
1.
After 4 weeks, GFP-expressing HAP stem cells formed GFP-expressing colonies (spheres).
-
2.
HAP stem cells isolated from the hair-follicle bulge area are negative for the keratinocyte marker keratin 15, and can differentiate into neurons , glia, keratinocytes , smooth muscle cells, and melanocytes in vitro. The HAP stem cells are positive for the stem cell marker CD34, as well as keratin 15-negative, suggesting their relatively undifferentiated state [3].
-
3.
After 2 months, the sciatic nerve has rejoined. At the healed juncture, of the nerve, the strong GFP fluorescence of the HAP stem cells can be observed. Implanting HAP stem cells into the gap region of the severed sciatic or tibial nerves greatly enhanced the rate of nerve regeneration and restoration of nerve function. The transplanted HAP stem cells transdifferentiated mostly into Schwann cells , which are known to support neuron regrowth. The treated mice regained the ability to walk essentially normally.
-
4.
Auto-fluorescence: It is important to minimize auto-fluorescence interference from the tissue and body fluids by using proper filters. Excitation filters should have a narrow band as close to 490 nm as possible to specifically excite GFP whose excitation peak is distinct from that of the skin , tissues, and fluid of the animal. In addition, proper band-pass emission filters should be used with a cutoff of approximately 515 nm.
-
5.
We severed the thoracic spinal cord of C57BL/6 immunocompetent mice and transplanted GFP-expressing HAP stem cells to the injury site. Most of the transplanted cells differentiated into Schwann cells that apparently facilitated repair of the severed spinal cord. The rejoined spinal cord reestablished extensive hind-limb locomotor performance [8].
-
6.
After 4 weeks, nestin-expressing HAP stem cells formed colonies.
-
7.
Human HAP stem cells can also be used to regenerate severed peripheral nerves in mice as described above.
-
8.
Previously, HAP stem cells were cultured for 1–2 months before transplantation to the injured nerve or spinal cord which would not be optimal for clinical application of these cells for nerve or spinal-cord repair , since the patient should be treated soon after injury. We subsequently addressed this issue by directly using the upper part of the hair follicle, which is highly enriched in HAP stem cells in the bulge area, without culture, for injection into the severed sciatic nerve in mice. After injection of upper part of the whisker, the implanted HAP stem cells grew and promoted joining of the severed nerve. The transplanted HAP stem cells differentiated mostly to glial cells forming myelin sheaths, which promoted axonal growth and functional recovery of the severed nerve. These results suggest that direct transplantation of the uncultured upper part of the hair follicle containing HAP stem cells is an important method to promote the recovery of peripheral-nerve injuries and has significant clinical potential [10].
References
Li L, Mignone J, Yang M, Matic M, Penman S, Enikolopov G et al (2003) Nestin expression in hair follicle sheath progenitor cells. Proc Natl Acad Sci U S A 100:9958–9961
Amoh Y, Li L, Yang M, Moossa AR, Katsuoka K, Penman S et al (2004) Nascent blood vessels in the skin arise from nestin-expressing hair follicle cells. Proc Natl Acad Sci U S A 101:13291–13295
Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM (2005) Multipotent nestin-positive, keratin-negative hair-follicle-bulge stem cells can form neurons. Proc Natl Acad Sci U S A 102:5530–5534
Amoh Y, Li L, Katsuoka K, Hoffman RM (2007) Chemotherapy targets the hair-follicle vascular network but not the stem cells. J Invest Dermatol 127:11–15
Yashiro M, Mii S, Aki R, Hamada Y, Arakawa N, Kawahara K et al (2015) From hair to heart: nestin-expressing hair-follicle-associated pluripotent (HAP) stem cells differentiate to beating cardiac muscle cells. Cell Cycle 14:2362–2366
Hoffman RM (2014) Nestin-expressing hair follicle-accessible-pluripotent (HAP) stem cells for nerve and spinal cord repair. Cells Tissues Organs 200:42–47
Amoh Y, Li L, Campillo R, Kawahara K, Katsuoka K, Penman S et al (2005) Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proc Natl Acad Sci U S A 102:17734–17738
Amoh Y, Li L, Katsuoka K, Hoffman RM (2008) Multipotent hair follicle stem cells promote repair of spinal cord injury and recovery of walking function. Cell Cycle 7:1865–1869
Amoh Y, Kanoh M, Niiyama S, Hamada Y, Kawahara K, Sato Y et al (2009) Human hair follicle multipotent stem (hfPS) cells promote regeneration of peripheral-nerve injury: An advantageous alternative to ES and iPS cells. J Cell Biochem 107:1016–1020
Amoh Y, Hamada Y, Aki R, Kawahara K, Hoffman RM, Katsuoka K (2010) Direct transplantation of uncultured hair-follicle multipotent stem (hfPS) cells promotes the recovery of peripheral nerve injury. J Cell Biochem 110:272–277
Liu F, Uchugonova A, Kimura H, Zhang C, Zhao M, Zhang L et al (2011) The bulge area is the major hair follicle source of nestin-expressing multipotent stem cells which can repair the spinal cord compared to the dermal papilla. Cell Cycle 10:830–839
Mii S, Duong J, Tome Y, Uchugonova A, Liu F, Amoh Y et al (2013) The role of hair follicle nestin-expressing stem cells during whisker sensory-nerve growth in long-term 3D culture. J Cell Biochem 114:1674–1684
Kajiura S, Mii S, Aki R, Hamada Y, Arakawa N, Kawahara K et al (2015) Cryopreservation of the hair follicle maintains pluripotency of nestin-expressing hair follicle-associated pluripotent stem cells. Tissue Eng Part C Methods 21:825–831
Yamauchi K, Yang M, Jiang P, Xu M, Yamamoto N, Tsuchiya H et al (2006) Development of real-time subcellular dynamic multicolor imaging of cancer-cell trafficking in live mice with a variable-magnification whole-mouse imaging system. Cancer Res 66:4208–4214
Amoh Y, Mii S, Aki R, Hamada Y, Kawahara K, Hoffman RM et al (2012) Multipotent nestin-expressing stem cells capable of forming neurons are located in the upper, middle and lower part of the vibrissa hair follicle. Cell Cycle 11:3513–3517
Amoh Y, Kanoh M, Niiyama S, Kawahara K, Satoh Y, Katsuoka K et al (2009) Human and mouse hair follicles contain both multipotent and monopotent stem cells. Cell Cycle 8:176–177
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Amoh, Y., Katsuoka, K., Hoffman, R.M. (2016). Peripheral-Nerve and Spinal-Cord Regeneration in Mice Using Hair-Follicle-Associated Pluripotent (HAP) Stem Cells. In: Hoffman, R. (eds) Multipotent Stem Cells of the Hair Follicle. Methods in Molecular Biology, vol 1453. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3786-8_4
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3786-8_4
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3784-4
Online ISBN: 978-1-4939-3786-8
eBook Packages: Springer Protocols