Abstract
Isolated whisker follicles from nestin-driven green fluorescent protein (ND-GFP) mice, containing hair-associated pluripotent (HAP) stem cells, were histocultured in three dimensions on Gelfoam® for 3 weeks for subsequent transplantation to the spinal cord in order to heal an induced injury with the HAP stem cells. The hair shafts were removed from Gelfoam®-histocultured whisker follicles, and the remaining parts of the whisker follicles, containing GFP-nestin-expressing (HAP) stem cells, were transplanted into the injured spinal cord of nude mice, along with the Gelfoam®. After 90 days, the mice were sacrificed and the spinal cord injuries were observed to have healed. ND-GFP expression was intense at the healed area of the spinal cord, as observed by fluorescence microscopy, demonstrating that the HAP stem cells were involved in healing the spinal cord. The transplanted whisker follicles produced remarkably long hair shafts in the spinal cord over 90 days and curved and enclosed the spinal cord. This result changes our concept of hair growth, demonstrating it is not limited to the skin and that hair growth appears related to HAP stem cells as both increased in tandem on the spinal cord.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
- Mice, Ectopic hair growth
- Gelfoam whisker culture
- Transplantation
- Spinal cord
- Nestin, HAP stem cells
- GFP
- Imaging
1 Introduction
We previously discovered nestin-expressing hair-follicle-associated pluripotent (HAP) stem cells , in the permanent upper hair follicle immediately below the sebaceous glands in the hair follicle bulge area (BA), in nestin-driven green fluorescent protein (GFP) (ND-GFP) transgenic mice. The ND-GFP-expressing HAP stem cells in the bulge area surrounded the hair shaft and were interconnected by short dendrites [1].
We subsequently demonstrated that ND-GFP HAP stem cells isolated from the BA could differentiate into neurons , glia, keratinocytes , smooth muscle cells, and melanocytes in vitro [2].
HAP stem cells from ND-GFP mice were transplanted into the gap region of severed sciatic nerves of nude mice. The transplanted HAP stem cells enhanced the rate of nerve regeneration and the restoration of nerve function. The HAP stem cells differentiated mostly into Schwann (glial) cells, which supported neuron regrowth [3].
HAP stem cells were subsequently transplanted to the injured spinal cord of nude mice. Most of the transplanted cells also differentiated into Schwann cells which facilitated repair of the severed spinal cord. The rejoined spinal cord resulted in extensive hind-limb locomotor performance recovery [4].
BA and dermal papilla (DP) HAP stem cells were histocultured on Gelfoam® and were separately transplanted to the injured spinal cord of nude mice. Both DP and BA ND-GFP cells differentiated into neuronal and glial cells after transplantation to the injured spinal cord. ND-GFP cells from both areas enhanced injury repair and locomotor recovery [5, 6].
Whiskers from ND-GFP mice were placed in 3D Gelfoam® histoculture . The whiskers produced β-III tubulin-positive fibers, consisting of ND-GFP-expressing HAP stem cells which extended up to 500 mm from the whisker nerve stump in Gelfoam® histoculture . These fibers had growth cones on their tips expressing F-actin indicating the fibers were growing axons which were highly enriched in ND-GFP HAP stem cells [7].
We previously demonstrated the surprising result that Gelfoam®-histocultured whisker follicles, transplanted to the injured spine, along with the Gelfoam® on which they were histocultured, sprouted long hair shafts from the spinal cord during the period the HAP stem cells rejoined the injured spinal cord (10). The present chapter describes a protocol for transplanting Gelfoam® whisker follicles to the injured spine.
2 Materials
2.1 Animals
-
1.
Nestin-GFP transgenic mice and non-transgenic nude mice (AntiCancer, Inc., San Diego, CA).
2.2 Instruments
-
1.
Stereomicroscope (MZ6, Leica, Buffalo Grove, IL).
-
2.
Scissors and forceps (Fisher Scientific, Hanover Park, IL).
-
3.
Needle holder (Fisher Scientific).
-
4.
Microscissors and microforceps (Fisher Scientific).
-
5.
Exel International Disposable Scalpels (Fisher Scientific).
-
6.
10 ml syringe (BD).
-
7.
Illumatool™ in vivo fluorescence imaging system (Lightools Research, Encinitas, CA).
-
8.
OV100 small animal fluorescence imaging system (Olympus Corp.).
-
9.
FV1000 confocal laser scanning microscope (Olympus Corp.).
2.3 Reagents and Consumable Items
-
1.
Gelfoam ® (Pfizer Inc., New York, NY).
-
2.
DMEM/F12 medium (GIBCO Life Technologies, Grand Island, NY).
-
3.
B-27 (GIBCO Life Technologies).
-
4.
N2 (GIBCO Life Technologies).
-
5.
Penicillin and streptomycin (GIBCO Life Technologies).
-
6.
Ketamine mixture: ketamine (100 mg/ml) 10 ml, xylazine (20 mg/ml) 10 ml, acepromazine (10 mg/ml) 4 ml, PBS 26 ml, total 50 ml.
-
7.
Six-well dish (Nest Biotechnology Co., Rahway, NJ).
-
8.
PBS (phosphate buffered saline) (GIBCO Life Technologies).
-
9.
0.9 % saline.
-
10.
Fixation solution: 4 % paraformaldehyde or 10 % buffered formalin acetate.
3 Methods
3.1 Isolation of Mouse Whisker Follicles
-
1.
Prepare the Gelfoam® culture medium before hair follicle Gelfoam® histoculture : DMEM/F12 medium containing B-27 (2.5 %), N2 (1 %) and 1 % penicillin and streptomycin.
-
2.
Use a disposable scalpel to cut Gelfoam® into approximately 1 cm × 1 cm and place into six-well dishes, and add 1 ml cell-culture medium to the Gelfoam® (see Note 1 ). Place the Gelfoam® with the cell culture medium in a 37 °C CO2 incubator overnight (see Note 2 ).
-
3.
Anesthetize nestin-GFP transgenic mice (6–8 weeks) with the ketamine mixture and cut the whisker hair shafts.
-
4.
Remove both of the whisker pads from the mice with sterilized scissors.
-
5.
Lay whisker pads on a black pad, expose the inner side of whisker pad and pin the whisker pad to the black pad.
-
6.
Under a stereo-microscope, carefully remove the attached tissue with microscissors and microforceps. Gently remove each whisker follicle by grasping the hair shaft near the skin surface and pulling firmly and smoothly. Put the freshly-isolated hair follicle in DMEM immediately (see Note 3 ).
-
7.
Collect all hair follicles and choose the hair follicles which are intact and transfer onto the prepared soaked Gelfoam® in the tissue-culture well. Each whisker follicle has one piece of Gelfoam® for support.
-
8.
Place the six-well dishes with the Gelfoam® histocultures in a 37 °C CO2 incubator for 3 weeks. Change the medium every 2–3 days (see Note 4 ).
-
9.
Use a confocal laser scanning microscope for two- (X,Y) and three-dimensional (3D, X,Y,Z) high-resolution imaging of the whiskera follicles in Gelfoam ® histoculture . Obtain fluorescence images using the 4×/0.10 Plan N, 10×/0.30 Plan-NEOFLUAR, 20×/0.50 UPlan FL N, and 20×/1.00w XLUMplan FL objectives.
3.2 Surgical Procedures for Spinal Cord Injury and Transplantation of Gelfoam Whisker Histocultures
-
1.
Anesthetize non-transgenic nude mice with the ketamine mixture.
-
2.
Place the mouse in a flat prone horizontal position. Identify the last rib by palpation. This allows one to estimate the location of the XIIIth thoracic vertebra (T13).
-
3.
Cut the skin overlaying the vertebral column.
-
4.
Observe and localize the spine process at T13 by palpating the XIIIth ribs, and identify T8 by counting spine processes cranially from T13 carefully, under an MZ6 stereo-microscope.
-
5.
Use fine scissors and forceps to separate the muscles along the spine under the stereo-microscope.
-
6.
Remove the spinous processes and lamina from T8 in order to achieve a partial laminectomy.
-
7.
Remove the right side of the spinal cord by using a 31 G insulin syringe needle. The lesion surface size should be 500 μm × 500 μm, and the depth of the lesion should be from the dorsal to the abdominal surface of the spinal cord.
-
8.
Cut Gelfoam® whisker cultures (3 weeks) into 500 μm × 500 μm pieces and introduce them into the spinal cord lesion site with fine forceps (see Note 5 ).
-
9.
Place the surgically-repaired mice back in their cages and maintain them for 90 days. Use fluorescence and bright light microscopy of the spinal cord to observe spinal cord repair and hair-shaft elongation. Euthanize animals at day 90 .
3.3 Whole-Animal Perfusion Fixation
-
1.
Anesthetize transplanted mice at day 90 with a ketamine mixture.
-
2.
Plate the mouse on a shallow tray with its back down. With sharp scissors, cut the skin and the muscles through the abdominal wall beneath the rib cage. Carefully separate the liver and other connective issue from the diaphragm.
-
3.
Hold and lift up the xiphoid process of the sternum with a needle holder, and cut both sides of the ribs up to the collarbone, and open up the thoracic cavity (see Note 6 ).
-
4.
Insert a needle into the left ventricle while holding the heart steady with your hand.
-
5.
Pump a small volume of saline into the heart with a 10 ml syringe (see Note 7 ) whereby the auricle of right atrium will become obviously larger and make a quick incision to allow the blood to flow freely.
-
6.
Keep pumping the 0.9 % saline into the left ventricle for 2–3 min until the solution coming out from the right atrium is not red and clear. The color of the liver will turn pale.
-
7.
Once blood has been cleared from the body, perfuse 4 % paraformaldehyde or 10 % buffered formalin acetate solution (10–20 min) (see Note 8 ).
-
8.
Stop the perfusion and excise the spinal cord. Under a stereomicroscope, cut the skin and the muscle, and remove the spine, and carefully excise the spinal cord. Place the specimens in vials containing the fixation solution and store at 4 °C for future histological procedures.
-
9.
Obtain fluorescence images of 90-day fixed spinal cords using an OV100 in vivo fluorescence imaging system (see Note 9 ).
4 Notes
-
1.
Gelfoam®, which is derived from gelatinized pig skin , provides a three-dimensional physiological scaffold for the hair follicle to attach and grow, both in vitro and in the injured spinal cord, which may provide nutritional factors for long-term hair shaft growth [5, 6, 8, 9]. Gelfoam® appears to preserve the integral hair follicle both in vitro and in vivo.
-
2.
The purpose of this step is to hydrate the Gelfoam® with culture medium.
-
3.
When isolating the hair follicles, try not to grasp and press the bulge area. Maintain the hair follicle intact and not pressed.
-
4.
The culture medium should be changed every 2–3 days. The interval time cannot exceed 1 week, otherwise the Gelfoam® will became thin and digested by 2–3 weeks.
-
5.
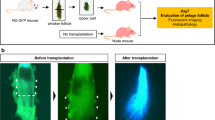
After Gelfoam® histoculture of whiskers for 3 weeks, elongated hair shafts were cut off and the whisker follicles, containing ND-GFP HAP stem cells , which had increased during histoculture, were transplanted along with the Gelfoam® into the injured spinal cord of nude mice (see Fig. 1).
Fig. 1 Transplantation of nestin-driven green fluorescent protein (ND-GFP )-expressing whisker follicle Gelfoam® histocultures to the injured spinal cord of nude mice. After Gelfoam® histoculture of isolated whisker hair follicles from ND-GFP mice for 3 weeks, the long hair shafts of the whisker follicle were cut off, and the follicle, along with the Gelfoam®, was transplanted into the injured nude-mouse spinal cord. The transplanted mice were sacrificed after 90 days. ND-GFP expression intensified by 90 days and expanded in the injured area of the spinal cord, which was apparently healed by ND-GFP expressing HAP stem cells within the whiskers. A total of seven mice were studied. The figure shows typical data
-
6.
Lift up the xiphoid process of stemum with a needle holder to avoid or minimize damage to the tissues in the thoracic cavity when cutting the ribs. Once the thoracic cavity is opened, the next 4–7 steps should be completed as soon as possible, since the mice will die soon.
-
7.
Alternatively, a perfusion pump or a flask placed upside down above the animal can be used.
-
8.
First perfuse the fixation solution quickly. When spontaneous movement occurs and the liver became hard, the perfusion is almost complete and then perform perfusion slowly to allow the fixation solution to flow through capillaries in order to fix distal tissue and nerve structures better.
-
9.
After 90 days, the mice are sacrificed. At this time, the spinal-cord lesion will be healed. ND-GFP expression will be visible and intense along the healed area of the spinal cord, indicating the HAP stem cells are viable and healed the injury. We previously reported that implantation of Gelfoam®-supported whisker histocultures to the injured spinal cord resulted in functional healing [5]. In the present experiment, the whiskers were histocultured for a longer period of time and the mice had a longer time after implantation before examination of their spinal cord. It was assumed that the spinal cord was functionally healed, as in our previous experiments [4, 5]. Unexpectedly, stout pigmented hair fibers were observed growing from the implanted whisker follicle Gelfoam® complex in the spinal cord (see Fig. 2). The hair shafts grew remarkably long in the spinal cord, as much as approximately 14 mm, and curved and enclosed the spinal cord (see Fig. 2, Mouse-3). A total of seven mice were implanted with Gelfoam® whisker histocultures and after examination, six mice showed ectopic hair growth (p = 0.001 compared to mice implanted with Gelfoam® only). The unanticipated results demonstrate the great potential of hair shaft growth after Gelfoam® histoculture and transplantation of the hair follicle in vivo, even at an ectopic site. The length of the hair shaft observed in mouse 3 of almost 14 mm suggests that the ectopic site of the spine can strongly stimulate hair growth .
Fig. 2 Ectopic hair growth in the spinal cord. Ninety days after transplantation of the 3-week Gelfoam® ND-GFP -expressing whisker histoculture s in the injured spinal cord, long hair shafts (arrows), were observed along and around the healed spinal cord. (a) Shows the elongated hair shafts that grew from whisker follicles, previously histocultured on Gelfoam®, transplanted into the injured spinal cord in three different mice at day 90 after surgery. Mouse 3 had the most remarkable hair shaft growth, which curved and enclosed the spinal cord. Arrows show the hair growth in the spinal cord. (b) Panels show the hair-shaft growth from the transplanted Gelfoam®-histoculture whisker follicles in the spine from mouse 3 at higher magnification from different views of the spinal cord (dorsal, right, and left side). The growing hair shaft reached a length of almost 14 mm and curved around the spinal cord. Arrows depict the hair shaft growing from the whisker hair follicles transplanted in the spine. Six out of seven mice implanted with Gelfoam® whisker histocultures showed extensive ectopic hair growth on the spine
References
Li L, Mignone J, Yang M et al (2003) Nestin expression in hair follicle sheath progenitor cells. Proc Natl Acad Sci U S A 100:9958–9961
Amoh Y, Li L, Katsuoka K et al (2005) Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc Natl Acad Sci U S A 102:5530–5534
Amoh Y, Li L, Campillo R et al (2005) Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proc Natl Acad Sci U S A 102:17734–17738
Amoh Y, Li L, Katsuoka K et al (2008) Multipotent hair follicle stem cells promote repair of spinal cord injury and recovery of walking function. Cell Cycle 7:1865–1869
Liu F, Uchugonova A, Kimura H et al (2011) The bulge area is the major hair follicle source of nestin-expressing pluripotent stem cells which can repair the spinal cord compared to the dermal papilla. Cell Cycle 10:830–839
Hoffman RM (2014) Nestin-expressing hair follicle-accessible pluripotent stem cells for nerve and spinal cord repair. Cells Tissues Organs 200:42–47
Mii S, Duong J, Tome Y et al (2013) The role of hair follicle nestin-expressing stem cells during whisker sensory-nerve growth in long-term 3D culture. J Cell Biochem 114:1674–1684
Duong J, Mii S, Uchugonova A et al (2012) Real-time confocal imaging of trafficking of nestin-expressing multipotent stem cells in mouse whiskers in long-term 3-D histoculture. In Vitro Cell Dev Biol Anim 48:301–305
Mii S, Uehara F, Yano S et al (2013) Nestin-expressing stem cells promote nerve growth in long-term 3-dimensional Gelfoam(R)-supported histoculture. PLoS One 8:e67153
Cao W, Li L, Mii S, Amoh Y, Liu F, Hoffman RM (2015) Long-term extensive ectopic hair growth on the spinal cord of mice from transplanted whisker follicles. PLoS One 10:e0133475
Acknowledgements
This studies described in the present chapter were supported by the National Institute of Neurological Disorders and Stroke grant NS086217.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Cao, W., Liu, F., Amoh, Y., Hoffman, R.M. (2016). Protocols for Ectopic Hair Growth from Transplanted Whisker Follicles on the Spinal Cord of Mice. In: Hoffman, R. (eds) Multipotent Stem Cells of the Hair Follicle. Methods in Molecular Biology, vol 1453. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3786-8_14
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3786-8_14
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3784-4
Online ISBN: 978-1-4939-3786-8
eBook Packages: Springer Protocols