Abstract
Several pathogenic fungi, including Cryptococcus gattii, Histoplasma capsulatum, Coccidioides immitis, and Penicillium marneffei, cause serious infectious diseases in immunocompetent humans. However, currently, prophylactic and therapeutic vaccines are not clinically used. In particular, C. gattii is an emerging pathogen and thus far protective immunity against this pathogen has not been well characterized. Experimental vaccines such as component and attenuated live vaccines have been used as tools to study protective immunity against fungal infection. Recently, we developed a dendritic cell (DC)-based vaccine to study protective immunity against pulmonary infection by highly virulent C. gattii strain R265 that was clinically isolated from bronchial washings of infected patients during the Vancouver Island outbreak. In this approach, bone marrow-derived DCs (BMDCs) are pulsed with heat-killed C. gattii and then transferred into mice prior to intratracheal infection. This DC vaccine significantly increases interleukin 17A (IL-17A)-, interferon gamma (IFN-γ)-, and tumor necrosis factor alpha (TNF-α)-producing T cells in the lungs and spleen and ameliorates the pathology, fungal burden, and mortality following C. gattii infection. This approach may result in the development of a new means of controlling lethal fungal infections. In this chapter, we describe the procedures of DC vaccine preparation and murine pulmonary infection model for analysis of immune response against C. gattii.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
- Cryptococcosis
- Cryptococcus gattii
- Acapsular mutant
- Glucuronoxylomannan
- Fungal vaccine
- Bone marrow-derived dendritic cells
- DC vaccine
- T-cell-inducing vaccine
- Pulmonary infection
1 Introduction
Cryptococcus gattii is an emerging fungal pathogen that infects immunocompetent humans via the respiratory tract. This pathogen can colonize the lungs and often disseminates to the brain, causing cryptococcosis , a life-threatening infectious disease with a mortality rate of 8–20 %. Although C. gattii infection is typically endemic in tropical areas such as Australia and Papua New Guinea, outbreaks have recently been reported on Vancouver Island and in surrounding areas [1–4]. To promote awareness of this outbreak, public health working groups have been organized in the Centers for Disease Control and Prevention of the United States and British Columbia [5, 6].
It is a key feature of highly virulent strains of C. gattii that they do not induce a strong inflammatory response after infection. Previous studies showed less migration of leukocytes and lower amounts of inflammatory cytokines in the lungs of mice experimentally infected with the C. gattii R265 strain that was clinically isolated during the Canadian outbreak than in those infected with Cryptococcus neoformans standard strain H99 [7–9]. A smaller amount of inflammatory cytokines was also observed in the cerebrospinal fluid of humans infected with C. gattii [10, 11]. A recent study induced a mixed infection of C. neoformans strain H99 and highly virulent C. gattii strain R265. Consequently, R265 infection inhibited chemokine expression and the infiltration of Th1 and Th17 cells in the lungs of mice infected with H99 [12]. These data suggest that the highly virulent C. gattii strain can suppress the inflammatory response rather than evade it.
This immunosuppressive response might be explained by the characteristics of cell components of C. gattii. It is known that C. gattii as well as C. neoformans cells are enveloped with capsular polysaccharides consisting of glucuronoxylomannan (GXM) and galactoxylomannan (GalXM) [13]. These polysaccharides not only protect yeast cells from environmental stress but can also be released in culture supernatant, where they are known as exopolysaccharides [13]. GXM and GalXM account for approximately 90 % and 10 %, respectively, of the total exopolysaccharides of C. neoformans [14, 15]. These polysaccharides can play an inhibitory role in numerous immune response s including cytokine production and leukocyte migration in vitro and in vivo [16]. Administration of purified GXM can inhibit inflammatory responses and improve symptoms in experimental endotoxin shock and rheumatoid arthritis [17, 18]. The results of these reports suggest that capsular polysaccharides play a role in the virulence of C. gattii.
The high virulence and immunosuppressive behavior of C. gattii often interfere with the analysis of protective immunity against C. gattii infection. In other words, the experimental “loss-of-function” approach using gene knockout mice may interfere with the study of protective immunity, owing to the immunosuppressive effect. A few studies have demonstrated protective immunity against highly virulent C. gattii using gene knockout mice, and this immunity remains to be elucidated [19, 20].
Recently, we demonstrated protective immunity against C. gattii infection by a gain-of-function approach using a dendritic cell (DC)-based vaccine [21]. DCs play a central role in T-cell activation [22] and can also be used as antigen delivery systems for vaccines against cancers or infections [23, 24]. Adoptive transfer of DCs pulsed with fungal cells or with fungal RNA has also been used as a means of assessing T-cell-mediated immunity against pathogenic fungi [25–27]. In our study, mouse bone marrow -derived DCs (BMDCs) were pulsed with heat-killed C. gattii and were then adoptively transferred into mice prior to intratracheal C. gattii infection. This DC vaccine markedly increased interleukin 17A (IL-17A)-, interferon gamma (IFN-γ)-, and tumor necrosis factor alpha (TNF-α)-producing T cells in the lungs and spleen and ameliorated pathology, fungal burden, and mortality following C. gattii infection [21].
In this chapter, we describe an experimental DC vaccine used to study protective immunity against the fungal pathogen C. gattii. As described above, capsular components are known to suppress several immune response s by DCs [28, 29]. Accordingly, we used a capsule-deficient mutant of C. gattii (cap60 deletion mutant, ∆cap60 strain) as a vaccine antigen . To evaluate the vaccine’s efficacy, serum antibody titer, T-cell response to antigen restimulation, and protective effect against infection are generally measured. We also describe the pulmonary infection model used to evaluate the efficacy of the DC vaccine.
2 Materials
2.1 Preparation of Heat-Killed C. gattii for Vaccine Antigen
-
1.
C. gattii ∆cap60 or other acapsular strains.
-
2.
Yeast extract peptone dextrose (YPD) medium: 1 % [weight/volume (w/v)] yeast extract, 2 % (w/v) Bacto-Peptone, and 2 % (w/v) dextrose, sterilized by autoclaving. Add 2 % (w/v) agar to prepare YPD agar plate.
-
3.
50-mL Conical tubes with screw cap with 0.2-μm filter membrane (Greiner Bio-One).
-
4.
Sterile and endotoxin-free Dulbecco’s phosphate-buffered saline (DPBS; Invitrogen).
-
5.
Disposable hemocytometer.
2.2 Preparation of BMDCs
-
1.
C57BL/6J mice (Japan SLC).
-
2.
RPMI 1640 complete medium: RPMI 1640 medium (Sigma) supplemented with 10 % fetal bovine serum (FBS), 1 % streptomycin–penicillin solution (Sigma; 10,000 units of penicillin and 10 mg/mL of streptomycin), 44 μM 2-mercaptoethanol.
-
3.
Murine granulocyte–macrophage colony-stimulating factor (mGM-CSF; PeproTech).
-
4.
10-cm-diameter Petri dishes: Untreated for cell culture.
-
5.
26 G × 1/2 needle (TERUMO).
-
6.
2.5-mL syringe (TERUMO).
-
7.
70-μm Cell strainer (Corning).
-
8.
Red blood cell (RBC) lysis buffer: Mix 9 volumes of 0.83 % (w/v) NH4Cl and 1 volume of 200 mM Tris–HCl pH 7.6, prior to use. Sterilize each stock solution by filtering through a sterile 0.2-μm membrane and store at 4 °C.
-
9.
Hemocytometer.
-
10.
Trypan blue solution, 0.4 % (w/v), sterile-filtered (Sigma).
2.3 Vaccination with DC Vaccine
-
1.
Heat-killed ∆cap60 strain (1 × 109 cells/mL).
-
2.
RPMI 1640 complete medium: RPMI 1640 medium (Sigma) supplemented with 10 % FBS, 1 % streptomycin–penicillin solution (Sigma; 10,000 U of penicillin and 10 mg/mL of streptomycin), 44 μM 2-mercaptoethanol.
-
3.
Mouse granulocyte–macrophage colony-stimulating factor (mGM-CSF; PeproTech).
-
4.
10-cm-diameter Petri dishes, untreated for cell culture.
-
5.
Sterile and endotoxin-free DPBS.
-
6.
Hemocytometer.
-
7.
Trypan blue solution, 0.4 % (w/v), sterile-filtered (Sigma).
-
8.
C57BL/6J mice (Japan SLC).
-
9.
30 G × 1/2 needle (BD) and 1-mL syringe (TERUMO).
2.4 Evaluation of DC Vaccine Efficacy (Murine Pulmonary Infection Model)
-
1.
Vaccinated and non-vaccinated C57BL/6J mice.
-
2.
C. gattii clinical isolate R265, highly virulent strain.
-
3.
YPD medium: 1 % (w/v) yeast extract, 2 % (w/v) Bacto-Peptone, and 2 % (w/v) dextrose, sterilized by autoclaving. Add 2 % (w/v) agar to prepare YPD agar plate.
-
4.
50-mL Conical tubes with screw cap with 0.2-μm filter membrane (Greiner Bio-One).
-
5.
Sterile and endotoxin-free DPBS (Invitrogen).
-
6.
Disposable hemocytometer.
-
7.
Vaporizer and isoflurane.
-
8.
24 G × 3/4 indwelling needle (TOP Corporation).
-
9.
Sterile stainless steel mesh (portable tea strainer with handle).
-
10.
2.5-mL Syringe (TERUMO).
3 Methods
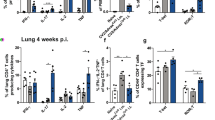
The procedural scheme and representative results are shown in Figs. 1 and 2, respectively.
Representative results of C. gattii murine pulmonary infection model. (a) Fungal burden in lungs. Heat-killed fungi (HK-R265 or HK-CAP60∆: 5 × 106 CFUs/mouse), bone marrow -derived dendritic cell (DC: 5 × 105 cells/mouse), and DC pulsed with heat-killed fungi (HK-R265/DC or HK-CAP60∆/DC: 5 × 105 cells/mouse) were administered intravenously twice 14 days and 1 day before the intratracheal infection of R265 (3 × 103 CFUs/mouse). Fungal burden in lungs was evaluated on day 14 postinfection. NS: nonsignificant difference vs. unvaccinated mice (None), * t-test p < 0.05 vs. None, # t-test p < 0.05 vs. DC. (b) Gross pathology of lungs. Lungs dissected from three mice at day 13 postinfection were fixed in 10 % formalin. The lungs of unvaccinated mice were swollen and showed awkward shapes and bumps. Lung homogenates of unvaccinated mice are usually very sticky and may contain higher amounts of the capsular component glucuronoxylomannan (GXM) produced by C. gattii cells
3.1 Preparation of Heat-Killed C. gattii for Vaccine Antigen
-
1.
Fungal strains are generally maintained at −80 °C in glycerol stocks. To revive C. gattii cells, scrape off splinters of solid ice from glycerol stock using a sterile toothpick or disposable inoculation loop and streak onto a YPD plate. Incubate the plate at 30 °C for 2 days.
-
2.
Pick five to ten colonies and inoculate into 10 mL of YPD liquid medium. Because C. gattii and other fungal cells strongly require oxygen for their propagation, aeration of culture containers should be ensured. It is recommended to use 50-mL conical tubes that have a screw cap with a 0.2-μm filter membrane.
-
3.
Shake YPD culture medium (170–200 rpm) overnight at 30 °C.
-
4.
Harvest yeast cells (5000 × g, 5–20 min) and wash twice with sterile DPBS.
-
5.
Resuspend yeast cells in a 1/10 volume ratio of DPBS. If cells are harvested from 10-mL cultures, cells are resuspended in 1 mL DPBS. At this point, approximately 2 × 109 cells/mL C. gattii suspension can be obtained. Using a hemocytometer, count cells, and adjust cell density to 1 × 109 cells/mL.
-
6.
Transfer cell suspension to a new thermal-tolerant conical tube and boil for 1 h to kill fungal cells. Vortex the suspension every 15 min (see Note 1 ). Do not further wash heat-treated cells.
-
7.
Spread the suspension onto YPD agar and incubate the plate at 30 °C for 7 days to verify that all C. gattii cells are dead.
-
8.
Dispense the heat-killed cells in a small quantity (500–1000 μL), and store at −20 °C.
3.2 Preparation of BMDCs
-
1.
Prepare two sterile 50-mL conical tubes and add 10 mL of RPMI 1640 complete medium to one and 40 mL of 70 % ethanol to the other. Place the tube containing medium in crushed ice until use (see Note 2 ).
-
2.
Euthanize one to three mice with carbon dioxide and harvest femurs and tibias from both legs using forceps and scissors. Using paper towels, remove surrounding muscles and tissues manually and carefully. Soak bones in 70 % ethanol (50-mL conical tube) for 10 s to sterilize them, and then transfer bones into the complete medium (50-mL conical tube) with forceps. Keep the conical tube containing the bones in crushed ice until the next step (see Note 3 ).
-
3.
Prepare three sterile 10-cm-diameter Petri dishes. Transfer medium and bones into the first Petri dish from the 50-mL conical tube. Because this medium contains a small amount of ethanol used for the sterilization described above, transfer bones to the second Petri dish and add 10–30 mL of fresh complete medium to the dish (see Note 4 ).
-
4.
Hold the bone with forceps above the second dish and cut off both ends (epiphyses) of each bone using scissors. Mince the epiphyses in the second dish. To obtain the marrow, repeatedly flush out each of the shafts with the complete medium in the second dish using a 26 G × 1/2 needle and 2.5-mL syringe. Collect the marrow clumps in the third dish (see Note 5 ).
-
5.
Prepare a sterile 50-mL conical tube and a 70-μm cell strainer, and set the strainer on the conical tube. Suspend the marrow clumps in the second and third dishes, and pass the suspension through the cell strainer. The marrow clumps will be trapped on the cell strainer and a single-cell suspension will be collected in the conical tube.
-
6.
Mash the marrow clumps on the cell strainer using the piston of a 2.5-mL syringe. Using 10 mL of fresh complete medium, rinse the second and third dishes. Pass the rinse fluid through the cell strainer. Verify that the membrane has been washed out completely.
-
7.
Centrifuge (300 × g, 5 min, 4 °C) the cell suspension and harvest all cells including RBCs. To lyse erythrocytes, resuspend the harvested cells in 2–4 mL of RBC lysis buffer. Incubate for 5 min at room temperature and then add 9 volumes of complete medium (18–36 mL) to stop the lysis reaction.
-
8.
Centrifuge (300 × g, 5 min, 4 °C) the cell suspension and resuspend in 10 mL of the complete medium. To remove debris, pass the suspension through a 70-μm cell strainer set on a 50-mL conical tube. Rinse the conical tube with 10 mL of the complete medium, and then pass the rinse fluid through the cell strainer. Finally, 20 mL of cell suspension will be obtained.
-
9.
Centrifuge (300 × g, 5 min, 4 °C) the cell suspension to remove supernatant, and resuspend in 10 mL of the complete medium.
-
10.
Count the viable cells using trypan blue dye. From one mouse, approximately 4 × 107 bone marrow cells can be obtained.
-
11.
Adjust the cell concentration to 3 × 106 cells/mL with the complete medium, and then add recombinant mGM-CSF at 10 ng/mL. Transfer 10 mL of suspension (3 × 107 cells) to a sterile 10-cm-diameter Petri dish (untreated for cell culture). Ten dishes can be prepared from the bone marrow suspension derived from one mouse.
-
12.
Incubate dishes for 3 days at 37 °C under 5 % CO2.
-
13.
On day 3, remove 6 mL of the medium in the culture and add 7 mL of fresh complete medium to the dish.
-
14.
On day 5, add 5 mL of fresh complete medium. On day 6, collect nonadherent cells in the 15-mL culture by flushing the medium against the dish with a pipette, and pool the cell suspension of each dish. Rinse each dish with 5 mL of complete medium, and pool the rinse fluid together with the initially collected suspension (see Note 6 ).
-
15.
Centrifuge (300 × g, 5 min, 4 °C) the cell suspension and resuspend in 10 mL of complete medium.
-
16.
The viable cells can then be counted as BMDCs. From one mouse, approximately 1 × 108 BMDCs can be obtained (see Note 7 ).
3.3 Vaccination with DC Vaccine
-
1.
Adjust the density of BMDCs to 1 × 106 cells/mL with complete medium and then add murine recombinant GM-CSF at 10 ng/mL and 5 × 106 cells/mL (MOI = 5) of heat-killed C. gattii (∆cap60 strain) to pulse BMDCs. Transfer 10 mL of suspension to a sterile 10-cm-diameter Petri dish (untreated for cell culture). Incubate dish for 24 h at 37 °C, under 5 % CO2 (see Notes 8 – 10 ).
-
2.
After incubation, BMDCs engulfing several acapsular C. gattii cells can be observed by microscopy. Collect nonadherent cells by flushing the medium against the dish with a pipette and pool the cell suspension of each dish. Rinse each dish with 10 mL of DPBS and pool the rinse fluid with the initially collected suspension.
-
3.
Centrifuge (300 × g, 5 min, 4 °C) the cell suspension, and wash harvested cells twice with sterile DPBS to remove residual mGM-CSF (see Note 11 ).
-
4.
Count the viable cells. BMDCs and C. gattii cells can be distinguished by differing cellular sizes. From 12 dishes, approximately 3 × 107 cells (60 injections) can be harvested. Adjust the density of BMDCs pulsed with the heat-killed ∆cap60 strain to 2.5 × 106 cells/mL with sterile DPBS, and keep the tube containing the suspension in crushed ice until next step (see Note 12 ).
-
5.
Inject 200 μL of suspension (5 × 105 cells) via the tail vein as DC vaccine using a 30 G × 1/2 needle and 1-mL syringe. DC vaccines are administered twice every 2 weeks.
3.4 Evaluation of Vaccine Efficacy (Murine Pulmonary Infection Model)
-
1.
Cultivate highly virulent C. gattii strain R265 in YPD liquid medium as described in Subheading 3.1.
-
2.
After cultivation, dispense 1 mL culture suspension into a 1.5-mL microcentrifuge tube. Harvest yeast cells (16,000 × g, 2 min) and wash twice with sterile DPBS (see Note 13 ).
-
3.
Resuspend yeast cells in 1 mL of DPBS. Using a hemocytometer, count viable cells and adjust cell density to 6 × 104 cells/mL. Dilute this suspension serially and spread onto YPD medium to determine the colony-forming units (CFUs) in this suspension. Keep the tube containing the suspension in crushed ice until use.
-
4.
Anesthetize mice using a vaporizer and isoflurane and intratracheally inject 50 μL of R265 suspension (3 × 103 CFUs/mouse) using a 24 G × 3/4 indwelling needle (see Note 14 ).
-
5.
On the day before mouse dissection, prepare 15-mL conical tubes to include 2-mL sterile DPBS, and then weigh the tubes.
-
6.
On day 14 post-infection, euthanize the mice by carbon dioxide inhalation and carefully and aseptically harvest their left and right lung lobes with forceps and scissors. Transfer dissected lungs into the 15-mL conical tubes containing 2-mL sterile DPBS described above. Before dissecting the next mouse, soak the forceps and scissors in 70 % ethanol to sterilize them. Keep the tubes containing lungs in crushed ice until next step.
-
7.
Weigh the tubes containing DPBS and harvested lungs. To calculate the lung weight, subtract the weight measured at step 5 from the weight measured at this step. The infected lungs of nonvaccinated mice weigh approximately 500–600 mg.
-
8.
Set the stainless steel mesh (autoclavable portable tea strainer with handle) on a sterile 10-cm-diameter Petri dish. Transfer the lungs and DPBS onto the steel mesh and manually homogenize using the piston of a 2.5-mL syringe. Collect the homogenates using a pipette and place back into the 15-mL conical tube (see Note 15 ).
-
9.
Add 3–4 mL of sterile DPBS onto the steel mesh to rinse the mesh, dish, and pipette, and pool the rinse fluid together with the initial collecting suspension. Add sterile DPBS to the suspension to a final volume of 5–6 mL. Keep the tube containing the suspension in crushed ice until next step.
-
10.
Serially dilute homogenates and spread 100 μL of the suspension onto YPD plates to determine the CFUs in this suspension. Incubate the plates at 30 °C for 24 h, after which the colonies can be counted. At day 14 postinfection, approximately 5 × 106 CFUs are detected in the lungs of nonvaccinated C57BL6 mice, in which sex, age, and individual differences are generally minor in this test. Thus, 30–200 colonies are detected on a YPD plate spread with 100 μL suspension of 103 diluting solution. Representative data of the fungal burden are shown in Fig. 2.
4 Notes
-
1.
Although several protocols recommend a 1-h incubation period at 60 °C, this often results in incomplete killing of fungal cells.
-
2.
For the preparation of BMDCs, ensure sterile practice. The protocol for BMDC preparation has also been described elsewhere [30].
-
3.
Select mouse sex depending on experiment design. Male mice have bigger bones and larger numbers of progenitor cells for BMDCs [30]. If DCs are transferred to female mice, female mice are preferably chosen to prepare BMDCs.
-
4.
It is recommended to use untreated Petri dishes for cell culture, which are generally used to prepare agar plates for microbial cultivation. If the cell-culture-grade dishes are used, BMDCs will adhere strongly to the dishes and the yield of BMDCs will be decreased.
-
5.
After the epiphyses are cut off, the bone shaft looks like a tube filled with red marrow. Marrow suspension, once flushed out, should not be aspirated again with the needle and syringe. Repeated flushing through the thin needle might damage the cells. The first dish should receive the uncut bone, the second dish is a reservoir of fresh medium and cut bone, and the third dish receives the marrow suspension flushed with fresh medium from the second dish.
-
6.
If the cells are harvested on day 7, add 10 mL of fresh complete medium at this point. One dish will contain 20 mL of culture medium.
-
7.
Harvested cells consist of CD11c+ and CD11c− cells. Depending on the experiment design, CD11c+ cells should be enriched using CD11c-MicroBeads and a MACS® Cell Separator (Miltenyi Biotec).
-
8.
To boost the immunological function of BMDCs, it is not always necessary to add toll-like receptor ligands or vaccine adjuvant such as lipopolysaccharide. Interestingly, when BMDCs were pulsed with ∆cap60 and α-galactosylceramide that is displayed on CD1d molecule-expressing BMDCs to stimulate natural killer T cells, the efficacy of DC-based vaccination against C. gattii infection was significantly decreased (unpublished data).
-
9.
Protein antigens of C. gattii stimulating T-cell responses have not yet been identified. A recent immune-blotting analysis has indicated that protein antigens are recognized by serum antibodies harvested from mice infected with C. gattii [31]. Two protein antigens, MP98 (synonym for Cda2: chitin deacetylase) and d25 (polysaccharide deacetylase) of C. neoformans, have been reported to induce T-cell responses [32–35]. Immunization using recombinant d25 protein induces a Th1 response and decreases fungal burden in organs and mortality after C. neoformans infection in an IFN-γ-dependent manner [34, 35]. In contrast, a recent report suggested that MP98-specific T cells in the lungs on day 14 postinfection predominantly expressed the Th2 cytokines IL-5 and IL-13 when restimulated for 6 h with phorbol myristate acetate and ionomycin. Induction of MP98-specific T cells required priming with interferon regulatory factor 4-expressing conventional DC in the lungs and also required the digested cell wall produced by chitotriosidase, a host intrinsic factor encoded by the Chit1 gene. This study also suggested that the digested fungal chitin stimulates pulmonary epithelial cells to release several Th2-inducing alarmins such as thymic-stromal lymphopoietin (TSLP), and results in Th2 polarization during C. neoformans infection. Indeed, conventional DC accumulated in lungs highly expressing TSLP receptors after C. neoformans infection. Furthermore, survival rate was improved in Chit1-deficient mice after C. neoformans infection, correlated with the decrease of MP98-specific Th2 cells in lungs [32]. The amino acid sequences of MP98 and d25 are also highly conserved in C. gattii. Depending on the experiment design, appropriate antigen proteins should be used.
-
10.
Capsular components are known to suppress several DC immune response s [28, 29]. We usually use heat-killed C. gattii capsule-deficient mutant (∆cap60) as vaccine antigen .
-
11.
Because C. gattii cells can also be precipitated with BMDCs by the centrifugation, they cannot be removed from the suspension.
-
12.
Because some cells pulsed with heat-killed ∆cap60 strain tend to adhere to the dish, cell yield tends to be greatly reduced. It was not tested in our study whether the adherent cells can be used for the vaccination; if they are, they should be gently collected by scraping the plates with a soft rubber spatula.
-
13.
It is difficult to precipitate yeast cells enveloped with capsular polysaccharide . Culture supernatant should be removed as carefully as possible.
-
14.
We usually use five mice per group for the fungal burden evaluation and eight mice per group for the mortality evaluation. Median survival time of nonvaccinated C57BL/6 mice infected with C. gattii R265 strain (3 × 103 CFUs) is 23–35 days, and mice begin to die on days 16–20 after infection. Thus, the fungal burden in lungs should be evaluated on days 3–15 after infection.
-
15.
Because the lung homogenates of unvaccinated mice are generally very sticky at day 14 postinfection, the homogenates including the residual solution in the pipette should be carefully collected. An automatic homogenizing mixer (cf. ULTRA-TURRAX® Tube Drive Control and DT-20-M tube, IKA) can also be used to prepare lung homogenate.
References
Chen S, Sorrell T, Nimmo G et al (2000) Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Australasian Cryptococcal Study Group. Clin Infect Dis 31:499–508
Galanis E, MacDougall L, Kidd S et al (2010) Epidemiology of Cryptococcus gattii, British Columbia, Canada, 1999–2007. Emerg Infect Dis 16:251–257
Smith RM, Mba-Jonas A, Tourdjman M et al (2014) Treatment and outcomes among patients with Cryptococcus gattii infections in the United States Pacific Northwest. PLoS One 9, e88875
Lizarazo J, Escandón P, Agudelo CI et al (2014) Retrospective study of the epidemiology and clinical manifestations of Cryptococcus gattii infections in Colombia from 1997–2011. PLoS Negl Trop Dis 8, e3272
BCCDC (2011) Environmental pathogens, Cryptococcus gattii. British Columbia annual summary of reportable diseases 2011, pp 112–113
CDC (2010) Emergence of Cryptococcus gattii, Pacific Northwest, 2004–2010. Morb Mortal Wkly Rep 59:865–868
Ngamskulrungroj P, Chang Y, Sionov E, Kwon-Chung KJ (2012) The primary target organ of Cryptococcus gattii is different from that of Cryptococcus neoformans in a murine model. mBio 3:e00103–e00112
Okubo Y, Wakayama M, Ohno H et al (2013) Histopathological study of murine pulmonary cryptococcosis induced by Cryptococcus gattii and Cryptococcus neoformans. Jpn J Infect Dis 66:216–221
Cheng P-Y, Sham A, Kronstad JW (2009) Cryptococcus gattii isolates from the British Columbia cryptococcosis outbreak induce less protective inflammation in a murine model of infection than Cryptococcus neoformans. Infect Immun 77:4284–4294
Einsiedel L, Gordon DL, Dyer JR (2004) Paradoxical inflammatory reaction during treatment of Cryptococcus neoformans var. gattii meningitis in an HIV-seronegative woman. Clin Infect Dis 39:e78–e82
Brouwer AE, Siddiqui AA, Kester MI et al (2007) Immune dysfunction in HIV-seronegative, Cryptococcus gattii meningitis. J Infect 54:e165–e168
Angkasekwinai P, Sringkarin N, Supasorn O et al (2014) Cryptococcus gattii infection dampens Th1 and Th17 responses by attenuating dendritic cell function and pulmonary chemokine expression in the immunocompetent hosts. Infect Immun 82:3880–3890
O'Meara TR, Alspaugh JA (2012) The Cryptococcus neoformans capsule: a sword and a shield. Clin Microbiol Rev 25:387–408
Frases S, Nimrichter L, Viana NB et al (2008) Cryptococcus neoformans capsular polysaccharide and exopolysaccharide fractions manifest physical, chemical, and antigenic differences. Eukaryot Cell 7:319–327
Cherniak R, Reiss E, Turner SH (1982) A galactoxylomannan antigen of Cryptococcus neoformans serotype A. Carbohydr Res 103:239–250
Vecchiarelli A, Pericolini E, Gabrielli E et al (2013) Elucidating the immunological function of the Cryptococcus neoformans capsule. Future Microbiol 8:1107–1116
Monari C, Bevilacqua S, Piccioni M et al (2009) A microbial polysaccharide reduces the severity of rheumatoid arthritis by influencing Th17 differentiation and proinflammatory cytokines production. J Immunol 183:191–200
Piccioni M, Monari C, Kenno S et al (2013) A purified capsular polysaccharide markedly inhibits inflammatory response during endotoxic shock. Infect Immun 81:90–98
Gibson JF, Johnston SA (2014) Immunity to Cryptococcus neoformans and C. gattii during cryptococcosis. Fungal Genet Biol 78:76–86
Mershon KL, Vasuthasawat A, Lawson GW et al (2009) Role of complement in protection against Cryptococcus gattii infection. Infect Immun 77:1061–1070
Ueno K, Kinjo Y, Okubo Y et al (2015) Dendritic cell-based immunization ameliorates pulmonary infection with highly virulent Cryptococcus gattii. Infect Immun 83:1577–1586
Steinman RM, Witmer MD (1978) Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc Natl Acad Sci U S A 75:5132–5136
Palucka K, Banchereau J (2013) Dendritic-cell-based therapeutic cancer vaccines. Immunity 39:38–48
García F, Climent N, Assoumou L et al (2011) A therapeutic dendritic cell-based vaccine for HIV-1 infection. J Infect Dis 203:473–478
d’Ostiani CF, Del Sero G, Bacci A et al (2000) Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J Exp Med 191:1661–1674
Bozza S, Perruccio K, Montagnoli C et al (2003) A dendritic cell vaccine against invasive aspergillosis in allogeneic hematopoietic transplantation. Blood 102:3807–3814
Roy RM, Klein BS (2012) Dendritic cells in antifungal immunity and vaccine design. Cell Host Microbe 11:436–446
Siegemund S, Alber G (2008) Cryptococcus neoformans activates bone marrow-derived conventional dendritic cells rather than plasmacytoid dendritic cells and down-regulates macrophages. FEMS Immunol Med Microbiol 52:417–427
Vecchiarelli A, Pietrella D, Lupo P et al (2003) The polysaccharide capsule of Cryptococcus neoformans interferes with human dendritic cell maturation and activation. J Leukoc Biol 74:370–378
Inaba K, Swiggard WJ, Steinman RM et al (2009) Isolation of dendritic cells. Curr Protoc Immunol 3.7.1–3.7.19
Chaturvedi AK, Hameed RS, Wozniak KL et al (2014) Vaccine-mediated immune responses to experimental pulmonary Cryptococcus gattii infection in mice. PLoS One 9, e104316
Wiesner DL, Specht CA, Lee CK et al (2015) Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection. PLoS Pathog 11, e1004701
Levitz SM, Nong S, Mansour MK et al (2001) Molecular characterization of a mannoprotein with homology to chitin deacetylases that stimulates T cell responses to Cryptococcus neoformans. Proc Natl Acad Sci U S A 98:10422–10427
Biondo C, Beninati C, Delfino D et al (2002) Identification and cloning of a cryptococcal deacetylase that produces protective immune responses. Infect Immun 70:2383–2391
Biondo C, Beninati C, Bombaci M et al (2003) Induction of T helper type 1 responses by a polysaccharide deacetylase from Cryptococcus neoformans. Infect Immun 71:5412–5417
Acknowledgements
This chapter described work supported by Health Science Research Grants for Research on Emerging and Re-emerging Infectious Diseases (H25-Shinkou-Shitei-001, H25-Shinkou-Shitei-002, H25-Shinkou-Wakate-005, H25-Shinkou-Ippan-006, and H26-Shinkoujitsuyouka-Ippan-010) from the Ministry of Health, Labor and Welfare of Japan, by the Research program on Emerging and Re-emerging Infectious Diseases from Japan Agency for Medical Research and development, AMED, by KAKENHI (15K21644) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and by a grant from the NOVARTIS Foundation (Japan) for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Ueno, K., Urai, M., Ohkouchi, K., Miyazaki, Y., Kinjo, Y. (2016). Dendritic Cell-Based Vaccine Against Fungal Infection. In: Thomas, S. (eds) Vaccine Design. Methods in Molecular Biology, vol 1403. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3387-7_30
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3387-7_30
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3385-3
Online ISBN: 978-1-4939-3387-7
eBook Packages: Springer Protocols