Abstract
Busulfan is a commonly used antineoplastic agent to condition/ablate bone marrow cells before hematopoietic stem cell transplant. While intravenous (IV) formulations of busulfan are now available and have lower incidences of toxicity and treatment related mortality compared to oral dosing, it still displays large pharmacokinetic variability. As a result, studies have shown that therapeutic drug monitoring is clinically useful to minimize graft failure, disease reoccurrence, and toxicities like veno-occlusive disease and neurologic toxicity. Current methods for assaying busulfan include the use of GC/MS, HPLC, and LC-MS/MS. The clinical need for faster turnaround times and increased testing volumes has required laboratories to develop faster methods of analysis for higher throughput of samples. Therefore, we present a method for the quantification of busulfan in plasma using an ultrafast SPE-MS/MS which has much faster sample cycle times (<20 s per sample) and comparable analytical results to GC/MS.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
Busulfan (1, 4-butanediol dimethansulfonate) was approved by the US Food and Drug Administration (FDA) in 1999 for the treatment of chronic myelogenous leukemia ; it is also used in combination with other drugs as a conditioning agent prior to bone marrow transplantation [1]. Busulfan is a bifunctional antineoplastic whose mechanism of action includes alkylation and cross-linking of strands of DNA to prevent its replication [2]. Specifically, busulfan prefers to react with the N7 nitrogen of guanine or the N3 nitrogen of adenine [3, 4]. The functionality of busulfan allows it to form an intra-strand cross-link between adjacent nucleotides in a DNA chain [5, 6] which is accomplished by the SN2 reactions with the nucleotides on both of the functionalized carbons on the busulfan. The intra-strand crosslinks formed are believed to be responsible for busulfan’s cytotoxicity and are thought to inhibit DNA replication leading to eventual cell death (apoptosis). To minimize cytotoxicity while ensuring adequate concentrations of busulfan completely destroy the bone marrow, therapeutic drug monitoring is critical. Studies have shown that monitoring the busulfan area under the plasma drug concentration-time curve (AUC ) and steady-state concentrations (Css) have been related to therapeutic outcome [7].
Busulfan is commonly administered intravenously through a central venous catheter. It is given as a 2-h infusion, every 6 h for four consecutive days resulting in a total of 16 doses [8]. The usual adult dose is 0.8 mg/kg of ideal body weight or actual body weight. Intravenous dosing is usually guided by pharmacokinetic evaluation of the area under the curve (AUC) and clearance after the first dose [9]. Busulfan AUC and clearance are calculated after the quantification of busulfan concentration in plasma collected immediately after the termination of a 2-h intravenous infusion of 0.8 mg/kg busulfan, as well as 1 h, 2 h, and 4 h after the end of infusion. The AUC is calculated using the trapezoidal rule. The optimal dose is calculated based on the assumption that the ideal AUC is 1100 µM*min. AUC greater than 1500 µM*min is associated with hepatic veno-occlusive disease [10], and AUC less than 900 µM*min indicates incomplete bone marrow ablation. In order to facilitate monitoring, there is the need for fast turn–around-times (TAT) for the analysis of busulfan in plasma. Therefore, an ultrafast SPE/MS/MS method with a cycle time of 20 s per injection for the quantitation of busulfan in plasma is presented.
2 Materials
2.1 Samples
Heparinized plasma samples must be collected, placed on ice, and centrifuged as quickly as possible to prevent degradation [11] (see Note 1 ). However, once separated, samples stored refrigerated were stable up to 3 days and samples stored frozen (−70 °C) with up to five freeze-thaw cycles were stable up to 28 days. Following 24 h of storage at ambient temperature, the drug concentrations decrease significantly and was therefore considered not stable under ambient conditions [12].
2.2 Reagents
-
1.
Wash solution #1(clinical laboratory reagent water (CLRW) with 0.1 % formic acid): In a 2 L reagent bottle, add 2 L CLRW. Add 2 mL formic acid and mix well. It is stable for 1 month at 20–27 °C.
-
2.
Wash solution #2 (90 % acetonitrile with 10 % isopropanol): In a 2 L reagent bottle, add 1.8 L acetonitrile. Add 200 mL isopropanol and mix well. It is stable for 1 year at 20–27 °C.
-
3.
Mobile phase 1/reconstitution solution: In a 2.0 L reagent bottle add 2.0 L CLRW. Add 1.54 g ammonium acetate and mix well. Add 2.0 mL formic acid and mix well. Add 180 μL TFA and mix well. It is stable 1 month at 20–27 °C.
-
4.
Mobile phase 2 (50 % methanol/CLRW): In a 2.0 L reagent bottle add 1.0 L methanol. Add 1.0 L CLRW and mix well. It is stable 2 months at 20–27 °C.
-
5.
Mobile phase 3: In a 2 L reagent bottle, add 2.0 L methanol. Add 1.54 g ammonium acetate and mix well. Add 2 mL formic acid and mix well. Add 180 μL TFA and mix well. It is stable 1 year at 20–27 °C.
2.3 Standards and Calibrators
-
1.
Busulfan, Stock I (Sigma-Aldrich).
-
2.
Busulfan Stock II Standard (1.0 mg/mL): Accurately weigh out 10 mg of busulfan and transfer to a 10 mL volumetric flask. Bring to volume with acetone and mix thoroughly. Store at −10 to −35 °C protected from light. Stable for 1 year after preparation or until expiration of Stock 1 Standard, whichever comes first.
-
3.
Busulfan Intermediate Standard (100 μg/mL): Transfer 2.5 mL of Stock II standard solution (1.0 mg/mL) to a 25 mL volumetric flask. Bring to volume with methanol and mix thoroughly. Store at −10 to −35 °C protected from light. Stable for 1 year after preparation or until expiration of Stock II Standards, whichever comes first.
-
4.
Calibrators are prepared according to Table 1.
Table 1 Preparation of calibrators: the total volume is made to 50 mL with drug-free plasma
2.4 Quality Controls and Internal Standard
-
1.
Quality controls samples are prepared in house according to the Table 2.
Table 2 Preparation of quality control: the total volume is made to 50 mL with drug-free plasma -
2.
Primary internal standard: Busulfan-d4—Prepared using the method of Vassal and Gouyette [13] or can be bought commercially (Cambridge Isotope Laboratories).
-
3.
Busulfan-d4 Stock II Internal Standard, 1.0 mg/mL: Accurately weigh out 10 mg of busulfan internal standard and transfer to a 10 mL volumetric flask. Bring to volume with ethyl acetate and mix thoroughly. Store at −10 to −35 °C protected from light. Stable for 1 year after preparation or until expiration of Stock I Standard, whichever comes first.
-
4.
Busulfan-d4 Intermediate Internal Standard (100.0 μg/mL): Transfer 1 mL of Stock II standard (1.0 mg/mL) to a 10 mL volumetric flask. Bring to volume with methanol and mix thoroughly. Store at −10 to −35 °C protected from light. Stable for 1 year after preparation or until expiration of Stock II Standards, whichever comes first.
-
5.
Busulfan-d4 Working Internal Standard (2.0 μg/mL): Transfer 2 mL of the 100 μg/mL Intermediate Internal Standard to a 100 mL volumetric flask. Bring to volume with methanol and mix thoroughly. Transfer solution into ten amber vials and seal with screw caps utilizing a rubber/Teflon septum. Store at −10 to −35 °C. Stable for 1 year after preparation or until expiration of Stock II Standards, whichever comes first.
2.5 Supplies and Equipment
-
1.
96 Deep-well 1.5 mL plates (Nalgen Nunc).
-
2.
SPE cartridge C18 (Agilent RapidFire).
-
3.
Borosilicate glass test tubes; 16 × 125 mm and 16 × 100 mm.
-
4.
Agilent 1200 series HPLC using Agilent RapidFire liquid chromatography .
-
5.
Agilent 6400 series QQQ mass spec detector.
3 Methods
3.1 Stepwise Procedure
-
1.
Aliquot 100 μL of each standard, control, and patient sample into individually labeled 16 × 125 mm glass tubes.
-
2.
To each standard, control, and patient sample add:
-
(a)
50 μL of working internal standard.
-
(b)
3.0 mL of n-butyl chloride.
-
(a)
-
3.
Vortex for 2 min on multi-vortexer.
-
4.
Centrifuge at 2100 × g for 5 min.
-
5.
Transfer organic supernatant to individually labeled 16 × 100 glass tubes.
-
6.
Dry down under a gentle stream of nitrogen at ≤40 °C.
-
7.
Add 600 μL of reconstitution solution.
-
8.
Vortex for 10 s.
-
9.
Transfer extracts to the 96 deep-well plate.
-
10.
Inject 10 μL of each extract on a RapidFire-MS/MS system.
3.2 Data Analysis
-
1.
Samples were analyzed at the rate of 20 s per sample. Analytical conditions are described in Table 3.
Table 3 Analytical conditions for HPLC-MS-MS -
2.
MassHunter Triple Quadruple Acquisition software (B.04.01) with Qualitative Analysis (B.04.00) and Quantitative Analysis (B.04.00), and RapidFire Integrator Software.
-
3.
The ions used for identification and quantification are listed in Table 4.
Table 4 MRMs for busulfan and busulfan-D4 -
4.
The linearity/limit of quantitation of the method is 25–7500 ng/mL. Samples in which the drug concentrations exceed the upper limit of quantitation should be diluted with drug free plasma and retested.
-
5.
A typical calibration curve has correlation coefficient (r 2) of >0.99.
-
6.
Typical intra and inter-assay imprecision throughout the analytical range is <5 %.
-
7.
Quality control: The analytical run is considered acceptable if the calculated concentrations of drugs in the controls are within ±10 % of target values. The quantifying ion in the sample is considered acceptable if the ratios of qualifier ions to quantifying ion are within ±20 % of the ion ratios for the calibrators.
-
8.
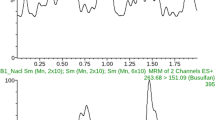
A typical Chromatogram is shown in Fig. 1.
3.3 Reporting
-
1.
The units, the AUC should be reported in µM*min, the clearance in (mL/min)/Kg, and dose in mg.
-
2.
Dosing is usually guided by pharmacokinetic evaluation of the area under the curve (AUC) and clearance calculation of AUC is performed using the trapezoidal rule, and clearance is the dose divided by the AUC .
4 Notes
-
1.
This step is particularly important to prevent degradation of the drug in whole blood and the possibility of reporting out a falsely decreased busulfan concentration.
References
Buggia I, Locatelli F, Regazzi MB, Zecca M (1994) Busulfan. Ann Pharmacother 28:1055–1062
Kohn KW (1996) Beyond DNA cross-linking: history and prospects of DNA-targeted cancer treatment--fifteenth Bruce F. Cain Memorial Award Lecture. Cancer Res 56:5533–5546
Brookes P, Lawley PD (1960) The reaction of mustard gas with nucleic acids in vitro and in vivo. Biochem J 77:478–484
Kohn KW, Hartley JA, Mattes WB (1987) Mechanisms of DNA sequence selective alkylation of guanine-N7 positions by nitrogen mustards. Nucleic Acids Res 15:10531–10549
Hurley LH (2002) DNA and its associated processes as targets for cancer therapy. Nat Rev Cancer 2:188–200
Newbold RF, Warren W, Medcalf AS, Amos J (1980) Mutagenicity of carcinogenic methylating agents is associated with a specific DNA modification. Nature 283:596–599
Slattery JT, Risler LJ (1998) Therapeutic monitoring of busulfan in hematopoietic stem cell transplantation. Ther Drug Monit 20:543–549
GlaxoSmithKline, Research Triangle Park, NC 27709. 2003
Baselt RC. Busulfan. In: Baselt RC, Ed. Disposition of toxic drugs and chemical in man. 9th ed. Foster, City, CA: Biomedical Publications; 2011. p. 218–220.
Slattery JT, Sanders JE, Buckner CD, Schaffer RL, Lambert KW, Langer FP, Anasetti C, Bensinger WI, Fisher LD, Appelbaum FR et al (1995) Graft-rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics. Bone Marrow Transplant 16:31–42
Moon SY, Lim MK, Hong S, Jeon Y, Han M, Song SH, Lim KS, Yu KS, Jang IJ, Lee JW et al (2014) Quantification of human plasma-busulfan concentration by liquid chromatography-tandem mass spectrometry. Ann Lab Med 34:7–14
Danso D, Jannetto PJ, Enger R, Langman LJ (2015) High-throughput validated method for the quantitation of busulfan in plasma using ultrafast SPE-MS/MS. Ther Drug Monit 37:319–324
Vassal G, Re M, Gouyette A (1988) Gas chromatographic-mass spectrometric assay for busulfan in biological fluids using a deuterated internal standard. J Chromatogr 428:357–361
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Langman, L.J., Danso, D., Robert, E., Jannetto, P.J. (2016). High-Throughput Quantitation of Busulfan in Plasma Using Ultrafast Solid-Phase Extraction Tandem Mass Spectrometry (SPE-MS/MS). In: Garg, U. (eds) Clinical Applications of Mass Spectrometry in Drug Analysis. Methods in Molecular Biology, vol 1383. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3252-8_10
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3252-8_10
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3251-1
Online ISBN: 978-1-4939-3252-8
eBook Packages: Springer Protocols