Abstract
The treatment of immune-mediated inflammatory diseases (IMIDs) has dramatically improved over the last two decades by the development of a series of targeted biological therapies. This paper focuses on new developments in the treatment of IMIDs. In particular, we discuss how different ways of targeting the same mediators can lead to different efficacy and safety profiles, using B cell targeting as example. In addition, we discuss the emerging field of ‘small molecules’ that target specifically intracellular processes related to cytokine signaling, cell activation, cell migration, and other processes relevant to tissue inflammation.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
Immune-mediated inflammatory diseases (IMIDs) encompasses disorders where tissue and organ inflammation is primarily d riven by aberrant immune responses. In contrast to the ‘secondary’ involvement of th e immune system in infectious diseases and oncology, the trigger of IMIDs is the immune system itself. Importantly, recent advances in our understanding of immunity and inflammation revealed that IMIDs can be driven not only by autoimmunity , defined here as abnormal responses of T and/or B lymphocytes against self-antigens, but also by auto-inflammation, this is self-directed tissue inflammation driven by aberrant or uncontrolled innate immune response triggered by local factors at tissues sites predisposed to disease. The former group encompasses diseases such rheumatoid arthritis (RA), type I diabetes, and systemic lupus erythematosis, whereas gout and sarcoidosis are examples of autoinflammatory diseases.
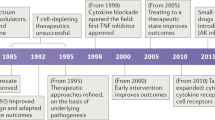
The treatment of IMIDs has dramatically improved over the last two decades by the development of a series of targeted biological therapies. Indeed, combined fundamental and translational immunology research has revealed that specific inflammatory mediators (in particular cytokines) and cells were ‘master switches’ in specific IMIDs and that targeting these cellular and molecular players with antibodie s or soluble receptors potently down-modulated chronic inflammation. The first and major success story is TNF blockade, which is very effective to treat a variety of IMIDs including RA, spondyloarthritis (SpA), psoriasis, and inflammatory bowel disease . Other major anti -cytokine therapies are directed towards IL-1 and IL-6 and, more recently, the IL-23/IL-17 pathway. Besides targeting cytokines, a second very successful approach was to target pathogenic cell subsets, with as prime example B cell depletion with the anti-CD20 antibody rituximab. Originally developed to treat lymphomas, this compound turned out to be also very effective in the treatment of RA and other autoimmune diseases. Thirdly and finally, pathogenic cell can not only be depleted but one can inhibit their interaction with other pathogenic cells (such as in the case of co-stimulation blockade by CTLA-4-Ig or abatacept) or with molecules directing their migration into target tissues (such as the anti-alpha4 integrin antibody natalizumab).
Existing and emerging therapies targeting cytokines, cells, and cellular interactions have been extensively described in the literature and are not reviewed in detail here. This chapter rather focuses on two specific new developments in the treatment of IMIDs. Firstly, we discuss how different ways of targeting the same mediators can lead to different efficacy and safety profiles, using B cell targeting as example. We discuss novel drugs beyond rituximab that target other B cell surface molecules, other B cell subsets, and B cell growth factors. Secondly, we discuss the emerging field of “sma ll molecules” that target specifically intracellular processes related to cytokine signaling, cell activation, cell migration, and other processes relevant to tissue inflammation.
2 Targeting B Cells
2.1 Targeting B Cells with Anti-CD20 Monoclonal Antibodies
B cells contribute to chroni c inflammatory disease by secreting cytokines, providing co-stimulatory signals to T cells, presenting antigen in the context of antibody production, and producing auto-antibodies. Therefore, selective depletion of these cells alters the immune response and reduces inflammation. Antibody-mediated depletion of B cells can be achieved via different mechanisms of which antibody-dependent cellular cytotoxicity (ADCC) and complement dependent cytotoxicity (CDC) are most widely used. The validity of this approach had been demonstrated by the use of the anti-CD20 antibody rituximab in RA [1] as well as ANCA-associated vasculitis [2], modest effects in SLE [3, 4], systemic sclerosis (SSc) [5], Sjogren’s syndrome (SS) [6], and multiple sclerosis (MS) [7]. Rituximab is currently tested in pemphigus, AIHA, and ITP [8].
Based on the efficacy and relatively good safety profile of rituximab (a rare but very severe complication of rituximab treatm ent is progressive multifocal leukoencephalopathy, a devastating demyelinating disease caused by reactivation of the JC virus [9]), other antibodies targeting CD20 are currently in development with the aim to improve the efficacy and safety profile (Fig. 1).
Surface molecules of B cells and plasma cells of soluble factors targeted in IMIDs. Schematic overview of B cell and plasma cell surface receptors or other molecules. The survival factors B cell activating factor (BAFF) and a proliferation-inducing ligand (APRIL) bind to their respective receptors on B cells (BAFF-receptor, BAFF-R) (=TNFRSF13C), and transmembrane activator and calcium-modulating ligand interactor, TACI (=TNFRSF13B) and plasma cells (B cell maturation antigen, BCMA (TNFRSF17), and TACI. Targeting APRIL and BAFF affects both B cells and plasma cells. B cells can be targeted specifically via the B cell restricted antigens CD20 and CD22. Targeting CD19 affects both B cells, plasmablasts, and a subset of plasma cells. Ab’s antibodies, BCR B cell receptor
Ofatumumab is a full y humanized IgG1 mAb which binds a CD20 epitope distinct from the binding site of rituximab. Ofatumumab has enhanced CDC activity compared to the other anti-CD20 mAbs [10]. In RA data from initial phase 1/2 and 3 studies point towards favorable effects on disease activity [11, 12]. In a phase 2 dose-finding study in MS ofatumumab treatment resulted in a substantial reduction in new and total lesions [13].
Ocrelizumab is a humanize d anti-CD20 mAb that binds a different but overlapping epitope from rituximab. It has similar CDC, but 2–5-fold increased ADCC [14]. Overall, it appears that fewer anti-drug antibody responses are elicited during ocrelizumab treatment. In RA patients ocrelizumab treatment resulted in reduced disease activity and a red uction in joint damage, however this was accompanied by an increased risk of infections which led to termination of development for RA [15, 16]. In SLE nephritis, overall renal response rates with ocrelizumab were numerically but not statistically significantly superior to those with placebo, while ocrelizumab treatment was associated with a higher rate of serious infections in the subgroup receiving background MMF [17]. Nevertheless, in MS after initial favorable results [18], additional clinical trials in different forms of this disease are still ongoing.
Veltuzumab is a humanized IgG1 anti-CD20 mAb with both structural and functional differences from rituximab. It has shown promising clinical activity in relapsing ITP [19] and is also bein g evaluat ed for RA [20], but no results have been disclosed yet.
2.2 Targeting Other B Cell Surface Molecules
CD19 is a B cell-restricted antigen that regulates the threshold for B cell activation and, in contrast to CD20, is maintained on plasmablasts and subsets of plasma cells (Fig. 1) [21]. Therefore, targeting CD19 is expected to have a more profound effect than anti-CD20 therapy [22]. MEDI-551 is a humanized IgG1 afucosylated mAb targeting CD19 with enhanced ADCC effector function [23]. It is currently under evaluation in clinical trials for systemic sclerosis (SSc) (Clinical Trials.gov: NCT00946699) and MS [24].
CD22 is considered to be a B cell ant igen (expressed on the majority of IgM+IgD+ B cells, but less so on germinal center B cells and plasma cells), which can also be detected on basophils and dendritic cells (Fig. 1) [25]. However, CD22 has been demonstrated to play an important role in the control of B cell activation, B cell survival, and cell-cycle progression following activation [26]. Epratuzumab is a humanized IgG1 mAb directed against CD22 w ith modest ADCC, but no CDC activity (most likely due to rapid internalization of CD22 after Ab binding) [27]. In an open-label phase 1/2 study in Sjogren’s syndrome (SS) epratuzumab treatment was well-tolerated and resulted in a moderate clinical responses [28]. A phase 2 study in SLE patients also demonstrated favorable clinical effects [29, 30]. Phase 3 trials in SLE are currently ongoing.
2.3 Targeting B Cell Survival Factors
Besides targeting B cell themselves, a novel strategy consists of targeting B cell growth and survivial factors. Indeed , B cell function and survival depends on various factors of which the TNF family members B-cell activating factor (BAFF or BlyS) and a proliferation induced ligand (APRIL) are probably m ost important in the context of autoimmune diseases. Interestingly, BAFF and APRIL also support plasma cell s urvival (Fig. 1) [31].
Belimumab is a fully human IgG1 mAb that selectively inhibits BAFF, which results in B cell apoptosis [32]. It is effective in SLE in patients with active, autoantibody positive disease [33] and was approved by the EMA and FDA for this indication in 2011. Belimumab was not very successful in RA [34], however its efficacy is currently under investigation for ITP, Waldenstrom’s macroglobulinemia, idiopathic membranous glomerulonephropathy, Sjogrens syndrome (SS), prevention of kidney transplant rejection, and myasthenia gravis (reviewed in [35]).
Tabalumab is a humanized IgG4 antibody that binds and neutralizes both soluble and membrane-bound BAFF [36]. A phase 2 dose-ranging study of subcutaneous tabalumab for the treatment of active RA patients with an inadequate response to methotrexate was successful [37]. Clinical t rials in SLE [38] and MS [39] are ongoing, but results have not been published yet.
Atacicept is a fusion protein soluble receptor construct of Transmembrane Activator and Calcium-modulating l igand Interactor (TACI) and the Fc part of human IgG1 (TACI-Ig) [40]. TACI is a receptor that is normally expressed both on B cells and on plasma cells and binds both BAFF and APRIL [41]. It has been tested in SLE [42] and RA [43, 44], but in general was not successful. In MS atacicept even worsened disease activity [45]. One explanation for this may be that atacicept also targets survival factors for regulatory B cells without full depletion of pathogenic B cells [46]. This example as well as the other emerging biological drugs discussed above in the context of B cell targeting illustrate well that different ways of approaching a therapeutic target can result in s tr ongly different efficacy and safety profiles.
3 Targeting Intracellular Signaling Pathways
Besides novel approaches to target extracellular molecules (including cytokines, growth factors, surface markers, co-stimulatory molecules, and adhesion molecules), intense efforts have been made in the last years in identifying intracellular targets, since all inflammatory responses are initiated by activ ation of intracellular signal transduction pathways. Examples of key molecules in these intracellular pathways are mitogen-activated protein kinases (MAPKs), nuclear factor-kappaB (NF-κB) activating kinases, Janus kinase (JAK), spleen tyrosine kinase (Syk), and phosphoinositide 3′kinase. Here we discuss the advances in targeting MAPKs, NF-κB, and JAKs as examples.
3.1 Targeting MAPK
The family of mitogen-activated protein kinases (MAPKs) play a central role in the regulation of various biological processes that are involved in immune responses, such as proliferation, differentiation, pro-inflammatory gene expression, and survival. MAPKs are activated in response to environmental stress factors, such as TLR ligands, cytokines, growth factors, and radiation. Subsequently, MAPKs induce s ignaling by phosphorylating specific target proteins. MAPKs consist of three main groups that all have specific roles in the regulation of cell function: p38 MAPKs, extracellular signal-regulated protein kinases (ERKs), and c-jun NH2 terminal kinases (JNKs). Recently, several additional atypical MAPKs such as ERK5, ERK3/4, ERK7/8, and Nemo-like kinase have been described [47], but these are less well studied and are not discussed here.
3.1.1 p38 Inhibitors
p38 has four isoforms (α, β, γ, and δ), of which p38α and p38β are ubiquitously expressed. Activation and phosphorylation of p38 is regulated by the upstream MAPK kinases (MKK)3 and MKK6 that are phosphorylated by m ultiple MKK kinases (MAP3Ks). Particularly p38α is a signaling molecule that regulates pro-inflammatory cytokine production (such as TNFα, IL-1β, and IL-6), which makes it an attractive target for many IMIDs including RA. Consequently, intense efforts have been made to develop small molecule p38 inhibitors. However, despite being effective in preclinical models of arthritis, to date clinical trials in RA have all failed due to poor efficacy or toxicity, including hepatotoxicity (reviewed in [48]). Yet, in inflammatory bow el disease (IBD) initial clinical trials with the p38 inhibitor Semapimod (CNI-1493) appeared promising [49] and follow-up studies have established a mild beneficial effect in a limited number of patients [50]. A potential explanation for th ese rather disappointing results may lie in the fact that p38 also has anti-inflammatory effects or that blocking one kinase may lead to compensatory effects in other kinases that regulate the same genes. Therefore, an alternative more effective strategy may be to block upstream kinases such as MKK3/6 [48].
3.1.2 ERK Inhibitors
The ERK family consists of two conventional MAPK, namely ERK 1 and ERK2, that are activated by the MAPKKs MEK1 and MEK2 in response to growth factors, including platelet-derived growth factor (PDGF) and epidermal growth factor (EGF). ERK1 and ERK2 are important for cell proliferation and differentiation [47]. FR180204, an ERK inhibitor, has been shown to be effective against mouse collagen-induced arthritis, a representative animal model of RA. The MEK1/2 inhibitors PD98059 and U0126 are not competitive with respect to ATP, but appear to physically interact with MEK1/2 thereby preventing phosphorylation and/or conformational transition that generates the activated enzyme. More recently, additional noncompetitive inhibitors of MEK1/2 with greater bioavailability (PD184352 and PD0325901) have been developed and entered clinical trials as potential anticancer agents (reviewed in [47]). However, no clinical trials in IMIDs have been performed so far.
3.1.3 JNK Inhibitors
The three JNK isoforms (JNK1, JNK2, and JNK3) are involved in many processes that contribute to chronic inflammation such as matrix metalloproteinase (MMP) and cytokine production, cell migration, and angio genesis [51, 52]. JNK1 and JNK2 are widely expressed, and therefore most attention of pharmaceutical companies has gone out to target these isoforms [51]. SP600125, a direct inhibitor of JNK activity, decreased paw swelling in rat adjuvant-induced arthritis, which was accompanied by a near-complete inhibition of radiographic damage [53]. However, this inhibitor lacked specificity and was replaced by more selective inhibitors. At present, several companies have JNK inhibitors that are in different stages of development, but no data of clinical trials in IMIDs have been reported.
3.2 NF-κB Inhibitors
The Nuclear Factor-kappaB (NF-κB) family of transcription factors is crucially involved in the regulation of immune responses in IMIDs (reviewed in [54]). NF-κB can be activated via two distinct pathways: the canonical pathway and the alternative or noncanonical pathway. The canonical pathway is most extensively studied and can be activated by stimulation of a variety of cell membrane receptors including tumor necrosis factor receptor (TNF-R), IL-1 receptor, and Toll-like receptors, in response to their respective pro-inflammatory ligands, as well as via triggering of classic immunoreceptors like the T-cell receptor (TCR) or the B-cell receptor (BCR). In this pathway, inhibitor of κB (IκB) kinase (IKK)β is required for NF-κB activation, whereas IKKα is redundant (reviewed in [55]). The cano nical NF-κB pathway is essential both in acute inflammatory responses and in chronic inflammatory diseases such as RA and inflammatory bowel disease [56]. In RA IKKβ is a key regulator of synovial inflammation and the importance of the canonical NF-κB pathway in arthritis is underlined by the beneficial effects of specific IKKβ inhibition in preclinical models of a rthritis [57, 58]. Fuelled by these results and beneficial effects of NF-κB inhibition in preclinical models of other inflammatory diseases, more than 700 compounds with inhibitory effects on NF-κB signaling have been reported [59]. However, clinical trials are hitherto lacking, presumably by fear of toxicity associated with global NF-κB inhibition or off-target effects. This could potentially be solved by selective targeting of the NF-κB inhibitor to a specific cell type, for instance using a multimodular recombinant protein that specifically binds to cytokine-activated endothelium, which has been demonstrated to work very elegantly under inflammatory conditions in vivo [60].
The noncanonical NF-κB pathway can be triggered by the activation of members of the TNF-receptor superfamily including the lymphotoxin β receptor (LTβ-R), CD40, B cell activating factor belonging to the TNF family (BAFF) receptor, and receptor activator of NF-κB (RANK). Of note, these receptors not only trigger the noncanonical NF-κB pathway, but simultaneously also the canonic al pathway. The noncanonical NF-κB pathway is strictly dependent on NF-κB inducing kinase (NIK) and IKKα homodimers, but does not involve IKKβ or IKKγ. Overall, this pathway is involved in lymphoid organ development and adaptive immune responses [61]. Recently, we established that noncanonical NF-κB signaling in endothelial cells stimulates pathological angiogenesis in chronic inflammation [62]. Conseque ntly, NIK inhibition using specific small molecule inhibitors could perhaps be an effective new treatment option for chronic inflammatory diseases [63].
3.3 Janus Kinase (JAK) Inhibitors
The Janus kinase (JAK) family consists of four members: JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2). JAKs associate with different cytokine receptors and via phosphorylation of tyrosine residues create docking sites for one or more signal transducer and activators of transcription (STAT) molecules. JAK1, JAK2, and TYK2 are ubiquitously expressed, whereas JAK3 is primarily expressed in hematopoietic cells. JAK1 and JAK3 convey signals from cytokine receptors that contain the IL-2 receptor common γ chain and mediate signaling by IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 , cytokines that are essential for the development and maturation of T cells. JAK2 is associated with hematopoietic growth factor receptors and cytokine receptors for IL-1, IL-6, and IL-17 that are critically involved in various aspects of immune cell function (reviewed in [64]). Consequently, inhibiting JAKs blocks multiple aspects of cytokine signaling, which makes them attractive targets for many IMIDs. Of all pr otein kinase inhibitors, JAK inhibitors have entered the clinic first. Tofacitinib (also known as CP-690550) is a potent JAK1 and JAK3 blocker, that also inhibits JAK2 to a certain extent. It was effective in preclinical models of arthritis and transplantation [65, 66]. Tofacitinib successively entered clinical trials, which demonstrated efficacy in RA [67, 68], IBD [69], and psoriasis [70]. In 2012 tofacitinib was approved for the treatment of RA in the USA, Japan and Russia. However, the European Medicines Agency (EMA) did not approve tofacitinib for RA, mainly due to concerns about the risk of serious infections. Nevertheless, EULAR included tofacitinib in their recommendations for the treatment of RA as a therapeutic option after biological treatment has failed [71].
4 Conclusion
The treatment of IMIDs continues to improve as we develop a better understanding of the pathogenesis of these diseases and the pathways that are suitable for targeting. Importantly, however, the clinical exploration of novel targeted therapies also contributes directly to our understanding of the functi on and role of specific pathways in vivo. This interaction between f undamental immunobiology and translational research has been key to many novel developments in the field of IMIDs.
These developments are not only related to an ongoing expansion of ‘classical’ target pathways (cytokines, growth factors, surface molecules, co-stimulation, adhesion) but also to fine-tuning of the way to approach these targets, as discussed for B cells. The key message here is that a single pathogenic pathway may operate in completely different ways depending on the exact immunological and tissue context. As we discussed recently for another key inflammatory pathways, the IL-23/IL-17 axis, studying the context of inflammation is as important as understanding the pathway to determine how, when and where this pathway should be optimally targeted [72, 73].
This may be further improved by new developments in recombinant antibody technology allows for the generation of bispecific antibodies that have the ability to bind to two different epitopes on the same or different antigens. This may have significant advantages over targeting one epitope, especially in complex multifaceted diseases [74], since with a single therapeutic entity two targets can be blocked or engaged. This approach has been rapidly adopted by the oncology and hematology field, and attempts are also made in the field of clinical immunology and rheumatology. An example of this is a bispecific hexavalent antibody comprising epratuzumab and veltuzumab (anti-CD22/CD20), which may lead to improved treatment of SLE and other IMIDs, but has not been formally tested yet [75].
Finally, new horizons are opening wit h completely novel targets such as the intracellular signaling pathways. This review discussed a few examples in order to highlight the enormous progresses and promises ahead of us, but is obviously far from complete. For example, there is crucial emerging knowledge in the role of epigenetic modifications in the initiation and maintenance of tissue inflammation and, accordingly, small molecules modifying for example DNA methylation and histone modifications are in (pre)clinical development [76]. To date, one clinical trial with a histone deacetylase (HDAC) inhibitor has been performed in systemic juvenile inflammatory arthritis. Oral administration of the nonselective HDACi givinostat (ITF2357) resulted in significant therapeutic benefit after 12 weeks, particularly with respect to arthritis activity, with a relatively good safety profile [77]. These and other new developments will continue to revolutionize the treat ment of IMIDs and contribute to the ongoing evolution from nonspecific im mune suppression to targeted immunomodulation and, ultimately, genuine disease modification and cure.
References
Edwards JC, Szczepanski L, Szechinski J et al (2004) Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 350:2572–2581
Stone JH, Merkel PA, Spiera R et al (2010) Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 363:221–232
Looney RJ, Anolik J, Sanz I (2010) A perspective on B-cell-targeting therapy for SLE. Mod Rheumatol 20:1–10
Looney RJ, Anolik JH, Campbell D et al (2004) B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum 50:2580–2589
Smith V, Piette Y, van Praet JT et al (2013) Two-year results of an open pilot study of a 2-treatment course with rituximab in patients with early systemic sclerosis with diffuse skin involvement. J Rheumatol 40:52–57
Meijer JM, Meiners PM, Vissink A et al (2010) Effectiveness of rituximab treatment in primary Sjogren’s syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 62:960–968
Hauser SL, Waubant E, Arnold DL et al (2008) B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 358:676–688
Bluml S, McKeever K, Ettinger R, Smolen J, Herbst R (2013) B-cell targeted therapeutics in clinical development. Arthritis Res Ther 15(Suppl 1):S4
Tan CS, Koralnik IJ (2010) Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol 9:425–437
Castillo J, Milani C, Mendez-Allwood D (2009) Ofatumumab, a second-generation anti-CD20 monoclonal antibody, for the treatment of lymphoproliferative and autoimmune disorders. Expert Opin Investig Drugs 18:491–500
Taylor PC, Quattrocchi E, Mallett S, Kurrasch R, Petersen J, Chang DJ (2011) Ofatumumab, a fully human anti-CD20 monoclonal antibody, in biological-naive, rheumatoid arthritis patients with an inadequate response to methotrexate: a randomised, double-blind, placebo-controlled clinical trial. Ann Rheum Dis 70:2119–2125
Ostergaard M, Baslund B, Rigby W et al (2010) Ofatumumab, a human anti-CD20 monoclonal antibody, for treatment of rheumatoid arthritis with an inadequate response to one or more disease-modifying antirheumatic drugs: results of a randomized, double-blind, placebo-controlled, phase I/II study. Arthritis Rheum 62:2227–2238
Sorensen PS, Lisby S, Grove R et al (2014) Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: a phase 2 study. Neurology 82:573–581
Kausar F, Mustafa K, Sweis G et al (2009) Ocrelizumab: a step forward in the evolution of B-cell therapy. Expert Opin Biol Ther 9:889–895
Rigby W, Tony HP, Oelke K et al (2012) Safety and efficacy of ocrelizumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a forty-eight-week randomized, double-blind, placebo-controlled, parallel-group phase III trial. Arthritis Rheum 64:350–359
Tak PP, Mease PJ, Genovese MC et al (2012) Safety and efficacy of ocrelizumab in patients with rheumatoid arthritis and an inadequate response to at least one tumor necrosis factor inhibitor: results of a forty-eight-week randomized, double-blind, placebo-controlled, parallel-group phase III trial. Arthritis Rheum 64:360–370
Mysler EF, Spindler AJ, Guzman R et al (2013) Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: results from a randomized, double-blind, phase III study. Arthritis Rheum 65:2368–2379
Kappos L, Li D, Calabresi PA et al (2011) Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet 378:1779–1787
Liebman HA, Saleh MN, Bussel JB et al (2013) Low-dose anti-CD20 veltuzumab given intravenously or subcutaneously is active in relapsed immune thrombocytopenia: a phase I study. Br J Haematol 162:693–701
Goldenberg DM, Morschhauser F, Wegener WA (2010) Veltuzumab (humanized anti-CD20 monoclonal antibody): characterization, current clinical results, and future prospects. Leuk Lymphoma 51:747–755
Tedder TF (2009) CD19: a promising B cell target for rheumatoid arthritis. Nat Rev Rheumatol 5:572–577
Yazawa N, Hamaguchi Y, Poe JC, Tedder TF (2005) Immunotherapy using unconjugated CD19 monoclonal antibodies in animal models for B lymphocyte malignancies and autoimmune disease. Proc Natl Acad Sci U S A 102:15178–15183
Herbst R, Wang Y, Gallagher S et al (2010) B-cell depletion in vitro and in vivo with an afucosylated anti-CD19 antibody. J Pharmacol Exp Ther 335:213–222
Deiss A, Brecht I, Haarmann A, Buttmann M (2013) Treating multiple sclerosis with monoclonal antibodies: a 2013 update. Expert Rev Neurother 13:313–335
Dorner T, Shock A, Smith KG (2012) CD22 and autoimmune disease. Int Rev Immunol 31:363–378
Tedder TF, Poe JC, Haas KM (2005) CD22: a multifunctional receptor that regulates B lymphocyte survival and signal transduction. Adv Immunol 88:1–50
Carnahan J, Stein R, Qu Z et al (2007) Epratuzumab, a CD22-targeting recombinant humanized antibody with a different mode of action from rituximab. Mol Immunol 44:1331–1341
Steinfeld SD, Tant L, Burmester GR et al (2006) Epratuzumab (humanised anti-CD22 antibody) in primary Sjogren’s syndrome: an open-label phase I/II study. Arthritis Res Ther 8:R129
Wallace DJ, Kalunian K, Petri MA et al (2014) Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study. Ann Rheum Dis 73:183–190
Wallace DJ, Gordon C, Strand V et al (2013) Efficacy and safety of epratuzumab in patients with moderate/severe flaring systemic lupus erythematosus: results from two randomized, double-blind, placebo-controlled, multicentre studies (ALLEVIATE) and follow-up. Rheumatology (Oxford) 52:1313–1322
Avery DT, Kalled SL, Ellyard JI et al (2003) BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest 112:286–297
Baker KP, Edwards BM, Main SH et al (2003) Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum 48:3253–3265
Navarra SV, Guzman RM, Gallacher AE et al (2011) Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 377:721–731
Stohl W, Merrill JT, McKay JD et al (2013) Efficacy and safety of belimumab in patients with rheumatoid arthritis: a phase II, randomized, double-blind, placebo-controlled, dose-ranging Study. J Rheumatol 40:579–589
Vincent FB, Saulep-Easton D, Figgett WA, Fairfax KA, Mackay F (2013) The BAFF/APRIL system: emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev 24:203–215
Davidson A (2010) Targeting BAFF in autoimmunity. Curr Opin Immunol 22:732–739
Genovese MC, Lee E, Satterwhite J et al (2013) A phase 2 dose-ranging study of subcutaneous tabalumab for the treatment of patients with active rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis 72:1453–1460
Stohl W (2014) Therapeutic targeting of the BAFF/APRIL axis in systemic lupus erythematosus. Expert Opin Ther Targets 18:473–489
Gensicke H, Leppert D, Yaldizli O et al (2012) Monoclonal antibodies and recombinant immunoglobulins for the treatment of multiple sclerosis. CNS Drugs 26:11–37
Gatto B (2008) Atacicept, a homodimeric fusion protein for the potential treatment of diseases triggered by plasma cells. Curr Opin Investig Drugs 9:1216–1227
Schneider P (2005) The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol 17:282–289
Dall’Era M, Chakravarty E, Wallace D et al (2007) Reduced B lymphocyte and immunoglobulin levels after atacicept treatment in patients with systemic lupus erythematosus: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating trial. Arthritis Rheum 56:4142–4150
van Vollenhoven RF, Kinnman N, Vincent E, Wax S, Bathon J (2011) Atacicept in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase II, randomized, placebo-controlled trial. Arthritis Rheum 63:1782–1792
Genovese MC, Kinnman N, de La BG, Pena RC, Tak PP (2011) Atacicept in patients with rheumatoid arthritis and an inadequate response to tumor necrosis factor antagonist therapy: results of a phase II, randomized, placebo-controlled, dose-finding trial. Arthritis Rheum 63:1793–1803
Kappos L, Hartung HP, Freedman MS et al (2014) Atacicept in multiple sclerosis (ATAMS): a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Neurol 13:353–363
Fernandez L, Salinas GF, Rocha C et al (2013) The TNF family member APRIL dampens collagen-induced arthritis. Ann Rheum Dis 72:1367–1374
Arthur JS, Ley SC (2013) Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol 13:679–692
Hammaker D, Firestein GS (2010) “Go upstream, young man”: lessons learned from the p38 saga. Ann Rheum Dis 69(Suppl 1):i77–i82
Hommes D, van den Blink B, Plasse T et al (2002) Inhibition of stress-activated MAP kinases induces clinical improvement in moderate to severe Crohn’s disease. Gastroenterology 122:7–14
Dotan I, Rachmilewitz D, Schreiber S et al (2010) A randomised placebo-controlled multicentre trial of intravenous semapimod HCl for moderate to severe Crohn’s disease. Gut 59:760–766
Salh B (2007) c-Jun N-terminal kinases as potential therapeutic targets. Expert Opin Ther Targets 11:1339–1353
Guma M, Firestein GS (2012) c-Jun N-terminal kinase in inflammation and rheumatic diseases. Open Rheumatol J 6:220–231
Han Z, Boyle DL, Chang L et al (2001) c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest 108:73–81
Hayden MS, Ghosh S (2011) NF-kappaB in immunobiology. Cell Res 21:223–244
Vallabhapurapu S, Karin M (2009) Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol 27:693–733
Tak PP, Firestein GS (2001) NF-kappaB: a key role in inflammatory diseases. J Clin Invest 107:7–11
Tak PP, Gerlag DM, Aupperle KR et al (2001) Inhibitor of nuclear factor kappaB kinase beta is a key regulator of synovial inflammation. Arthritis Rheum 44:1897–1907
Tas SW, Vervoordeldonk MJ, Hajji N, May MJ, Ghosh S, Tak PP (2006) Local treatment with the selective IkappaB kinase beta inhibitor NEMO-binding domain peptide ameliorates synovial inflammation. Arthritis Res Ther 8:R86
Kwak JH, Jung JK, Lee H (2011) Nuclear factor-kappa B inhibitors; a patent review (2006-2010). Expert Opin Ther Pat 21:1897–1910
Sehnert B, Burkhardt H, Wessels JT et al (2013) NF-kappaB inhibitor targeted to activated endothelium demonstrates a critical role of endothelial NF-kappaB in immune-mediated diseases. Proc Natl Acad Sci U S A 110:16556–16561
Sun SC (2012) The noncanonical NF-kappaB pathway. Immunol Rev 246:125–140
Noort AR, van Zoest KP, Weijers EM et al (2014) NF-kappaB inducing kinase is a key regulator of inflammation-induced and tumor-associated angiogenesis. J Pathol 234:375
Li K, McGee LR, Fisher B et al (2013) Inhibiting NF-kappaB-inducing kinase (NIK): discovery, structure-based design, synthesis, structure-activity relationship, and co-crystal structures. Bioorg Med Chem Lett 23:1238–1244
Shuai K, Liu B (2003) Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol 3:900–911
Milici AJ, Kudlacz EM, Audoly L, Zwillich S, Changelian P (2008) Cartilage preservation by inhibition of Janus kinase 3 in two rodent models of rheumatoid arthritis. Arthritis Res Ther 10:R14
Changelian PS, Flanagan ME, Ball DJ et al (2003) Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science 302:875–878
Fleischmann R, Kremer J, Cush J et al (2012) Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 367:495–507
Burmester GR, Blanco R, Charles-Schoeman C et al (2013) Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 381:451–460
Sandborn WJ, Ghosh S, Panes J et al (2012) Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med 367:616–624
Boy MG, Wang C, Wilkinson BE et al (2009) Double-blind, placebo-controlled, dose-escalation study to evaluate the pharmacologic effect of CP-690,550 in patients with psoriasis. J Invest Dermatol 129:2299–2302
Smolen JS, Landewe R, Breedveld FC et al (2014) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 73:492–509
Baeten DL, Kuchroo VK (2013) How Cytokine networks fuel inflammation: interleukin-17 and a tale of two autoimmune diseases. Nat Med 19:824–825
Yeremenko N, Paramarta JE, Baeten D (2014) The interleukin-23/interleukin-17 immune axis as a promising new target in the treatment of spondyloarthritis. Curr Opin Rheumatol 26:361–370
Byrne H, Conroy PJ, Whisstock JC, O’Kennedy RJ (2013) A tale of two specificities: bispecific antibodies for therapeutic and diagnostic applications. Trends Biotechnol 31:621–632
Rossi EA, Chang CH, Goldenberg DM (2014) Anti-CD22/CD20 Bispecific antibody with enhanced trogocytosis for treatment of Lupus. PLoS One 9:e98315
Grabiec AM, Reedquist KA (2013) The ascent of acetylation in the epigenetics of rheumatoid arthritis. Nat Rev Rheumatol 9:311–318
Vojinovic J, Damjanov N, D’Urzo C et al (2011) Safety and efficacy of an oral histone deacetylase inhibitor in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum 63:1452–1458
Acknowledgements
SWT is supported by a VENI grant and a Clinical Fellowship from the Netherlands Organization for Scientific Research (NWO/ZonMw), and grants from the Dutch Arthritis Foundation. DLB is supported by a VICI grant from the Netherlands Organization for Scientific Research (NWO), and grants from the Dutch Arthritis Foundation.
Disclosure of conflicts of interest: The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Tas, S.W., Baeten, D.L.P. (2016). Recent Advances in the Treatment of Immune-Mediated Inflammatory Diseases. In: Cuturi, M., Anegon, I. (eds) Suppression and Regulation of Immune Responses. Methods in Molecular Biology, vol 1371. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3139-2_9
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3139-2_9
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3138-5
Online ISBN: 978-1-4939-3139-2
eBook Packages: Springer Protocols