Abstract
Magnetic nanocomposites are hybrid structures consisting of an iron oxide (Fe3O4/γ-Fe2O3) superparamagnetic core and a coating shell which presents affinity for a specific target molecule. Within the scope of phosphopeptide enrichment, the magnetic core is usually first functionalized with an intermediate layer of silica or carbon to improve dispersibility and increase specific area, and then with an outer layer of a phosphate-affinity material. Fe3O4-coating materials include metal oxides, rare earth metal-based compounds, immobilized-metal ions, polymers, and many others. This chapter provides a generic overview of the different materials that can be found in literature and their advantages and drawbacks.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
Phosphorylation is a dynamic, abundant, and highly studied posttranslational modification of proteins, affecting approximately one-third of all proteins at any particular time [1]. It is involved in the regulation of a variety of cellular processes, such as cell cycle control, DNA damage responses, transcription, protein trafficking, metabolism, and programmed cell death [2, 1]. In the human proteome, phosphorylation occurs predominantly in serine (≈90 %), followed by threonine (≈10 %) and tyrosine (≈0.05 %), although the relative abundances of these residues depend on the methodology used for the quantification of phosphorylation events [3, 4].
Phosphorylation events are key players in cellular signaling, being involved in the occurrence of many human diseases, such as cancer [5], Alzheimer’s [6], Parkinson’s [7], cardiovascular diseases [8], schizophrenia [9], and many others. The accurate identification of phosphorylated proteins, determination of their phosphorylation sites and quantification of stoichiometry, and monitoring of temporal dynamics of protein phosphorylation in response to cellular perturbations, are fundamental to understand the mechanisms behind disease pathologies and inspire the development of novel biomarkers and therapeutic agents [10].
During the last decades, mass spectrometry (MS) has been the basis of enormous scientific breakthroughs and it has been playing a central role in the profiling of protein phosphorylation [3, 11, 12]. However, as phosphopeptides have comparably low ionization efficiencies and are usually present in sub-stoichiometric concentrations in biological samples, an enrichment step is required prior to MS analysis [13, 14].
A great number of enrichment methods have been reported in recent years, namely immunoprecipitation, immobilized metal affinity chromatography (IMAC ), metal oxide affinity chromatography (MOAC ), ion-exchange chromatography, chemical tagging and use of phosphate-affinity ligands [15].
Ion-exchange methodologies (strong ion exchange (SCX ) or strong anion exchange (SAX)) usually require high amounts of starting material and prolonged analysis times, due to the presence of multiple fractions [10]. In addition, due to the nonspecific adsorption behavior of SCX and SAX, these methods are more commonly used as pre-fractionation techniques in combination with other methods such as reversed phase-LC , IMAC or MOAC [16–18]. Recently, a mixed-bed resin comprising a blend of anion and cation exchangers (ACE) has been reported to increase phosphopeptide identification by 94 % when compared to SCX [19].
Methods based on the chemical modification of the phosphate group are usually labor-intensive and implicate several reaction steps, which makes them less useful for routine utilization [20].
Phospho-specific antibodies are expensive and difficult to produce due to the low immunogenicity of the phosphate group and its susceptibility to cleavage during the immunization process [21]. Nonetheless, antiphosphotyrosine antibodies have been successfully used for the detection of a variety of phosphorylated proteins, in contrast with antiphosphoserine and antiphosphothreonine antibodies, which are still far less specific [10, 15, 22].
Metal chelating methodologies include IMAC , MOAC , and some new metal chelating ligands (e.g., Phos-Tag™) (see Chapter 3). These methods are based on the coordination of the negatively charged phosphate group to positively charged metal ions, a main disadvantage being the nonspecific binding of acidic peptides to the metal-chelating resins [22]. Different research groups have been trying to circumvent this problem. O-methyl esterification of the carboxylic acid groups is a common approach, but has some limitations associated with the occurrence of side reactions and incomplete derivatization of the carboxylic groups [23–25]. Larsen and coworkers introduced a competitive binder—2,5-dihydroxy benzoic acid (2,5-DHB )—in buffers to reduce the nonspecific binding of acidic peptides to TiO2 microcolumns [25]. Ye and coworkers developed an optimized Fe(III) nitrilotriacetic acid (NTA) IMAC protocol by using 60 % (v/v) acetonitrile (ACN) in loading and washing buffers, since ACN has different effects on the degree of ionization of phosphate and carboxylic acid moieties [26].
Hybrid material s , which combine distinct properties of various materials, have been proven exquisitely efficient in the selective binding of phosphorylated peptides. In particular, composite materials in the nanomolar range (nanocomposites) present higher surface area to volume ratios, providing superior surface functionalization [27, 28]. This chapter will explore the application of different classes of nanocomposites in the phosphoproteomics field.
2 Magnetic Nanocomposites
As mentioned previously, metal oxides can be used to enrich phosphorylated species in peptide samples. Among them, iron oxide nanoparticles (MNPs) present unique properties, such as superparamagnetism, high magnetic susceptibility, high coercivity, and low Curie temperature [29]. Bare MNPs have been reported to selectively bind phosphorylated peptides from tryptic digests containing 1 pmol β-casein , cytochrome c, bovine serum albumin (BSA ), and horse heart myoglobin. However, this was only observed when the iron oxide was in the form of magnetite (Fe3O4), as maghemite (γ-Fe2O3) beads did not facilitate phosphopeptide binding. The reasons behind the superior performance of Fe3O4 over γ-Fe2O3 are not yet fully understood, but have been speculated to be related to structural differences and magnetic properties of those materials [30].
In addition to their easy manipulation by an external magnetic field, their surface can be functionalized with a variety of organic and inorganic materials, which not only protect them against oxidation and erosion by acids and bases, but also tailor their surface in terms of charge, hydrophobicity, and chemical functionality [31, 32]. The process of phosphopeptide enrichment using magnetic nanocomposites is illustrated in Fig. 1.
Enrichment of phosphorylated peptides using magnetic nanocomposites: (1) incubation of magnetic nanocomposites with tryptic protein digest samples; (2) magnetic separation of phosphopeptide-bound magnetic nanocomposites; (3) washing step to remove nonspecifically adsorbed non-phosphorylated peptides; (4a) phosphopeptide-bound magnetic nanocomposites can be spotted onto a MALDI target and directly analyzed by MS or (4b) phosphopeptides may be eluted from particles and analyzed either by MALDI or ESI MS
2.1 Metal Oxide-Based Magnetic Nanocomposites
2.1.1 Titanium Dioxide (TiO2 )
TiO2 was the first metal oxide to be reported for the enrichment of phosphorylated peptides. TiO2-based materials are commercially available and TiO2-based protocols are common practice in many laboratories, due to their simplicity and efficiency (see Table 1) (see Chapters 9, 12, 17). Chen and Chen used a two-step sol–gel process to produce TiO2-coated MNPs (Fe3O4@TiO2) combining the convenient separation ability of MNPs with the phosphopeptide trapping capacity of TiO2. In addition, Fe3O4@TiO2 magnetic nanoparticles were effectively used as surface-assisted laser desorption/ionization (SALDI) MS matrices [33]. However, they obtained composite materials of ill-defined structure, which compromised their bias towards phosphorylated peptides, as nonspecific binding of acidic peptides was also observed. Later on, Li and coworkers addressed this issue by coating the particles with a carbon layer in an intermediate reaction step in order to reduce nonspecific adsorption. Their three-step synthesis route consisted of: (1) synthesis of MNPs via solvothermal reaction; (2) MNPs coating with a thin layer of carbon (≈20 nm thickness); and (3) absorption of nanosized titanium oligomers and conversion into titanium by calcination [34, 35].
In 2010, Lu and coworkers reported the synthesis of self-assembled mesoporous TiO2 nanocrystal structures with high adsorption capacity, low detection limit (10 fmol for a β-casein tryptic digest), high selectivity, high water dispersibility, and high chemical and mechanical stability. The process of fabrication of these mesoporous nanocrystal clusters consists of the synthesis and self-assembly of TiO2 nanocrystals; coating with a thin layer of silica to avoid aggregation; calcination at high temperatures to improve mechanical stability and remove organic surfactants; and silica removal by etching. One of the advantages of this self-assembly process is that multiple components may be added to the clusters, which was demonstrated by the successful fabrication of γ-Fe2O3/TiO2 composite clusters to facilitate separation. Both TiO2 and γ-Fe2O3/TiO2 colloidal nanocrystal clusters presented similar hydrodynamic diameters between 50 and 200 nm [36].
2.1.2 Zirconium Dioxide (ZrO2)
A fast method using ZrO2-coated MNPs as concentrating probes was developed to enrich samples in phosphopeptides in only 30 s employing vigorous mixing by pipetting. In addition, these particles can function as microwave absorbers and facilitate enzymatic digestion (digestion time ≈15 s sonication + 1 min microwave heating) [37].
MNPs coated with both TiO2 and ZrO2 (Fe3O4@TiO2-ZrO2) present improved phosphopeptide trapping ability when compared to Fe3O4@TiO2 or Fe3O4@ZrO2 alone and efficiently enrich samples in both mono- and multi-phosphorylated peptides [38]. These metal oxides have complementary properties: ZrO2 is more selective towards mono-phosphorylated peptides whereas TiO2 preferentially binds to multi-phosphorylated peptides [39].
Very recently, yolk-shell magnetic supraparticles coated with ZrO2 (MSP@ZrO2) have been reported to selectively enrich phosphopeptides both from standard phosphoprotein tryptic digests and biological samples [40]. Supraparticles are originated from the assembly of MNPs and have enhanced magnetic responsiveness and preserved superparamagnetism [41]. The yolk-shell architecture consists of nanoparticle core inside a hollow shell, presenting low mass density which improves dispersibility and enhances the adsorbing efficiency of phosphopeptides when compared to solid Fe3O4@ZrO2 microspheres (see Fig. 2) [40]. A total of 33 phosphopeptides containing 49 phosphorylation sites mapped to 33 phosphoproteins were identified in human saliva, which was a better result when compared to zinc oxide (ZnO)-coated MNPs. Nonetheless, ZnO-coated MNPs presented a detection limit 3 orders of magnitude lower (2.5 fmol for β-casein tryptic digest) and an enrichment time 60 times lower than the yolk-shell MSP@ZrO2 [40, 42]. In addition, TiO2 -coated MNPs presented similar phosphopeptide trapping performance as ZnO-coated MNPs for the same human saliva tryptic digest [42].
Synthesis of yolk–shell MSP@ZrO2. Magnetic supraparticles synthesized by a solvothermal reaction are modified with a polymeric layer of polymethylacrylic acid (PMAA), followed by functionalization with Zr(OH)4. Calcination of MSP@PMAA@PMAA-Zr(OH)4 removes the polymeric layer and leads to the formation of hollow crystalline ZrO2 spheres with movable MSP core
2.1.3 Aluminum Oxide (Al2O3)
Al2O3-coated magnetic beads have been described in literature as effective phosphopeptide affinity and sensing tools. Al2O3-coated MNPs have trapping capacities of 60 μg of phosphopeptides per milligram of particles, and a detection limit of 25 fmol for human protein phosphatase inhibitor 1 (PPI 1) tryptic digest. The entire process of enrichment and MALDI MS analysis takes approximately 5 min [43]. Al2O3-coated MNPs modified with a fluorophore—riboflavin-5′-monophosphate (RFMP-Fe3O4@Al2O3) have been used as affinity and sensing probes for phosphorylated fibrinopeptide A, a peptide which exists in elevated levels in patients with gastric and ovarian cancers and is known to exist in phosphorylated form to an extent of 20–30 % in human blood. RFMP molecules immobilized at the surface of the particles exchange with phosphorylated fibrinopeptide A in solution, allowing the quantitative analysis of the solution by fluorescence spectroscopy. Particles with the trapped phosphorylated peptide may subsequently be analyzed qualitatively by mass spectrometry [44].
2.1.4 Other Metal Oxides
Niobium pentoxide (Nb2O5) efficiently enriches phosphopeptides from standard tryptic protein mixtures and cellular lysates with 50 % overlap in peptide sequence when compared to TiO2 , which translates into a high degree of orthogonality between both metal oxides. In addition, Nb2O5 and TiO2 presented similar recovery efficiencies of phosphopeptides between 50 and 100 % [45]. Nb2O5-coated MNPs are able to trap phosphorylated peptides from tryptic digests of caseins, serum and cell lysate in only 1 min using microwave heating and with a detection limit of 5 fmol [46].
Tantalum pentoxide (Ta2O5)-coated MNPs present divergent phosphopeptide trapping selectivity when compared to TiO2-coated MNPs, with a larger number of unique phosphopeptides being identified by Ta2O5-coated MNPs [47]. The addition of 2,5-DHB to the loading solution contributes to the enhanced selectivity towards phosphorylated species, a concept that had been introduced by Larsen and coworkers [25, 47].
Other metal oxides, such as gallium oxide (Ga2O3) [48] and tin dioxide (SnO2) [49], have also been combined with Fe3O4 and used in phosphoproteomic experiments.
The sensitivity and enrichment time of different metal oxide-coated MNPs are presented in Table 2. However, it should be taken into consideration that this comparison between different materials is not always straightforward, as experiments are not always performed in the same conditions and in some cases use different instrumentation. One interesting detail to point out is that Fe3O4 magnetic particles are excellent microwave absorbers. Microwave heating can be applied both during tryptic digestion and enrichment steps, which in addition to the ease of separation of MNPs makes the entire process extremely time-efficient.
2.2 Rare Earth Metal-Based Magnetic Nanocomposites
Due to the strong affinity between rare earth metal ions and phosphate moieties, a variety of different materials based on this type of metals have been developed for phosphopeptide enrichment. Rare metal ions (hard acids) are able to coordinate oxygen atoms (hard bases) of phosphate groups through mono- or multi-dentate bonds, which make the phosphorous atoms more electropositive and more susceptible to nucleophilic attack by hydroxyl groups. This process results in the cleavage of phosphate-ester bonds and consequent dephosphorylation of the peptides [50].
Very recently, rare earth vanadate-coated MNPs (γ-Fe2O3@REVO4; RE = Sm, Dy, Ho) were used for the first time to enrich phosphorylated peptides from tryptic digests of standard proteins and human serum. The three rare earth metals tested presented similar morphologies, saturation magnetization values, and selectivity towards phosphorylated peptides. The sensitivity was slightly worse for γ-Fe2O3@HoVO4 (200 fmol for a β-casein tryptic digest) when compared to γ-Fe2O3@SmVO4 and γ-Fe2O3@DyVO4 (100 fmol). Particles could be used up to five times without significant loss of binding capacity or selectivity [50].
Rare earth metal oxides, such as CeO2, are also starting to be explored in the field of phosphoproteomics. MNPs coated with an intermediate layer of silica and an outer layer of mesoporous CeO2 (Fe3O4@SiO2@mCeO2) are multifunctional probes, as they have phosphate-affinity, magnetic properties, and they catalyze the dephosphorylation of phosphopeptides, which results in a specific neutral loss of n × 80 Da that can be detected in the MS spectra [51]. The same number of phosphorylated peptides and their correspondent label ions (with decreased masses of n × 80 Da) from a tryptic digest of β-casein could be identified using lanthanum silicate coated MNPs (Fe3O4@La x Si y O5). Moreover, the relative intensity of the multi-phosphorylated peptide at m/z 3122 Da was much higher than for Fe3O4@SiO2@mCeO2. This is probably related with the differences in the catalytic efficiency of La and Ce towards phosphate hydrolysis: milder dephosphorylation facilitating phosphopeptide identification [52].
Very recently, a 3D flowerlike structure composed of a γ-Fe2O3 magnetic core coated with a shell of ammonium fluoride and lutetium fluoride (γ-Fe2O3@xNH4F.yLuF3) was used for the selective capture of phosphopeptides from β-casein digest, nonfat milk tryptic digest and human serum. These 3D nanostructured architectures have unique properties such as highly specific surface areas, low density, and large open pores [53].
A yolk-shell nanostructure composed of a Fe3O4 magnetic core and an yttrium phosphate (YPO4) hollow porous affinity shell (Fe3O4@hYPO4) is another example of success in the application of rare metal ions for the enrichment of phosphopeptides, where a detection limit of 10 fmol was determined for β-casein tryptic digests [54]. A distinct approach consisting of coating an ultrathin YPO4 shell on polyacrylate capped Fe3O4 (PA-Fe3O4@YPO4) allowed faster adsorption/desorption dynamics, and low nonspecific binding [55]. Table 3 presents the sensitivity and enrichment times of different rare earth metal-based magnetic nanocomposites.
2.3 IMAC-Based Magnetic Nanocomposites
Despite some inherent disadvantages, such as nonspecific binding and metal leaching, IMAC still plays a prominent role in many purification and enrichment processes. In IMAC, transition metal ions are immobilized onto a solid support using a chelating ligand, such as iminodiacetic acid (IDA), nitrilotriacetic acid (NTA), or tris(carboxymethyl)ethylene diamine (TED). The selection of metal ion depends on the application: divalent cations (Cu2+, Ni2+, Zn2+, Co2+) are used for the purification of histidine-tagged proteins, whereas trivalent ions (Al3+, Ga3+, Fe3+) and the tetravalent ion Zr4+ are used for phosphopeptide enrichment [56]. Rare earth metals, such as La3+, Ho3+, Er3+, and Ce4+, have also been successfully used for the capture of phosphorylated peptides using IMAC [57, 58].
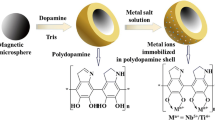
A common method of functionalizing the surface of nanoparticles with IDA groups is by reacting IDA with a silane coupling agent such as 3-glycidoxypropyltrimethoxysilane (GLYMO). GLYMO-IDA can then be grafted onto the surface of silica-coated Fe3O4, providing chelate sites for the immobilization of metal ions. The presence of silica prevents iron leaching at acidic pH, and helps dispersing the particles in solution, besides providing functional groups at the surface of the particles [59]. GLYMO-IDA has already been used to attach Fe3+ and Ce4+ ions at the surface of magnetic silica nanocomposites [58, 60, 59]. Ce4+-magnetic silica nanocomposites provided higher phosphopeptide selectivity than Fe3+-magnetic silica [58].
A different methodology consisted of coating the magnetic iron oxide core with poly(2-hydroxyethylmethacrylate-co-glycidyl methacrylate) (P(HEMA–GMA)), a polymer which endows both hydrophilicity to prevent nonspecific binding of peptides, and oxirane functional groups able to react with diethyl ester of IDA. Fe3+ and Ga3+ immobilized IDA-modified magnetic nanoparticles successfully enriched the samples in both mono- and multi-phosphorylated peptides with minor interference of acidic peptides [61].
Ga3+ and Zr4+ immobilized NTA-modified magnetic nanoparticles are able to detect as little as 50 fmol phosphopeptides from tryptic digests of α- and β-casein s, with the entire process taking less than 10 min. Fe3O4 particles are first silanized and coated with succinic anhydride, and then functionalized with NTA using carbodiimide chemistry. Fe3O4/NTA/Zr4+ presents higher phosphopeptide binding capacity than Fe3O4/NTA/Ga3+ owning to the fact that Zr4+ presents higher coordination number and consequently provides a larger number of phosphopeptide binding sites [62].
The urgent need for rapid automated systems that combine pre-concentration and selective extraction of phosphorylated species from complex samples, allowing qualitative and quantitative analysis, led to the appearance of high-throughput platforms. With this purpose, a robotic platform that manipulates magnetic beads in a 96-well format, providing highly selective automated enrichment of phosphopeptides and rapid evaluation of experimental parameters, such as metal/chelator combinations, buffer composition, and sample clean-up conditions, was recently described. A combination of six metal ions (Fe3+, Ga3+, Al3+, Zn2+, Cu2+, ZrO2+) and two chelating agents (IDA and NTA) were screened. Generally speaking NTA outperformed IDA, and best results in terms of number of phosphopeptides identified and selectivity were found for magnetic particles functionalized with NTA and either Fe3+ or Ga3+. Experiments were conducted in parallel in 96-well plates and completed in approximately 45 min [63].
Considerable efforts have been made to surpass problems associated with low ligand density and metal-leaching observed for traditional IDA and NTA linkers. Very recently, Ti4+-immobilized multilayer polysaccharide coated magnetic nanoparticles have been reported as an exquisite alternative, presenting an extremely low detection limit of 0.5 fmol, large binding capacities (100 mg/g), an enrichment recovery of 85 %, and rapid magnetic separation (10 s). In addition, these nanocomposites were effective in the enrichment of human serum and nonfat milk. The Fe3O4 core is coated with two layers of silica, which is further functionalized first with a thick multilayer polysaccharide consisting of hyaluronate (HA) and chitosan (CS), and second with titanium phosphate (Fe3O4@SiO2@(HA/CS)10-Ti4+). The multilayer polysaccharide provides both hydrophilic properties and larger number of immobilized Ti4+ ions [64].
Recently, Zhang and coworkers have reported the use of adenosine triphosphate (ATP ) as chelating ligand. ATP presents two main advantageous features: (1) it is hydrophilic and therefore minimizes nonspecific binding of non-phosphorylated peptides through hydrophobic interactions; and (2) it is able to immobilize metal ions through intermolecular and intramolecular forces providing cross-linked metal-phosphonate sites for the binding of phosphorylated peptides. Using on-target enrichment it was possible to identify peptides from β-casein tryptic digests at the attomole level [65].
Table 4 presents the sensitivity and enrichment times of different IMAC -based magnetic nanocomposites.
2.4 Polymer-Based Magnetic Nanocomposites
As mentioned in the previous section, coating nanoparticles with polymers may present several advantages, as they tune surface chemistry by introducing different functional groups, help preventing particle agglomeration and reduce nonspecific binding. The choice of polymer is extremely important and several factors must be carefully considered, such as charge, hydrophobicity, molecular weight, conformation, biodegradation, degree of surface coverage [32, 66, 67].
Chen and coworkers have used polyethylenimine (PEI), a branched polymer with a high-density of amine groups and strong protonation capacity, to coat Fe3O4 MNPs. PEI-coated MNPs were able to enrich phosphopeptides from tryptic digests of protein mixtures consisting of 0.07 % (mol/mol) phosphoproteins in only 1 min. The sensitivity of the method was determined to be 5 fmol using α- and β-casein tryptic digests. PEI-coated MNPs are positively charged within a wide pH range (3–11) and interact electrostatically with the negatively charged phosphate groups. However, similar to other enrichment methods, factors such as the composition of the binding buffer have visible effects on phosphopeptide binding. When 100 % ACN/0.1 % TFA (v/v) was used as binding solvent, both mono- and multi-phosphorylated peptides were detected on the MS spectra . Increasing the water content of the binding solvent (50 % ACN/0.1 % TFA (v/v)) led to the identification of higher intensity peaks correspondent to multi-phosphorylated peptides, but the mono-phosphorylated species did not appear on spectra. This is speculated to be related to the fact that hydration promotes phosphopeptide binding and, therefore, the preferential binding of multiply phosphorylated peptides. A two-step treatment using both solvents, first 100 % ACN/0.1 % TFA (v/v) and then 50 % ACN/0.1 % TFA (v/v), provided improved results as both mono- and multi-phosphorylated peptides could be identified with higher intensities [68].
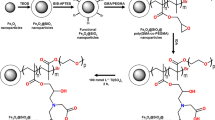
A new method coined “polymer-based metal ion affinity capture (PolyMAC )” has been revolutionizing the concept of phosphopeptide enrichment. The method consists of functionalizing a soluble nanopolymer (polyamidoamine (PAMAM) dendrimer generation 4) with phosphonate groups which in turn chelates Ti4+ ions. The nanopolymer is also modified with aldehyde (“handle”) groups. In total, the PolyMAC-Ti4+ reagent contains 35 Ti4+ and 6 aldehyde groups, but this number can be tailored by adapting the reaction conditions. PolyMAC-Ti4+ captures phosphopeptides in solution phase and is then immobilized onto a solid support, such as agarose or magnetic beads, through the formation of a hydrazone bond between its aldehyde groups and hydrazine groups at the surface of the solid support (see Fig. 3). PolyMAC showed better performance in terms of number of unique phosphosites identified and enrichment selectivity when compared to other commercially available TiO2 and IMAC reagents (see Table 1) [69].
2.5 Other Magnetic Nanocomposites
The number of novel magnetic nanocomposites with affinity for phosphorylated peptides is increasing day to day. This section describes some of the recently reported novel composites which showed great potential for phosphopeptide enrichment.
During the last decade, a few phosphate-affinity ligands based on metal chelation have been reported in the literature [15]. Among them, Phos-tag excelled by its competitiveness to other commercially available traditional implemented IMAC and MOAC based materials. Phos-tag consists of an alkoxide-bridged dinuclear Zn(II) complex with 1,3-bis(pyridin-2-ylmethylamino)propan-2-olate which is able to chelate phosphate moieties through the divalent metal ion (see also Chapter 3). Zn2+-Phos-tag was attached to N-hydroxysuccinimide-activated agarose-coated magnetic beads using a 15-atom amine-terminated spacer (see Fig. 4). One of the advantages of this method is that it allows the use of buffers at physiological pH. Phosphate binding capacity was 4 μmol phenyl phosphate dianion per milliliter Zn2+-Phos-tag magnetic beads. Beads can be used up to 15 times without loss of performance and phosphopeptide recovery yields of nearly 100 % [70].
The chemical stability and excellent mechanical and electronic properties of carbon nanotubes (CNTs) boosted their application in numerous scientific fields [71]. CNTs have been combined with several solid supports, such as gold and magnetic nanoparticles, because they help preventing macroscopic particle agglomeration. Recently, magnetic CNTs were synthesized via a hydrothermal method and then modified with TiO2 (MagCNT@TiO2). TiO2 was added in order to increase the surface area and due to its phosphate-affinity properties. The composites presented a sensitivity of 20 fmol for a tryptic β-casein digest and could be reused up to ten times [72].
Fe3O4 particles derivatized with octadecyltrimethoxysilane (C18-functionalized magnetic beads) have been used to capture both phosphorylated and non-phosphorylated peptides, which can subsequently be selectively desorbed and analyzed by MS without the need of an elution step. C18 binds non-phosphorylated peptides through hydrophobic interactions, while the Fe3O4 core is able to chelate phosphorylated peptides. Desalting is achieved by washing the particles with 0.1 % formic acid (v/v). After desalting, particles can be resuspended in α-cyano-4-hydroxy-cinnamic acid (α-CHCA) matrix and spotted onto the MALDI target for the identification of non-phosphorylated peptides. The analysis of phosphorylated peptides requires two additional washing steps with 75 % ACN/0.25 % H2SO4 and 75 % ACN/1 % NH4OH in order to remove the non-phosphorylated peptides bound to the particles. The washed particles are then resuspended in 2,5-DHB with 1 % H3PO4 and spotted onto the MALDI plate. The choice of an appropriate MALDI matrix is critical for a sensitive and accurate identification of both phosphorylated and non-phosphorylated peptides [73].
3 Conclusions
Magnetic nanocomposite s are promising materials in the phosphoproteomics field, because they combine the superparamagnetic properties of the Fe3O4/γ-Fe2O3 core with the phosphate-affinity properties of different coating materials. In addition, Fe3O4/γ-Fe2O3 nanoparticles are able to absorb microwave radiation, allowing faster enzymatic digestion and shorter incubation periods. Therefore, these multifunctional composites are optimized for high-throughput protocols, which are both time and cost effective.
References
López E, Cho W (2012) Phosphoproteomics and lung cancer research. Int J Mol Sci 13:12287–12314
Rigbolt KT, Blagoev B (2012) Quantitative phosphoproteomics to characterize signaling networks. Semin Cell Dev Biol 23:863–871
Mann M, Ong S-E, Grønborg M et al (2002) Analysis of protein phosphorylation using mass spectrometry: deciphering the phosphoproteome. Trends Biotechnol 20:261–268
Engholm-Keller K, Larsen MR (2013) Technologies and challenges in large-scale phosphoproteomics. Proteomics 13:910–931
Harsha H, Pandey A (2010) Phosphoproteomics in cancer. Mol Oncol 4:482–495
Iqbal K, Liu F, Gong C-X et al (2010) Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res 7:656
Braithwaite SP, Stock JB, Mouradian MM (2012) α-Synuclein phosphorylation as a therapeutic target in Parkinson’s disease. Rev Neurosci 23:191–198
Kotlo K, Johnson KR, Grillon JM et al (2012) Phosphoprotein abundance changes in hypertensive cardiac remodeling. J Proteomics 77:1–13
Jaros JA, Martins-de-Souza D, Rahmoune H et al (2012) Protein phosphorylation patterns in serum from schizophrenia patients and healthy controls. J Proteomics 76:43–55
Nita-Lazar A, Saito-Benz H, White FM (2008) Quantitative phosphoproteomics by mass spectrometry: past, present, and future. Proteomics 8:4433–4443
Eyrich B, Sickmann A, Zahedi RP (2011) Catch me if you can: mass spectrometry‐based phosphoproteomics and quantification strategies. Proteomics 11:554–570
Palumbo AM, Smith SA, Kalcic CL et al (2011) Tandem mass spectrometry strategies for phosphoproteome analysis. Mass Spectrom Rev 30:600–625
Macek B, Mann M, Olsen JV (2009) Global and site-specific quantitative phosphoproteomics: principles and applications. Annu Rev Pharmacol 49:199–221
Beltran L, Cutillas PR (2012) Advances in phosphopeptide enrichment techniques for phosphoproteomics. Amino Acids 43:1009–1024
Batalha IL, Lowe CR, Roque AC (2012) Platforms for enrichment of phosphorylated proteins and peptides in proteomics. Trends Biotechnol 30:100–110
Villén J, Gygi SP (2008) The SCX/IMAC enrichment approach for global phosphorylation analysis by mass spectrometry. Nat Protoc 3:1630–1638
Zarei M, Sprenger A, Metzger F et al (2011) Comparison of ERLIC–TiO2, HILIC–TiO2, and SCX–TiO2 for global phosphoproteomics approaches. J Proteome Res 10:3474–3483
Nühse TS, Stensballe A, Jensen ON et al (2003) Large-scale analysis of in vivo phosphorylated membrane proteins by immobilized metal ion affinity chromatography and mass spectrometry. Mol Cell Proteomics 2:1234–1243
Motoyama A, Xu T, Ruse CI et al (2007) Anion and cation mixed-bed ion exchange for enhanced multidimensional separations of peptides and phosphopeptides. Anal Chem 79:3623–3634
Pina AS, Batalha ÍL, Roque ACA (2014) Affinity tags in protein purification and peptide enrichment: an overview. In: Labrou NE (ed) Protein downstream processing, vol 1129, Methods in molecular biology. Humana Press, Totowa, NJ, pp 147–168. doi:10.1007/978-1-62703-977-2_14
Cai D, Lee A, Chiang C-M et al (2011) Peptoid ligands that bind selectively to phosphoproteins. Bioorg Med Chem Lett 21:4960–4964
Fíla J, Honys D (2012) Enrichment techniques employed in phosphoproteomics. Amino Acids 43:1025–1047
Ficarro SB, McCleland ML, Stukenberg PT et al (2002) Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae. Nat Biotechnol 20:301–305
Pinkse MW, Uitto PM, Hilhorst MJ et al (2004) Selective isolation at the femtomole level of phosphopeptides from proteolytic digests using 2D-NanoLC-ESI-MS/MS and titanium oxide precolumns. Anal Chem 76:3935–3943
Larsen MR, Thingholm TE, Jensen ON et al (2005) Highly selective enrichment of phosphorylated peptides from peptide mixtures using titanium dioxide microcolumns. Mol Cell Proteomics 4:873–886
Ye J, Zhang X, Young C et al (2010) Optimized IMAC–IMAC protocol for phosphopeptide recovery from complex biological samples. J Proteome Res 9:3561–3573
Najam-ul-Haq M, Jabeen F, Hussain D et al (2012) Versatile nanocomposites in phosphoproteomics: a review. Anal Chim Acta 747:7–18
Zhu Y, Stubbs LP, Ho F et al (2010) Magnetic nanocomposites: a new perspective in catalysis. ChemCatChem 2:365–374
Wu W, He Q, Jiang C (2008) Magnetic iron oxide nanoparticles: synthesis and surface functionalization strategies. Nano Res Lett 3:397–415
Lee A, Yang HJ, Lim ES et al (2008) Enrichment of phosphopeptides using bare magnetic particles. Rapid Commun Mass Spectrum 22:2561–2564
Lu AH, Salabas EL, Schüth F (2007) Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed 46:1222–1244
Veiseh O, Gunn JW, Zhang M (2010) Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv Drug Deliv Rev 62:284–304
Chen C-T, Chen Y-C (2005) Fe3O4/TiO2 core/shell nanoparticles as affinity probes for the analysis of phosphopeptides using TiO2 surface-assisted laser desorption/ionization mass spectrometry. Anal Chem 77:5912–5919
Li Y, Xu X, Qi D et al (2008) Novel Fe3O4@TiO2 core–shell microspheres for selective enrichment of phosphopeptides in phosphoproteome analysis. J Proteome Res 7:2526–2538
Li Y, Wu J, Qi D et al. (2008) Novel approach for the synthesis of Fe3O4@TiO2 core–shell microspheres and their application to the highly specific capture of phosphopeptides for MALDI-TOF MS analysis. Chem Commun 564–566
Lu Z, Duan J, He L et al (2010) Mesoporous TiO2 nanocrystal clusters for selective enrichment of phosphopeptides. Anal Chem 82:7249–7258
Lo C-Y, Chen W-Y, Chen C-T et al (2007) Rapid enrichment of phosphopeptides from tryptic digests of proteins using iron oxide nanocomposites of magnetic particles coated with zirconia as the concentrating probes. J Proteome Res 6:887–893
Li W, Deng Q, Fang G et al (2013) Facile synthesis of Fe3O4@TiO2–ZrO2 and its application in phosphopeptide enrichment. J Mater Chem B 1:1947–1961
Kweon HK, Håkansson K (2006) Selective zirconium dioxide-based enrichment of phosphorylated peptides for mass spectrometric analysis. Anal Chem 78:1743–1749
Ma W, Zhang C, Zhang Y et al (2014) Magnetic MSP@ZrO2 microspheres with yolk-shell structure: designed synthesis and application in highly selective enrichment of phosphopeptides. Langmuir 30:6602
Guo J, Yang W, Wang C (2013) Magnetic colloidal supraparticles: design, fabrication and biomedical applications. Adv Mater 25:5196–5214
Chen W-Y, Chen Y-C (2010) Functional Fe3O4@ZnO magnetic nanoparticle-assisted enrichment and enzymatic digestion of phosphoproteins from saliva. Anal Bioanal Chem 398:2049–2057
Chen C-T, Chen W-Y, Tsai P-J et al (2007) Rapid enrichment of phosphopeptides and phosphoproteins from complex samples using magnetic particles coated with alumina as the concentrating probes for MALDI MS analysis. J Proteome Res 6:316–325
Chen C-T, Chen Y-C (2010) Functional magnetic nanoparticle-based label free fluorescence detection of phosphorylated species. Chem Commun 46:5674–5676
Ficarro SB, Parikh JR, Blank NC et al (2008) Niobium (V) oxide (Nb2O5): application to phosphoproteomics. Anal Chem 80:4606–4613
Lin H-Y, Chen W-Y, Chen Y-C (2009) Iron oxide/niobium oxide core–shell magnetic nanoparticle-based phosphopeptide enrichment from biological samples for MALDI MS analysis. J Biomed Nanotechnol 5:215–223
Qi D, Lu J, Deng C et al (2009) Development of core-shell structure Fe3O4@ Ta2O5 microspheres for selective enrichment of phosphopeptides for mass spectrometry analysis. J Chromatogr A 1216:5533–5539
Li Y, Lin H, Deng C et al (2008) Highly selective and rapid enrichment of phosphorylated peptides using gallium oxide-coated magnetic microspheres for MALDI-TOF-MS and nano-LC-ESI-MS/MS/MS analysis. Proteomics 8:238–249
Qi D, Lu J, Deng C et al (2009) Magnetically responsive Fe3O4@C@SnO2 core–shell microspheres: synthesis, characterization and application in phosphoproteomics. J Phys Chem C 113:15854–15861
Wang Z-G, Cheng G, Liu Y-L et al (2013) Magnetic γ-Fe2O3@REVO4 (RE = Sm, Dy, Ho) affinity microspheres for selective capture, fast separation and easy identification of phosphopeptides. J Mater Chem B 1:1491–1500
Cheng G, Zhang J-L, Liu Y-L et al (2011) Synthesis of novel Fe3O4@SiO2@CeO2 microspheres with mesoporous shell for phosphopeptide capturing and labeling. Chem Commun 47:5732–5734
Cheng G, Liu Y-L, Zhang J-L et al (2012) Lanthanum silicate coated magnetic microspheres as a promising affinity material for phosphopeptide enrichment and identification. Anal Bioanal Chem 404:763–770
Wang Z-G, Cheng G, Liu Y-L et al (2013) Novel 3D flowerlike hierarchical γ-Fe2O3@xNH4F · yLuF3 core–shell microspheres tailor-made by a phase transformation process for the capture of phosphopeptides. J Mater Chem B 1:4845–4854
Cheng G, Liu Y-L, Wang Z-G et al (2013) Yolk–shell magnetic microspheres with mesoporous yttrium phosphate shells for selective capture and identification of phosphopeptides. J Mater Chem B 1:3661–3669
Sun Y, Wang H-F (2013) Ultrathin-yttrium phosphate-shelled polyacrylate-ferriferrous oxide magnetic microspheres for rapid and selective enrichment of phosphopeptides. J Chromatogr A 1316:62–68
Block H, Maertens B, Spriestersbach A et al (2009) Immobilized-metal affinity chromatography (IMAC): a review. Method Enzymol 463:439–473
Mirza MR, Rainer M, Messner CB et al (2013) A new type of metal chelate affinity chromatography using trivalent lanthanide ions for phosphopeptide enrichment. Analyst 138:2995–3004
Li Y, Qi D, Deng C et al (2008) Cerium ion-chelated magnetic silica microspheres for enrichment and direct determination of phosphopeptides by matrix-assisted laser desorption ionization mass spectrometry. J Proteome Res 7:1767–1777
Xu X, Deng C, Gao M et al (2006) Synthesis of magnetic microspheres with immobilized metal ions for enrichment and direct determination of phosphopeptides by matrix-assisted laser desorption ionization mass spectrometry. Adv Mater 18:3289–3293
Tan F, Zhang Y, Mi W et al (2008) Enrichment of phosphopeptides by Fe3+-immobilized magnetic nanoparticles for phosphoproteome analysis of the plasma membrane of mouse liver. J Proteome Res 7:1078–1087
Novotna L, Emmerova T, Horak D et al (2010) Iminodiacetic acid-modified magnetic poly (2-hydroxyethyl methacrylate)-based microspheres for phosphopeptide enrichment. J Chromatogr A 1217:8032–8040
Li Y-C, Lin Y-S, Tsai P-J et al (2007) Nitrilotriacetic acid-coated magnetic nanoparticles as affinity probes for enrichment of histidine-tagged proteins and phosphorylated peptides. Anal Chem 79:7519–7525
Ficarro SB, Adelmant G, Tomar MN et al (2009) Magnetic bead processor for rapid evaluation and optimization of parameters for phosphopeptide enrichment. Anal Chem 81:4566–4575
Xiong Z, Zhang L, Fang C et al (2014) Ti4+-immobilized multilayer polysaccharide coated magnetic nanoparticles for highly selective enrichment of phosphopeptides. J Mater Chem B 2:4473
Zhang L, Zhao Q, Liang Z et al (2012) Synthesis of adenosine functionalized metal immobilized magnetic nanoparticles for highly selective and sensitive enrichment of phosphopeptides. Chem Commun 48:6274–6276
Sun C, Lee JSH, Zhang M (2008) Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev 60:1252–1265
Dias AMGC, Hussain A, Marcos AS et al (2011) A biotechnological perspective on the application of iron oxide magnetic colloids modified with polysaccharides. Biotechnol Adv 29:142–155
Chen C-T, Wang L-Y, Ho Y-P (2011) Use of polyethylenimine-modified magnetic nanoparticles for highly specific enrichment of phosphopeptides for mass spectrometric analysis. Anal Bioanal Chem 399:2795–2806
Iliuk AB, Martin VA, Alicie BM et al (2010) In-depth analyses of kinase-dependent tyrosine phosphoproteomes based on metal ion-functionalized soluble nanopolymers. Mol Cell Proteomics 9:2162–2172
Tsunehiro M, Meki Y, Matsuoka K et al (2013) A Phos-tag-based magnetic-bead method for rapid and selective separation of phosphorylated biomolecules. J Chromatogr B 925:86–94
Fang G, Gao W, Deng Q et al (2012) Highly selective capture of phosphopeptides using a nano titanium dioxide–multiwalled carbon nanotube nanocomposite. Anal Biochem 423:210–217
Yan Y, Zheng Z, Deng C et al (2014) Selective enrichment of phosphopeptides by titania nanoparticles coated magnetic carbon nanotubes. Talanta 118:14–20
Hsiao H-H, Hsieh H-Y, Chou C-C et al (2007) Concerted experimental approach for sequential mapping of peptides and phosphopeptides using C18-functionalized magnetic nanoparticles. J Proteome Res 6:1313–1324
Lin H-Y, Chen W-Y, Chen Y-C (2009) Iron oxide/tantalum oxide core–shell magnetic nanoparticle-based microwave-assisted extraction for phosphopeptide enrichment from complex samples for MALDI MS analysis. Anal Bioanal Chem 394:2129–2136
Acknowledgments
The authors thank the financial support from Fundação para a Ciência e a Tecnologia, Ministério da Educação e da Ciência, Portugal, through projects no. Pest-OE/EQB/LA0004/2011, PEst-C/EQB/LA0006/2013, PTDC/EBB-BIO/102163/2008, PTDC/EBBBIO/118317/2010 and SFRH/BD/64427/2009 for I.L.B.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Batalha, Í.L., Roque, A.C.A. (2016). Phosphopeptide Enrichment Using Various Magnetic Nanocomposites: An Overview. In: von Stechow, L. (eds) Phospho-Proteomics. Methods in Molecular Biology, vol 1355. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-3049-4_13
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3049-4_13
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-3048-7
Online ISBN: 978-1-4939-3049-4
eBook Packages: Springer Protocols