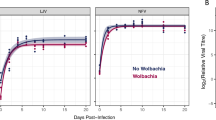
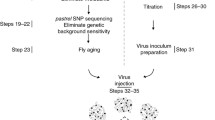
Abstract
Spread of Wolbachia infections in host populations may be enhanced by Wolbachia-conferred protection from viral pathogens. Wolbachia-infected Drosophila melanogaster survive the pathogenic effects of positive-sense single-stranded RNA virus infections at a higher rate than the flies without Wolbachia. The protection can occur with or without detectable reduction in virus titer. For the comparisons to be meaningful, Wolbachia-harboring and Wolbachia-free insects need to be genetically matched, and original populations of gut microbiota need to be restored after the removal of Wolbachia using antibiotics. Here, I describe the procedures needed to detect Wolbachia-conferred antiviral protection against Drosophila C virus measured as the difference in survival and viral titer between flies with and without Wolbachia.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Teixeira L, Ferreira A, Ashburner M (2008) The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol 6:e1000002
Hedges LM, Brownlie JC, O’Neill SL et al (2008) Wolbachia and virus protection in insects. Science 322:702
Moreira LA, Iturbe-Ormaetxe I, Jeffery JA et al (2009) A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium. Cell 139:1268–1278
Utarini A, Indriani C, Ahmad RA et al (2021) Efficacy of Wolbachia-infected mosquito deployments for the control of dengue. N Engl J Med 384:2177–2186
Anders KL, Indriani C, Ahmad RA et al (2018) The AWED trial (applying Wolbachia to eliminate dengue) to assess the efficacy of Wolbachia-infected mosquito deployments to reduce dengue incidence in Yogyakarta, Indonesia: study protocol for a cluster randomized controlled trial. Trials 19:302
Nazni WA, Hoffmann AA, NoorAfizah A et al (2019) Establishment of Wolbachia strain wAlbB in Malaysian populations of Aedes aegypti for dengue control. Curr Biol 29:4241–4248.e5
Rancès E, Ye YH, Woolfit M et al (2012) The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog 8:e1002548
Bourtzis K, Pettigrew MM, O’Neill SL (2000) Wolbachia neither induces nor suppresses transcripts encoding antimicrobial peptides. Insect Mol Biol 9:635–639
Chrostek E, Marialva MSP, Yamada R et al (2014) High anti-viral protection without immune upregulation after interspecies Wolbachia transfer. PLoS One 9:e99025
Ant TH, Herd CS, Geoghegan V et al (2018) The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti. PLoS Pathog 14:e1006815
Beckmann J, Ronau J, Hochstrasser M (2017) A Wolbachia deubiquitylating enzyme induces cytoplasmic incompatibility. Nat Microbiol 2:1–7
LePage DP, Metcalf JA, Bordenstein SR et al (2017) Prophage WO genes recapitulate and enhance Wolbachia-induced cytoplasmic incompatibility. Nature 543:243–247
Adams KL, Abernathy DG, Willett BC et al (2021) Wolbachia cifB induces cytoplasmic incompatibility in the malaria mosquito vector. Nat Microbiol 6:1575–1582
Cogni R, Ding SD, Pimentel AC et al (2021) Wolbachia reduces virus infection in a natural population of Drosophila. Commun Biol 4:1327
Chrostek E, Marialva MSP, Esteves SS et al (2013) Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: a phenotypic and phylogenomic analysis. PLoS Genet 9:e1003896
Pais IS, Valente RS, Sporniak M et al (2018) Drosophila melanogaster establishes a species-specific mutualistic interaction with stable gut-colonizing bacteria. PLoS Biol 16:e2005710
Johnson KN, Christian PD (1999) Molecular characterization of Drosophila C virus isolates. J Invertebr Pathol 73:248–254
Reed LJ, Muench H (1938) A simple method of estimating fifty percent endpoints. Am J Epidemiol 27:493–497
Chrostek E, Martins N, Marialva MS et al (2021) Wolbachia-conferred antiviral protection is determined by developmental temperature. MBio 12
Ferreira ÁG, Naylor H, Esteves SS et al (2014) The toll-dorsal pathway is required for resistance to viral oral infection in Drosophila. PLoS Pathog 10:e1004507
Chrostek E, Teixeira L (2015) Mutualism breakdown by amplification of Wolbachia genes. PLoS Biol 13:e1002065
Zhou W, Rousset F, O’Neill S (1998) Phylogeny and PCR–based classification of Wolbachia strains using wsp gene sequences. Proc R Soc B Biol Sci 265:509–515
Schneider I (1972) Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol 27:353–365
Deddouche S, Matt N, Budd A et al (2008) The DExD/H-box helicase Dicer-2 mediates the induction of antiviral activity in Drosophila. Nat Immunol 9:1425–1432
Brun G and Plus N (1978) The viruses of Drosophila, In: Ashburner M, Wright, TRF, The genetics and biology of Drosophila, pp. 625–702 Academic Press, New York
Layton EM, On J, Perlmutter JI et al (2019) Paternal grandmother age affects the strength of Wolbachia-induced cytoplasmic incompatibility in Drosophila melanogaster. MBio 10
Merkling SH and Rij RP van (2015) Analysis of resistance and tolerance to virus infection in Drosophila. Nat Protoc 10:1084–1097
Bio-Rad Laboratories I Real-Time PCR Applications Guide. https://www.bio-rad.com/webroot/web/pdf/lsr/literature/Bulletin_5279.pdf
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Ridley EV, Wong ACN, Douglas AE (2013) Microbe-dependent and nonspecific effects of procedures to eliminate the resident microbiota from Drosophila melanogaster. Appl Environ Microbiol 79:3209–3214
Ballard JWO, Melvin RG (2007) Tetracycline treatment influences mitochondrial metabolism and mtDNA density two generations after treatment in Drosophila. Insect Mol Biol 16:799–802
Webster CL, Waldron FM, Robertson S et al (2015) The discovery, distribution, and evolution of viruses associated with Drosophila melanogaster. PLoS Biol 13:e1002210
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Chrostek, E. (2024). Procedures for the Detection of Wolbachia-Conferred Antiviral Protection in Drosophila melanogaster. In: Fallon, A.M. (eds) Wolbachia. Methods in Molecular Biology, vol 2739. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3553-7_14
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3553-7_14
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3552-0
Online ISBN: 978-1-0716-3553-7
eBook Packages: Springer Protocols