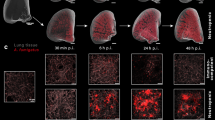
Abstract
Aspergillus fumigatus and Cryptococcus neoformans species infections are two of the most common life-threatening fungal infections in the immunocompromised population. Acute invasive pulmonary aspergillosis (IPA) and meningeal cryptococcosis are the most severe forms affecting patients with elevated associated mortality rates despite current treatments. As many unanswered questions remain concerning these fungal infections, additional research is greatly needed not only in clinical scenarios but also under controlled preclinical experimental settings to increase our understanding concerning their virulence, host–pathogen interactions, infection development, and treatments. Preclinical animal models are powerful tools to gain more insight into some of these needs. However, assessment of disease severity and fungal burden in mouse models of infection are often limited to less sensitive, single-time, invasive, and variability-prone techniques such as colony-forming unit counting. These issues can be overcome by in vivo bioluminescence imaging (BLI). BLI is a noninvasive tool that provides longitudinal dynamic visual and quantitative information on the fungal burden from the onset of infection and potential dissemination to different organs throughout the development of disease in individual animals. Hereby, we describe an entire experimental pipeline from mouse infection to BLI acquisition and quantification, readily available to researchers to provide a noninvasive, longitudinal readout of fungal burden and dissemination throughout the course of infection development, which can be applied for preclinical studies into pathophysiology and treatment of IPA and cryptococcosis in vivo.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Rodrigues ML, Nosanchuk JD (2020) Fungal diseases as neglected pathogens: a wake-up call to public health officials. PLoS Negl Trop Dis 14(2):e07964. https://doi.org/10.1371/JOURNAL.PNTD.0007964
Nature Microbiology Editorial (2017) Stop neglecting fungi. Nat Microbiol 2(8):1–2. https://doi.org/10.1038/nmicrobiol.2017.120
The Fungal Infection Trust (GAFFI, Global action fund for fungal infections) (2017) How common are fungal diseases?. http://www.gaffi.org/media/fact-. Accessed 25 Mar 2022
Latgé JP, Chamilos G (2020) Aspergillus fumigatus and aspergillosis in 2019. Clin Microbiol Rev 33(1):e00140. https://doi.org/10.1128/CMR.00140-18
Verweij PE, Gangneux J-P, Bassetti M et al (2020) Diagnosing COVID-19-associated pulmonary aspergillosis. The Lancet Microbe. https://doi.org/10.1016/s2666-5247(20)30027-6
Vanderbeke L, Spriet I, Breynaert C et al (2018) Invasive pulmonary aspergillosis complicating severe influenza: epidemiology, diagnosis and treatment. Curr Opin Infect Dis 31(6):471–480. https://doi.org/10.1097/QCO.0000000000000504
GAFFI (2020) Fungal disease frequency – Gaffi | Gaffi – Global action fund for fungal infections. Global Action Fund for Fungal Infection. https://gaffi.org/why/fungal-disease-frequency/. Published 2020. Accessed 25 Mar 2022
Resendiz-Sharpe A, Lagrou K et al (2018) Triazole resistance surveillance in Aspergillus fumigatus. Med Mycol 56(suppl_1):S83–S92. https://doi.org/10.1093/mmy/myx144
Nywening AV, Rybak JM, Rogers PD et al (2020) Mechanisms of triazole resistance in aspergillus fumigatus. Environ Microbiol 22(12):4934–4952. https://doi.org/10.1111/1462-2920.15274
Resendiz-Sharpe A, Mercier T, Lestrade PPA et al (2019) Prevalence of voriconazole-resistant invasive aspergillosis and its impact on mortality in haematology patients. J Antimicrob Chemother 74(9):2759–2766. https://doi.org/10.1093/jac/dkz258
Lestrade PP, Bentvelsen RG, Schauwvlieghe AFAD et al (2019) Voriconazole-resistant Aspergillosis Voriconazole resistance and mortality in invasive aspergillosis: a multicenter retrospective cohort study. Clin Infect Dis CID 68(9):1463–1471. https://doi.org/10.1093/cid/ciy859
Iyer KR, Revie NM, Fu C et al (2021) Treatment strategies for cryptococcal infection: challenges, advances and future outlook. Nat Rev Microbiol 19(7):454–466. https://doi.org/10.1038/S41579-021-00511-0
Lin X, Heitman J (2006) The biology of the Cryptococcus neoformans species complex. Annu Rev Microbiol 60:69–105. https://doi.org/10.1146/annurev.micro.60.080805.142102
Perfect JR, Dismukes WE, Dromer F et al (2010) Clinical practice guidelines for the management of Cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 50(3):291–322. https://doi.org/10.1086/649858
Spec A, Mejia-Chew C, Powderly WG et al (2018) EQUAL Cryptococcus score 2018: a European Confederation of Medical Mycology score derived from current guidelines to measure quality of clinical cryptococcosis management. Open Forum Infect Dis 5(11):ofy299. https://doi.org/10.1093/OFID/OFY299
Desoubeaux G, Cray C (2017) Rodent models of invasive aspergillosis due to aspergillus fumigatus: still a long path toward standardization. Front Microbiol 8:841. https://doi.org/10.3389/fmicb.2017.00841
Cundell T (2015) The limitations of the colony-forming unit in microbiology. Eur Pharm Rev 20(6):11–13
Olsen RA, Bakken LR (1987) Viability of soil bacteria: optimization of plate-counting technique and comparison between total counts and plate counts within different size groups. Microb Ecol 13(1):59–74. https://doi.org/10.1007/BF02014963
Papon N, Courdavault V, Lanoue A et al (2014) Illuminating fungal infections with bioluminescence. PLoS Pathog 10(7):1–4. https://doi.org/10.1371/journal.ppat.1004179
Vanherp L, Ristani A, Poelmans J et al (2019) Sensitive bioluminescence imaging of fungal dissemination to the brain in mouse models of cryptococcosis. DMM Dis Model Mech 12(6):dmm039123. https://doi.org/10.1242/dmm.039123
Poelmans J, Himmelreich U, Vanherp L et al (2018) A multimodal imaging approach enables in vivo assessment of antifungal treatment in a mouse model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother 62(7):e00240. https://doi.org/10.1128/AAC.00240-18
Defosse TA, Courdavault V, Coste AT et al (2018) A standardized toolkit for genetic engineering of CTG clade yeasts. J Microbiol Methods 144:152–156. https://doi.org/10.1016/J.MIMET.2017.11.015
Vande Velde G, Kucharíková S, Van Dijck P et al (2014) Bioluminescence imaging of fungal biofilm development in live animals. Meth Mol Biol 1098:153–167. https://doi.org/10.1007/978-1-62703-718-1_13
Van Dyck K, Van Dijck P, Vande VG (2020) Bioluminescence imaging to study mature biofilm formation by Candida spp. and antifungal activity in vitro and in vivo. In: Ripp S (ed) Bioluminescent imaging: methods and protocols, pp 127–143. https://doi.org/10.1007/978-1-4939-9940-8_9
Brock M (2012) Application of bioluminescence imaging for in vivo monitoring of fungal infections. Int J Microbiol 2012:956794. https://doi.org/10.1155/2012/956794
Seldeslachts L, Jacobs C, Tielemans B et al (2021) Overcome double trouble: Baloxavir Marboxil suppresses influenza thereby mitigating secondary invasive pulmonary Aspergillosis. J Fungi (Basel, Switzerland) 8(1):1. https://doi.org/10.3390/JOF8010001
Vande Velde G, Kucharíková S, Van Dijck P, Himmelreich U (2018) Bioluminescence imaging increases in vivo screening efficiency for antifungal activity against device-associated Candida albicans biofilms. Int J Antimicrob Agents 52(1):42–51. https://doi.org/10.1016/J.IJANTIMICAG.2018.03.007
Jacobsen ID, Lüttich A, Kurzai O et al (2014) In vivo imaging of disseminated murine Candida albicans infection reveals unexpected host sites of fungal persistence during antifungal therapy. J Antimicrob Chemother 69(10):2785–2796. https://doi.org/10.1093/jac/dku198
Galiger C, Brock M, Jouvion G et al (2013) Assessment of efficacy of antifungals against aspergillus fumigatus: value of real-time bioluminescence imaging. Antimicrob Agents Chemother 57(7):3046–3059. https://doi.org/10.1128/AAC.01660-12
Dorsaz S, Coste AT, Sanglard D (2017) Red-shifted firefly luciferase optimized for Candida albicans in vivo bioluminescence imaging. Front Microbiol 8:01478. https://doi.org/10.3389/fmicb.2017.01478
Binder U, Navarro-Mendoza MI, Naschberger V et al (2018) Generation of a Mucor circinelloides reporter strain—a promising new tool to study antifungal drug efficacy and Mucormycosis. Genes (Basel) 9(12):613. https://doi.org/10.3390/genes9120613
Vande Velde G, Kucharíková S, Schrevens S et al (2014) Towards non-invasive monitoring of pathogen-host interactions during Candida albicans biofilm formation using in vivo bioluminescence. Cell Microbiol 16(1):115–130. https://doi.org/10.1111/cmi.12184
Avci P, Karimi M, Sadasivam M et al (2018) In-vivo monitoring of infectious diseases in living animals using bioluminescence imaging. Virulence 9:28. https://doi.org/10.1080/21505594.2017.1371897
Vecchiarelli A, d’Enfert C (2012) Shedding natural light on fungal infections. Virulence 3(1):15–17. https://doi.org/10.4161/viru.3.1.19247
Reséndiz Sharpe A, Peres Ds Silva R, Geib E et al (2022) Longitudinal multimodal imaging-compatible mouse model of triazole sensitive and resistant invasive pulmonary aspergillosis. Dis Model Mech 15:dmm049165
Seldeslachts L, Vanderbeke L, Fremau A et al (2021) Early oseltamivir reduces risk for influenza-associated aspergillosis in a double-hit murine model. Virulence 12(1):2493–2508. https://doi.org/10.1080/21505594.2021.1974327
Marx JO, Vudathala D, Murphy L et al (2014) Antibiotic administration in the drinking water of mice. J Am Assoc Lab Anim Sci 53(3):301–306. PMID: 24827573 PMCID: PMC4128569
Acknowledgements
This work was supported by the Flemish Research Foundation (Fonds Wetenschappelijk Onderzoek, FWO, grants 1506114 N and G057721N). E.V. acknowledges support by an aspirant mandate from the FWO (ISF2222N).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Resendiz-Sharpe, A., Vanhoffelen, E., Velde, G.V. (2023). Bioluminescence Imaging, a Powerful Tool to Assess Fungal Burden in Live Mouse Models of Infection. In: Drummond, R.A. (eds) Antifungal Immunity. Methods in Molecular Biology, vol 2667. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3199-7_15
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3199-7_15
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3198-0
Online ISBN: 978-1-0716-3199-7
eBook Packages: Springer Protocols