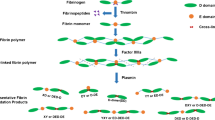
Abstract
Measuring D-dimer is commonly used as a surrogate to indicate a clot-forming process, with subsequent lysis. This test has two primary intended uses: (1) as aid to diagnosis of various conditions and (2) venous thromboembolism (VTE) exclusion. If the manufacturer cites a VTE exclusion claim, the D-dimer test must only be used in evaluating patients with a non-high or unlikely pretest probability for pulmonary embolism and deep vein thrombosis. D-dimer kits with only aid to diagnosis claim should not be used for VTE exclusion. The intended use of the D-dimer may vary by region, and readership should consult manufacturer instructions for use to assure proper use of the assay. In this chapter, several methods for measuring D-dimer will be described.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- 4PLC:
-
four-parameter logistic curve
- CI:
-
confidence interval
- CLIA:
-
chemiluminescent immunoassay
- CLSI:
-
Clinical and Laboratory Standards Institute
- CV:
-
coefficient of variation
- DDi:
-
D-dimer
- DEA:
-
diethanolamine
- DIC:
-
disseminated intravascular coagulation
- DVT:
-
deep vein thrombosis
- EDTA:
-
ethylenediaminetetraacetic acid
- ELFA:
-
enzyme-linked immunosorbent fluorescent assay
- ELISA:
-
enzyme-linked immunosorbent assay
- FbDP:
-
fibrin degradation products
- FDP:
-
fibrin(ogen) degradation products
- FEU:
-
fibrinogen equivalent unit
- HAMA:
-
human anti-mouse antibodies
- HCV:
-
hepatitis C virus
- HIV:
-
human immunodeficiency virus
- ID:
-
identification
- IFU:
-
instructions for use
- IU:
-
international units
- LIA:
-
latex immunoassay
- MLE:
-
Master Lot Entry
- NPV:
-
negative predictive value
- PC:
-
control line or control zone
- PE:
-
pulmonary embolism
- POC:
-
point of care
- PPP:
-
platelet-poor plasma
- PTP:
-
pretest probability
- RFV:
-
relative fluorescence value
- RLUs:
-
relative light units
- SPR:
-
Solid Phase Receptacle
- T:
-
test line or test zone
- TMB:
-
tetramethylbenzidine
- VTE:
-
venous thromboembolism
References
Horan JT, Francis CW (2001) Fibrin degradation products, fibrin monomer and soluble fibrin in disseminated intravascular coagulation. Semin Thromb Hemost 27:657–666
Ratky SM, Martin MJ, Gordon YB, Baker LRI, Leslie J, Chard T (1975) A comparison between radioimmunoassay and other immunological techniques for the measurement of fibrinogen/fibrin degradation products in serum. Brit J of Haematol 30:145–149
Cooke ED, Bowcock SA, Pilcher MF, Ibbotson RM, Gordon YB, Sola CM et al (1975) Serum fibrin(ogen) degradation products in diagnosis of deep-vein thrombosis and pulmonary embolism after hip surgery. Lancet 12;2(7924):51–54
Clinical and Laboratory Standards Institute (CLSI) (2011) Quantitative D-dimer for the exclusion of venous thromboembolic disease; approved guideline. CLSI document H59-A. CLSI, Wayne
Suzuki K, Wada H, Imai H, Iba T, Thachil J, Toh CH; Subcommittee on Disseminated Intravascular Coagulation (2018) A re-evaluation of the D-dimer cut-off value for making a diagnosis according to the ISTH overt-DIC diagnostic criteria: communication from the SSC of the ISTH. J Thromb Haemost 16(7):1442–1444
Johnson ED, Schell JC, Rodgers GM (2019) The D-dimer assay. Am J Hematol 94:833–839
Kearon C, Akl EA (2014) Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood 123:1794–1801
Rodger MA, Le Gal G, Anderson DR, Schmidt J, Pernod G, Kahn SR, et al; REVERSE II Study Investigators (2017) Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ 356:j1065
Kearon C, Kahn SR (2020) Long-term treatment of venous thromboembolism. Blood 135:317–325
Thachil J, Juffermans NP, Ranucci M, Connors JM, Warkentin TE, Ortel TL, Levi M, Iba T, Levy JH (2020) ISTH DIC subcommittee communication on anticoagulation in COVID-19. J Thromb Haemost 18:2138–2144
Schouten HJ, Geersing GJ, Koek HL, Zuithoff NP, Janssen KJ, Douma RA et al (2013) Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ 3(346):f2492
Righini M, Van Es J, Den Exter PL, Roy PM, Verschuren F, Ghuysen A et al (2014) Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA 311:1117–1124
Wells PS, Anderson DR, Bormanis J, Guy F, Mitchell M, Gray L et al (1999) Application of a diagnostic clinical model for the management of hospitalized patients with suspected deep-vein thrombosis. Thromb Haemost 81:493–497
Wells PS, Anderson DR, Rodger M, Ginsberg JS, Kearon C, Gent M et al (2000) Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost 83:416–420
Gosselin RC, Owings JT, Kehoe J, Anderson JT, Dwyre DM, Jacoby RC et al (2003) Comparison of six D-dimer methods in patients suspected of deep vein thrombosis. Blood Coagul Fibrinolysis 14:545–550
Longstaff C, Adcock D, Olson JD, Jennings I, Kitchen S, Mutch N et al (2016) Harmonisation of D-dimer – a call for action. Thromb Res 137:219–220
Thachil J, Longstaff C, Favaloro EJ, Lippi G, Urano T, Kim PY; SSC Subcommittee on Fibrinolysis of the International Society on Thrombosis and Haemostasis (2020) The need for accurate D-dimer reporting in COVID-19: communication from the ISTH SSC on fibrinolysis. J Thromb Haemost 18:2408–2411
Lippi G, Favaloro EJ (2020) D-dimer measurement in COVID-19: silver bullet or clinical distraction? Thromb Res 196:635–637
HemosIL™ D-Dimer HS Instructions for Use. Product 0020007700; revision 5; April 2010. Instrumentation Laboratory, Medford
HemosIL® D-Dimer 500 Instructions for Use. Product 0020301000; revision 3; February 2017. Instrumentation Laboratory, Medford
Olson JD, Cunningham MT, Higgins RA, Eby CS, Brandt JT (2013) D-dimer: simple test, tough problems. Arch Pathol Lab Med 137:1030–1038
Clinical and Laboratory Standards Institute (CLSI) (2008) Collection, transport, and processing of blood specimens for testing plasma-based coagulation assays and molecular hemostasis assays; Approved guideline – fifth edition. CLSI document H21-A5. CLSI, Wayne
Gosselin RC, Marlar RA (2019) Preanalytical variables in Coagulation testing: setting the stage for accurate results. Semin Thromb Hemost 45:433–448
Asserachrom® D-Di Instructions for use. Product 00947; July 2015. Diagnostica Stago, Asnières sur Seine, France
Vidas® D-Dimer Exclusion II™ (DEX2) Instructions for Use. Product #30 455. Revision B, Jun 2011. bioMérieux SA. Marcy-l’Etoile, France
STA® – Liastest® D-Di U.S. Instructions for Use. U.S. Specific package insert. (Product # 00515). Jan 2017. Diagnostica Stago, Asnières sur Seine, France
HemosIL® AcuStar D-Dimer Instructions for Use (Product 0009802000). Revision 7, Nov 2016. Instrumentation Laboratory, Bedford MA
Clinical and Laboratory Standards Institute (CLSI). (2006) Preparation and testing of reagent water in the clinical laboratory. Approved guideline, 4th Edition CLSI document C3-A4. CLSI, Wayne, PA
D-dimer Rapid Test Cassette Package insert. Home Health UK. Number 145638302. Effective date: 2018-05-17
HemosIL® AcuStar™ D-Dimer Controls Instructions for Use. (Product #0009802100). Revision 7, Dec 2016. Instrumentation Laboratory, Bedford MA
Favaloro EJ, Chapman K, Mohammed S, Vong R, Pasalic L (2023) Automated and rapid ADAMTS13 testing using chemiluminescence: utility for identification or exclusion of TTP and beyond. In: Favaloro EJ, Gosselin RC (eds) Hemostasis and thrombosis: methods and protocols. Methods in molecular biology. Springer, Cham, pp 487–504. 10.1007/978-1-0716-3175-1_32
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Gosselin, R.C. (2023). Protocols for D-Dimer Measurements for Aid to Diagnosis or Exclusion of Venous Thromboembolism. In: Favaloro, E.J., Gosselin, R.C. (eds) Hemostasis and Thrombosis. Methods in Molecular Biology, vol 2663. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3175-1_8
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3175-1_8
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3174-4
Online ISBN: 978-1-0716-3175-1
eBook Packages: Springer Protocols