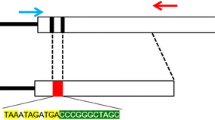
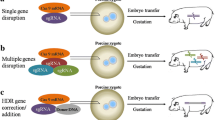
Abstract
Pigs have anatomical and physiological characteristics similar to humans; therefore, genetically modified pigs have the potential to become a valuable bioresource in biomedical research. In fact, considering the increasing need for translational research, pigs are useful for studying intractable diseases, organ transplantation, and regenerative medicine as large-scale experimental animals with excellent potential for extrapolation to humans. With the advent of zinc finger nucleases (ZFNs), breakthroughs in genome editing tools such as transcription activator-like effector nucleases (TALENs) and clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR associated protein 9 (Cas9) have facilitated the efficient generation of genetically modified pigs. Genome editing has been used in pigs for more than 10 years; now, along with knockout pigs, knock-in pigs are also gaining increasing importance. In this chapter, we describe the establishment of gene-modified cells (nuclear donor cells), which are necessary for gene knockout and production of knock-in pigs via somatic cell nuclear transplantation, as well as the production of gene knockout pigs using a simple cytoplasmic injection method.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Matsunari H, Nagashima H (2009) Application of genetically modified and cloned pigs in translational research. J Reprod Dev 55:225–230
Luo Y, Lin L, Bolund L et al (2012) Genetically modified pigs for biomedical research. J Inherit Metab Dis 35:695–713
Perleberg C, Kind A, Schnieke A (2018) Genetically engineered pigs as models for human disease. Dis Model Mech 11:dmm030783
Kim AJ, Xu N, Umeyama K, Hulin A et al (2020) Deficiency of circulating monocytes ameliorates the progression of myxomatous valve degeneration in Marfan syndrome. Circulation 141:132–146
Watanabe M, Nakano K, Matsunari H et al (2013) Generation of interleukin-2 receptor gamma gene knockout pigs from somatic cells genetically modified by zinc finger nuclease-encoding mRNA. PlosOne 8:e76478
Watanabe M, Umeyama K, Nakano K et al (2022) Generation of heterozygous PKD1 mutant pigs exhibiting early-onset renal cyst formation. Lab Invest 102:560–569
Polejaeva IA, Chen SH, Vaught TD et al (2000) Cloned pigs produced by nuclear transfer from adult somatic cells. Nature 407:86–90
Onishi A, Iwamoto M, Akita T et al (2000) Pig cloning by microinjection of fetal fibroblast nuclei. Science 289:1188–1190
Lai L, Kolber-Simonds D, Park KW et al (2002) Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science 295:1089–1092
Shiue YL, Yang JR, Liao YJ et al (2016) Derivation of porcine pluripotent stem cells for biomedical research. Theriogenology 86:176–181
Kim YG, Cha J, Chandrasegaran S (1996) Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci U S A 93:1156–1160
Boch J, Scholze H, Schornack S et al (2009) Breaking the code of DNA binding specificity of TAL-type III effectors. Science 326:1509–1512
Christian M, Cermak T, Doyle EL et al (2010) Targeting DNA double-strand breaks with TAL effector nucleases. Genetics 186:757–761
Cong L, Ran FA, Cox D et al (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823
Mali P, Yang L, Esvelt KM (2013) RNA-guided human genome engineering via Cas9. Science 339:823–826
Watanabe M, Umeyama K, Matsunari H et al (2010) Knockout of exogenous EGFP gene in porcine somatic cells using zinc-finger nucleases. Biochem Biophys Res Commun 402:14–18
Whyte JJ, Zhao J, Wells KD et al (2011) Gene targeting with zinc finger nucleases to produce cloned eGFP knockout pigs. Mol Reprod Dev 78:2
Hauschild J, Petersen B, Santiago Y et al (2011) Efficient generation of a biallelic knockout in pigs using zinc-finger nucleases. Proc Natl Acad Sci U S A 108:12013–12017
Carlson DF, Tan W, Lillico SG et al (2012) Efficient TALEN-mediated gene knockout in livestock. Proc Natl Acad Sci U S A 109:17382–17387
Hai T, Teng F, Guo R et al (2014) One-step generation of knockout pigs by zygote injection of CRISPR/Cas system. Cell Res 24:372–375
Umeyama K, Watanabe K, Watanabe M et al (2016) Generation of heterozygous fibrillin-1 mutant cloned pigs from genome-edited foetal fibroblasts. Sci Rep 6:24413
Miyagawa S, Matsunari H, Watanabe M et al (2015) Generation of α1,3-galactosyltransferase and cytidine monophospho-N-acetylneuraminic acid hydroxylase gene double-knockout pigs. J Reprod Dev 61:449–457
Nagashima H, Matsunari H (2016) Growing human organs in pigs-A dream or reality? Theriogenology 86:422–426
Watanabe M, Nakano K, Uchikura A et al (2019) Anephrogenic phenotype induced by SALL1 gene knockout in pigs. Sci Rep 9:8016
Matsunari H, Watanabe M, Hasegawa K et al (2020) Compensation of disabled organogenesis in genetically modified pig fetuses by blastocyst complementation. Stem Cell Rep 14:21–23
Rao S, Fujimura T, Matsunari H et al (2016) Efficient modification of the myostatin gene in porcine somatic cells and generation of knockout piglets. Mol Reprod Dev 83:61–70
Whitworth KM, Rowland RR, Ewen CL et al (2016) Gene-edited pigs are protected from porcine reproductive and respiratory syndrome virus. Nat Biotechnol 34:20–22
Xie Z, Pang D, Yuan H et al (2018) Genetically modified pigs are protected from classical swine fever virus. PLoS Pathog 14:e1007193
Tanihara F, Takemoto T, Kitagawa E et al (2016) Somatic cell reprogramming-free generation of genetically modified pigs. Sci Adv 2:e1600803
Sato M, Koriyama M, Watanabe S et al (2015) Direct injection of CRISPR/Cas9-related mRNA into cytoplasm of parthenogenetically activated porcine oocytes causes frequent mosaicism for indel mutations. Int J Mol Sci 16:17838–17856
Peng J, Wang Y, Jiang J et al (2015) Production of human albumin in pigs through CRISPR/Cas9-mediated knockin of human cDNA into swine albumin locus in the zygotes. Sci Rep 5:16705
Mehravar M, Shirazi A, Nazari M et al (2019) Mosaicism in CRISPR/Cas9-mediated genome editing. Dev Biol 445:152–162
Nottle MB, Salvaris EJ, Fisicaro N et al (2017) Targeted insertion of an anti-CD2 monoclonal antibody transgene into the GGTA1 locus in pigs using FokI-dCas9. Sci Rep 7:8383
Yan S, Tu Z, Liu Z et al (2018) A Huntingtin knockin pig model recapitulates features of selective neurodegeneration in Huntington’s disease. Cell 173:989–1002.e13
Zou X, Ouyang H, Yu T et al (2019) Preparation of a new type 2 diabetic miniature pig model via the CRISPR/Cas9 system. Cell Death Dis 10:823
Tanihara F, Hirata M, Otoi T (2021) Current status of the application of gene editing in pigs. J Reprod Dev 67:177–187
Kurome M, Kessler B, Wuensch A et al (2015) Nuclear transfer and transgenesis in the pig. Methods Mol Biol 1222:37–5925
Sato M, Ohtsuka M, Miura H et al (2012) Determination of the optimal concentration of several selective drugs useful for generating multi-transgenic porcine embryonic fibroblasts. Reprod Domest Anim 47:759–765
Doyon Y, Choi VM, Xia DF et al (2010) Transient cold shock enhances zinc-finger nuclease-mediated gene disruption. Nat Methods 7:459–460
Matsunari H, Watanabe M, Umeyama K et al (2012) Cloning of homozygous α1,3-galactosyltransferase gene knock-out pigs by somatic cell nuclear transfer. In: Miyagawa S (ed) Xenotransplantation. InTech, Croatia
Kikuchi K, Nagai T, Kashiwazaki N et al (1998) Cryopreservation and ensuing in vitro fertilization ability of boar spermatozoa from epididymides stored at 4 degrees C. Theriogenology 50:615–623
Singh P, Schimenti JC, Bolcun-Filas E (2015) A mouse geneticist's practical guide to CRISPR applications. Genetics 199:1–15
Acknowledgment
This work was supported by a Grant-in-Aid for Scientific Research (A) from the Japan Society for the Promotion of Science (No. 15H02480 to Hiroshi Nagashima), the Meiji University International Institute for Bio-Resource Research (MUIIBR to Hiroshi Nagashima), the Nakauchi Stem Cell and Organ Regeneration Project, the Exploratory Research for Advanced Technology (ERATO), the Japan Science and Technology Agency (JST), the Leading Advanced Projects for Medical Innovation (LEAP), and the Advanced Research and Development Programs for Medical Innovation (AMED).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Watanabe, M., Nagashima, H. (2023). Genome Editing of Pig. In: Hatada, I. (eds) Genome Editing in Animals. Methods in Molecular Biology, vol 2637. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3016-7_21
Download citation
DOI: https://doi.org/10.1007/978-1-0716-3016-7_21
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-3015-0
Online ISBN: 978-1-0716-3016-7
eBook Packages: Springer Protocols