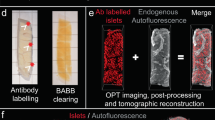
Abstract
The rodent pancreas is the prevalent model system for preclinical diabetes research. However, due to the compound endocrine–exocrine organization of the gland, with the endocrine islets of Langerhans scattered by the thousands throughout the much greater exocrine parenchyma, stereological assessments of endocrine cell mass, commonly insulin-producing ß-cells, are exceedingly challenging. In recent years, optical mesoscopic imaging techniques such as optical projection tomography (OPT) and light sheet fluorescence microscopy (LSFM) have seen dramatic developments, enabling 3D visualization of fluorescently labeled cells in mm- to cm-sized tissues with μm resolution. Here we present a protocol for 3D visualization and “absolute” quantitative assessments of, for example, islet mass throughout the volume of rodent pancreata with maintained spatial context.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Sharpe J, Ahlgren U, Perry P, Hill B, Ross A, Hecksher-Sorensen J et al (2002) Optical projection tomography as a tool for 3D microscopy and gene expression studies. Science 296(5567):541–545. https://doi.org/10.1126/science.1068206
Dodt HU, Leischner U, Schierloh A, Jahrling N, Mauch CP, Deininger K et al (2007) Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain. Nat Methods 4(4):331–336. https://doi.org/10.1038/nmeth1036
Huisken J, Swoger J, Del Bene F, Wittbrodt J, Stelzer EH (2004) Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science 305(5686):1007–1009. https://doi.org/10.1126/science.1100035
Keller PJ, Schmidt AD, Wittbrodt J, Stelzer EH (2008) Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science 322(5904):1065–1069. https://doi.org/10.1126/science.1162493
Alanentalo T, Hahn M, Willekens SMA, Ahlgren U (2021) Mesoscopic optical imaging of the pancreas-revisiting pancreatic anatomy and pathophysiology. Front Endocrinol (Lausanne) 12:633063. https://doi.org/10.3389/fendo.2021.633063
Alanentalo T, Asayesh A, Morrison H, Loren CE, Holmberg D, Sharpe J et al (2007) Tomographic molecular imaging and 3D quantification within adult mouse organs. Nat Methods 4(1):31–33. https://doi.org/10.1038/nmeth985
Serra-Navarro B, Fernandez-Ruiz R, Garcia-Alaman A, Pradas-Juni M, Fernandez-Rebollo E, Esteban Y et al (2021) Gsalpha-dependent signaling is required for postnatal establishment of a functional beta-cell mass. Mol Metab 53:101264. https://doi.org/10.1016/j.molmet.2021.101264
Hahn M, van Krieken PP, Nord C, Alanentalo T, Morini F, Xiong Y et al (2020) Topologically selective islet vulnerability and self-sustained downregulation of markers for beta-cell maturity in streptozotocin-induced diabetes. Commun Biol 3(1):541. https://doi.org/10.1038/s42003-020-01243-2
Grong E, Nord C, Arbo IB, Eriksson M, Kulseng BE, Ahlgren U et al (2017) The effect of hypergastrinemia following sleeve gastrectomy and pantoprazole on type 2 diabetes mellitus and beta-cell mass in Goto-Kakizaki rats. J Endocrinol Investig. https://doi.org/10.1007/s40618-017-0793-9
Parween S, Kostromina E, Nordd C, Eriksson M, Linddström P, Ahlgren U (2016) Intra-islet lesions and lobular variations in β-cell mass expansion in ob/ob mice revealed by 3D imaging of intact pancreas. Sci Rep 6. https://doi.org/10.1038/srep34885
Grong E, Kulseng B, Arbo IB, Nord C, Eriksson M, Ahlgren U et al (2016) Sleeve gastrectomy, but not duodenojejunostomy, preserves total beta-cell mass in Goto-Kakizaki rats evaluated by three-dimensional optical projection tomography. Surg Endosc 30(2):532–542. https://doi.org/10.1007/s00464-015-4236-4
Van de Casteele M, Leuckx G, Baeyens L, Cai Y, Yuchi Y, Coppens V et al (2013) Neurogenin 3+ cells contribute to beta-cell neogenesis and proliferation in injured adult mouse pancreas. Cell Death Dis 4:e523. https://doi.org/10.1038/cddis.2013.52
Alanentalo T, Hornblad A, Mayans S, Karin Nilsson A, Sharpe J, Larefalk A et al (2010) Quantification and three-dimensional imaging of the insulitis-induced destruction of beta-cells in murine type 1 diabetes. Diabetes 59(7):1756–1764. https://doi.org/10.2337/db09-1400
Hörnblad A, Nord CSP, Ahnfeldt-Rønne J, Ahlgren U (2016) The pancreas. In: Baldock R, Bard J, Davidson DR, Moriss-Kay G (eds) Kaufman’s Atlas of mouse development supplement: with coronal images. Academic/Elsevier, London, pp 85–94
Hornblad A, Eriksson AU, Sock E, Hill RE, Ahlgren U (2011) Impaired spleen formation perturbs morphogenesis of the gastric lobe of the pancreas. PLoS One 6(6):e21753. https://doi.org/10.1371/journal.pone.0021753
Asayesh A, Sharpe J, Watson RP, Hecksher-Sorensen J, Hastie ND, Hill RE et al (2006) Spleen versus pancreas: strict control of organ interrelationship revealed by analyses of Bapx1-/- mice. Genes Dev 20(16):2208–2213. https://doi.org/10.1101/gad.381906
Hornblad A, Cheddad A, Ahlgren U (2011) An improved protocol for optical projection tomography imaging reveals lobular heterogeneities in pancreatic islet and beta-cell mass distribution. Islets 3(4):204–208. https://doi.org/10.4161/isl.3.4.16417
Vallejo Ramirez PP, Zammit J, Vanderpoorten O, Riche F, Ble FX, Zhou XH et al (2019) OptiJ: open-source optical projection tomography of large organ samples. Sci Rep 9(1):15693. https://doi.org/10.1038/s41598-019-52065-0
Voigt FF, Kirschenbaum D, Platonova E, Pages S, Campbell RAA, Kastli R et al (2019) The mesoSPIM initiative: open-source light-sheet microscopes for imaging cleared tissue. Nat Methods 16(11):1105–1108. https://doi.org/10.1038/s41592-019-0554-0
Wong MD, Dazai J, Walls JR, Gale NW, Henkelman RM (2013) Design and implementation of a custom built optical projection tomography system. PLoS One 8(9):e73491. https://doi.org/10.1371/journal.pone.0073491
Eriksson AU, Svensson C, Hornblad A, Cheddad A, Kostromina E, Eriksson M et al (2013) Near infrared optical projection tomography for assessments of beta-cell mass distribution in diabetes research. J Vis Exp 71:e50238. https://doi.org/10.3791/50238
Cheddad A, Svensson C, Sharpe J, Georgsson F, Ahlgren U (2012) Image processing assisted algorithms for optical projection tomography. IEEE Trans Med Imaging 31(1):1–15. https://doi.org/10.1109/TMI.2011.2161590
Koley D, Bard AJ (2010) Triton X-100 concentration effects on membrane permeability of a single HeLa cell by scanning electrochemical microscopy (SECM). Proc Natl Acad Sci U S A 107(39):16783–16787. https://doi.org/10.1073/pnas.1011614107
Campbell-Thompson M, Tang SC (2021) Pancreas optical clearing and 3-D microscopy in health and diabetes. Front Endocrinol (Lausanne) 12:644826. https://doi.org/10.3389/fendo.2021.644826
Acknowledgement
We are grateful to previous and current lab members and collaborators for their invaluable contributions to the presented protocol which was developed with support of the Swedish Research Council, the European Commission, the Kempe Foundations, the Novo Nordisk Foundation, the Swedish Diabetes Foundation, the Juvenile Diabetes Research Foundation (Barndiabetesfonden), Diabetes Wellness (Sweden) and Umeå University.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Hahn, M., Ahlgren, U. (2023). 3D Optical Molecular Imaging of the Rodent Pancreas by OPT and LSFM. In: Moore, A., Wang, P. (eds) Type-1 Diabetes. Methods in Molecular Biology, vol 2592. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2807-2_1
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2807-2_1
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2806-5
Online ISBN: 978-1-0716-2807-2
eBook Packages: Springer Protocols