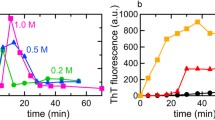
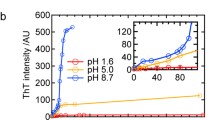
Abstract
Inducing protein aggregation in vitro under various formulation and stress conditions may lead to an increased understanding of the different association routes a protein can undergo. However, a range of factors can affect the aggregation process, often leading to heterogenous samples and experimental irreproducibility between labs. Here, we present detailed methods to reproducibly form homogenous samples of superstructures: amyloid-like fibrils, spherulites, and particulates from human insulin. We discuss pitfalls and good practice in the lab, with the aim of creating awareness on the potential sources of artefacts for protein stability and aggregation studies.
Authors Minna Groenning and Vito Foderà share the last authorship of the manuscript.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Fodera V, Zaccone A, Lattuada M et al (2013) Electrostatics controls the formation of amyloid superstructures in protein aggregation. Phys Rev Lett 111(10):1–5. https://doi.org/10.1103/PhysRevLett.111.108105
Wälchli R, Vermeire P-J, Massant J et al (2019) Accelerated aggregation studies of monoclonal antibodies: considerations for storage stability. J Pharm Sci 109(1):595–602. https://doi.org/10.1016/j.xphs.2019.10.048
Rosenberg AS, Worobec AS (2004) A risk-based approach to immunogenicity concerns of therapeutic protein products, part 2: considering host-specific and product-specific factors impacting immunogenicity. BioPharm Int 17(12):34–42
Hermeling S, Schellekens H, Maas C et al (2006) Antibody response to aggregated human interferon alpha2b in wild-type and transgenic immune tolerant mice depends on type and level of aggregation. J Pharm Sci 95(5):1084–1096. https://doi.org/10.1002/jps.20599
Vetri V, Fodera V (2015) The route to protein aggregate superstructures: particulates and amyloid-like spherulites. FEBS Lett 589(19A):2448–2463. https://doi.org/10.1016/j.febslet.2015.07.006
Vestergaard B, Groenning M, Roessle M et al (2007) A helical structural nucleus is the primary elongating unit of insulin amyloid fibrils. PLoS Biol 5(5):1089–1097. https://doi.org/10.1371/journal.pbio.0050134
Jansen R, Dzwolak W, Winter R (2005) Amyloidogenic self-assembly of insulin aggregates probed by high resolution atomic force microscopy. Biophys J 88(2):1344–1353. https://doi.org/10.1529/biophysj.104.048843
Ahmad A, Uversky VN, Hong D et al (2005) Early events in the fibrillation of monomeric insulin. J Biol Chem 280(52):42669–42675. https://doi.org/10.1074/jbc.M504298200
Fodera V, Donald AM (2010) Tracking the heterogeneous distribution of amyloid spherulites and their population balance with free fibrils. Eur Phys J E 33(4):273–282. https://doi.org/10.1140/epje/i2010-10665-4
De Luca G, Galparsoro DF, Sancataldo G et al (2020) Probing ensemble polymorphism and single aggregate structural heterogeneity in insulin amyloid self-assembly. J Coll Int Sci 574(1):229–240. https://doi.org/10.1016/j.jcis.2020.03.107
Krebs MRH, Macphee CE, Miller AF et al (2004) The formation of spherulites by amyloid fibrils of bovine insulin. Proc Natl Acad Sci USA 101(40):14420–14424. https://doi.org/10.1073/pnas.0405933101
Fodera V, Vetri V, Wind TS et al (2014) Observation of the early structural changes leading to the formation of protein superstructures. J Phys Chem Lett 5(18):3254–3258. https://doi.org/10.1021/jz501614e
Krebs MRH, Devlin GL, Donald AM (2007) Protein particulates: another generic form of protein aggregation? Biophys J 92(4):1336–1342. https://doi.org/10.1529/biophysj.106.094342
Vetri V, D’amico M, Fodera V et al (2011) Bovine serum albumin protofibril-like aggregates formation: solo but not simple mechanism. Arch Biochem Biophys 508(1):13–24. https://doi.org/10.1016/j.abb.2011.01.024
Pedersen MN, Fodera V, Horvath I et al (2015) Direct correlation between ligand-induced alpha-synuclein oligomers and amyloid-like fibril growth. Sci Rep 5(10422):1–11. https://doi.org/10.1038/srep10422
Heijna MCR, Theelen MJ, Van Enckevort WJP et al (2007) Spherulitic growth of hen egg-white lysozyme crystals. J Phys Chem B 111(7):1567–1573. https://doi.org/10.1021/jp0643294
Jiang Y, Shi K, Xia D et al (2011) Protein spherulites for sustained release of interferon: preparation, characterization and in vivo evaluation. J Pharm Sci 100(5):1913–1922. https://doi.org/10.1002/jps.22403
Lambrecht MA, Jansens KJA, Rombouts I et al (2019) Conditions governing food protein amyloid fibril formation. Part II: milk and legume proteins. Com Rev Food Sci Food Safe 18(1):1277–1291. https://doi.org/10.1111/1541-4337.12465
Scheidt T, Łapińska U, Kumita JR et al (2019) Secondary nucleation and elongation occur at different sites on Alzheimer’s amyloid-β aggregates. Sci Adv 5(4):1–9. https://doi.org/10.1126/sciadv.aau3112
Krebs MRH, Domike KR, Donald AM (2009) Protein aggregation: more than just fibrils. Biochem Soc Trans 37(4):682–686. https://doi.org/10.1042/BST0370682
Acknowledgments
The work was funded, and all protein material was provided by Novo Nordisk A/S. For use of the TEM, we acknowledge the Core Facility for Integrated Microscopy, Faculty of Health and Medical Sciences, University of Copenhagen. For use of the MFI image evaluation software, we acknowledge Jesper Søndergaard Marino from Novo Nordisk A/S. Illustrations presented in the chapter were created with Biorender.com. V.F. also acknowledges the VILLUM FONDEN for the Villum Young Investigator Grant “Protein Superstructures as Smart Biomaterials (ProSmart)” 2018–2023 (project number: 19175). The authors acknowledge Marco van de Weert (University of Copenhagen) for inspiring discussions on reproducibility in protein stability and aggregation studies.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Thorlaksen, C., Neergaard, M.B., Groenning, M., Foderà, V. (2023). Reproducible Formation of Insulin Superstructures: Amyloid-Like Fibrils, Spherulites, and Particulates. In: Cieplak, A.S. (eds) Protein Aggregation. Methods in Molecular Biology, vol 2551. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2597-2_20
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2597-2_20
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2596-5
Online ISBN: 978-1-0716-2597-2
eBook Packages: Springer Protocols