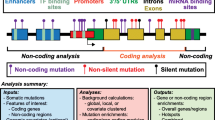
Abstract
Accurate detection of somatic mutations in genetically heterogeneous tumor cell populations using next-generation sequencing remains challenging. We have developed MuSE, Mutation calling using a Markov Substitution model for Evolution, a novel approach for modeling the evolution of the allelic composition of tumor and normal tissue at each reference base. It adopts a sample-specific error model to depict inter-tumor heterogeneity, which greatly improves the overall accuracy. Here, we describe the method and provide a tutorial on the installation and application of MuSE.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, Miller CA, Mardis ER, Ding L, Wilson RK (2012) VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res 22(3):568–576. https://doi.org/10.1101/gr.129684.111
Reumers J, De Rijk P, Zhao H, Liekens A, Smeets D, Cleary J, Van Loo P, Van Den Bossche M, Catthoor K, Sabbe B, Despierre E, Vergote I, Hilbush B, Lambrechts D, Del-Favero J (2011) Optimized filtering reduces the error rate in detecting genomic variants by short-read sequencing. Nat Biotechnol 30(1):61–68. https://doi.org/10.1038/nbt.2053
Roth A, Ding J, Morin R, Crisan A, Ha G, Giuliany R, Bashashati A, Hirst M, Turashvili G, Oloumi A, Marra MA, Aparicio S, Shah SP (2012) JointSNVMix: a probabilistic model for accurate detection of somatic mutations in normal/tumour paired next-generation sequencing data. Bioinformatics 28(7):907–913. https://doi.org/10.1093/bioinformatics/bts053
Saunders CT, Wong WS, Swamy S, Becq J, Murray LJ, Cheetham RK (2012) Strelka: accurate somatic small-variant calling from sequenced tumor-normal sample pairs. Bioinformatics 28(14):1811–1817. https://doi.org/10.1093/bioinformatics/bts271
Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, Gabriel S, Meyerson M, Lander ES, Getz G (2013) Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol 31(3):213–219. https://doi.org/10.1038/nbt.2514
Ewing AD, Houlahan KE, Hu Y, Ellrott K, Caloian C, Yamaguchi TN, Bare JC, P’ng C, Waggott D, Sabelnykova VY, participants I-TDSMCC, Kellen MR, Norman TC, Haussler D, Friend SH, Stolovitzky G, Margolin AA, Stuart JM, Boutros PC (2015) Combining tumor genome simulation with crowdsourcing to benchmark somatic single-nucleotide-variant detection. Nat Methods 12(7):623–630. https://doi.org/10.1038/nmeth.3407
Meyerson M, Gabriel S, Getz G (2010) Advances in understanding cancer genomes through second-generation sequencing. Nat Rev Genet 11(10):685–696. https://doi.org/10.1038/nrg2841
Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, Raine K, Latimer C, Santos CR, Nohadani M, Eklund AC, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C (2012) Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366(10):883–892. https://doi.org/10.1056/NEJMoa1113205
Fan Y, Xi L, Hughes DST, Zhang JJ, Zhang JH, Futreal PA, Wheeler DA, Wang WY (2016) MuSE: accounting for tumor heterogeneity using a sample-specific error model improves sensitivity and specificity in mutation calling from sequencing data. Genome Biol 17:178. https://doi.org/10.1186/s13059-016-1029-6
ICGC/TCGA Pan-cancer Analysis of Whole Genomes Consortium (2020) Pan-cancer analysis of whole genomes. Nature 578(7793):82–93. https://doi.org/10.1038/s41586-020-1969-6
Ellrott K, Bailey MH, Saksena G, Covington KR, Kandoth C, Stewart C, Hess J, Ma S, Chiotti KE, McLellan M, Sofia HJ, Hutter C, Getz G, Wheeler D, Ding L, Grp MW, Network CGAR (2018) Scalable Open Science approach for mutation calling of tumor exomes using multiple genomic pipelines. Cell Syst 6(3):271–281. https://doi.org/10.1016/j.cels.2018.03.002
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Ji, S., Montierth, M.D., Wang, W. (2022). MuSE: A Novel Approach to Mutation Calling with Sample-Specific Error Modeling. In: Ng, C., Piscuoglio, S. (eds) Variant Calling. Methods in Molecular Biology, vol 2493. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2293-3_2
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2293-3_2
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2292-6
Online ISBN: 978-1-0716-2293-3
eBook Packages: Springer Protocols