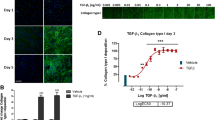
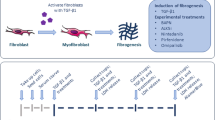
Abstract
Excessive deposition of type I collagen follows in the wake of chronic inflammation processes in dysregulated tissue healing and causes fibrosis that can ultimately lead to organ failure. While the development of antifibrotic drugs is targeting various upstream events in collagen matrix formation (synthesis, secretion, deposition, stabilization, remodeling), the evaluation of drug effects would use as net read-out of the above effects the presence of a deposited collagen matrix by activated cells, mainly myofibroblasts. Conventional methods comprise lengthy and labor-intensive protocols for the quantification of deposited collagen, some with sensitivity and/or specificity issues. Here we describe the Scar-in-a-Jar assay, an in vitro fibrosis model for anti-fibrotic drug testing that benefits from a substantially accelerated extracellular matrix deposition employing macromolecular crowding and a collagen-producing cell type of choice (e.g., lung fibroblasts like WI-38). The system can be aided by activating compounds such as transforming growth factor-β1, a classical inducer of the myofibroblast phenotype in fibroblasts. Direct image analysis of the well plate not only eliminates the need for matrix extraction or solubilization methods, but also allows for direct imaging and monitoring of phenotypical markers and offers the option for high-content screening applications when adapted to well formats compatible with a screening format.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Sundarakrishnan A, Chen Y, Black LD, Aldridge BB, Kaplan DL (2018) Engineered cell and tissue models of pulmonary fibrosis. Adv Drug Deliv Rev 129:78–94. https://doi.org/10.1016/j.addr.2017.12.013
Neuman RE, Logan MA (1950) The Determination of Hydroxyproline. J Biol Chem 184:299–306
Woessner JF (1961) The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 93:440–447. https://doi.org/10.1016/0003-9861(61)90291-0
Hofman K, Hall B, Cleaver H, Marshall S (2011) High-throughput quantification of hydroxyproline for determination of collagen. Anal Biochem 417:289–291. https://doi.org/10.1016/j.ab.2011.06.019
da Silva CML, Spinelli E, Rodrigues SV (2015) Fast and sensitive collagen quantification by alkaline hydrolysis/hydroxyproline assay. Food Chem 173:619–623. https://doi.org/10.1016/j.foodchem.2014.10.073
Kindt E, Gueneva-Boucheva K, Rekhter MD, Humphries J, Hallak H (2003) Determination of hydroxyproline in plasma and tissue using electrospray mass spectrometry. J Pharm Biomed Anal 33:1081–1092. https://doi.org/10.1016/S0731-7085(03)00359-5
Junqueira LCU, Bignolas G, Brentani RR (1979) Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 11:447–455. https://doi.org/10.1007/BF01002772
Lareu RR, Zeugolis DI, Abu-Rub M, Pandit A, Raghunath M (2010) Essential modification of the Sircol Collagen Assay for the accurate quantification of collagen content in complex protein solutions. Acta Biomater 6:3146–3151. https://doi.org/10.1016/j.actbio.2010.02.004
Huizen NA, Ijzermans JNM, Burgers PC, Luider TM (2020) Collagen analysis with mass spectrometry. Mass Spectrom Rev 39(4):309–335. https://doi.org/10.1002/mas.21600
Seo W-Y, Kim J-H, Baek D-S, Kim S-J, Kang S, Yang WS, Song J-A, Lee M-S, Kim S, Kim Y-S (2017) Production of recombinant human procollagen type I C-terminal propeptide and establishment of a sandwich ELISA for quantification. Sci Rep 7:15946. https://doi.org/10.1038/s41598-017-16290-9
Lareu RR, Subramhanya KH, Peng Y, Benny P, Chen C, Wang Z, Rajagopalan R, Raghunath M (2007) Collagen matrix deposition is dramatically enhanced in vitro when crowded with charged macromolecules: the biological relevance of the excluded volume effect. FEBS Lett 581:2709–2714. https://doi.org/10.1016/j.febslet.2007.05.020
Clemons TD, Bradshaw M, Toshniwal P, Chaudhari N, Stevenson AW, Lynch J, Fear MW, Wood FM, Iyer KS (2018) Coherency image analysis to quantify collagen architecture: implications in scar assessment. RSC Adv 8:9661–9669. https://doi.org/10.1039/C7RA12693J
Bernstein DM, Toth B, Rogers RA, Kling DE, Kunzendorf P, Phillips JI, Ernst H (2020) Evaluation of the dose-response and fate in the lung and pleura of chrysotile-containing brake dust compared to TiO2, chrysotile, crocidolite or amosite asbestos in a 90-day quantitative inhalation toxicology study—Interim results Part 2: histopathological examination, Confocal microscopy and collagen quantification of the lung and pleural cavity. Toxicol Appl Pharmacol 387:114847. https://doi.org/10.1016/j.taap.2019.114847
Khorasani H, Zheng Z, Nguyen C, Zara J, Zhang X, Wang J, Ting K, Soo C (2011) A quantitative approach to scar analysis. Am J Pathol 178:621–628. https://doi.org/10.1016/j.ajpath.2010.10.019
Stoller P, Celliers PM, Reiser KM, Rubenchik AM (2003) Quantitative second-harmonic generation microscopy in collagen. Appl Opt 42:5209. https://doi.org/10.1364/AO.42.005209
Mostaço-Guidolin L, Rosin NL, Hackett T-L (2017) Imaging collagen in scar tissue: developments in second harmonic generation microscopy for biomedical applications. Int J Mol Sci 18:1772. https://doi.org/10.3390/ijms18081772
Clark RAF, McCoy GA, Folkvord JM, McPherson JM (1997) TGF-β1 stimulates cultured human fibroblasts to proliferate and produce tissue-like fibroplasia: a fibronectin matrix-dependent event. J Cell Physiol 170:69–80. https://doi.org/10.1002/(SICI)1097-4652(199701)170:1<69::AID-JCP8>3.0.CO;2-J
Pohlers D, Brenmoehl J, Löffler I, Müller CK, Leipner C, Schultze-Mosgau S, Stallmach A, Kinne RW, Wolf G (2009) TGF-β and fibrosis in different organs—molecular pathway imprints. Biochim Biophys Acta BBA Mol Basis Dis 1792:746–756. https://doi.org/10.1016/j.bbadis.2009.06.004
Xu Q, Norman JT, Shrivastav S, Lucio-Cazana J, Kopp JB (2007) In vitro models of TGF-β-induced fibrosis suitable for high-throughput screening of antifibrotic agents. Am J Physiol-Ren Physiol 293:F631–F640. https://doi.org/10.1152/ajprenal.00379.2006
Strikoudis A, Cieślak A, Loffredo L, Chen Y-W, Patel N, Saqi A, Lederer DJ, Snoeck H-W (2019) Modeling of fibrotic lung disease using 3D organoids derived from human pluripotent stem cells. Cell Rep 27:3709–3723. e5. https://doi.org/10.1016/j.celrep.2019.05.077
Wilkinson DC, Alva-Ornelas JA, Sucre JMS, Vijayaraj P, Durra A, Richardson W, Jonas SJ, Paul MK, Karumbayaram S, Dunn B, Gomperts BN (2017) Development of a three-dimensional bioengineering technology to generate lung tissue for personalized disease modeling. Stem Cells Transl Med 6:622–633. https://doi.org/10.5966/sctm.2016-0192
Mastikhina O, Moon B-U, Williams K, Hatkar R, Gustafson D, Mourad O, Sun X, Koo M, Lam AYL, Sun Y, Fish JE, Young EWK, Nunes SS (2020) Human cardiac fibrosis-on-a-chip model recapitulates disease hallmarks and can serve as a platform for drug testing. Biomaterials 233:119741. https://doi.org/10.1016/j.biomaterials.2019.119741
Asmani M, Velumani S, Li Y, Wawrzyniak N, Hsia I, Chen Z, Hinz B, Zhao R (2018) Fibrotic microtissue array to predict anti-fibrosis drug efficacy. Nat Commun 9:2066. https://doi.org/10.1038/s41467-018-04336-z
Neuhaus V, Danov O, Konzok S, Obernolte H, Dehmel S, Braubach P, Jonigk D, Fieguth H-G, Zardo P, Warnecke G, Martin C, Braun A, Sewald K (2018) Assessment of the cytotoxic and immunomodulatory effects of substances in human precision-cut lung slices. J Vis Exp 135:57042. https://doi.org/10.3791/57042
Cedilak M, Banjanac M, Belamarić D, Paravić Radičević A, Faraho I, Ilić K, Čužić S, Glojnarić I, Eraković Haber V, Bosnar M (2019) Precision-cut lung slices from bleomycin treated animals as a model for testing potential therapies for idiopathic pulmonary fibrosis. Pulm Pharmacol Ther 55:75–83. https://doi.org/10.1016/j.pupt.2019.02.005
Huang X, Li L, Ammar R, Zhang Y, Wang Y, Ravi K, Thompson J, Jarai G (2018) Molecular characterization of a precision-cut rat lung slice model for the evaluation of antifibrotic drugs. Am J Physiol Lung Cell Mol Physiol 316:L348–L357. https://doi.org/10.1152/ajplung.00339.2018
Lehmann M, Buhl L, Alsafadi HN, Klee S, Hermann S, Mutze K, Ota C, Lindner M, Behr J, Hilgendorff A, Wagner DE, Königshoff M (2018) Differential effects of Nintedanib and Pirfenidone on lung alveolar epithelial cell function in ex vivo murine and human lung tissue cultures of pulmonary fibrosis. Respir Res 19:175. https://doi.org/10.1186/s12931-018-0876-y
Chen C, Peng Y, Wang Z, Fish P, Kaar J, Koepsel R, Russell A, Lareu R, Raghunath M (2009) The scar-in-a-Jar: studying potential antifibrotic compounds from the epigenetic to extracellular level in a single well: scar-in-a-Jar to assess potential antifibrotics. Br J Pharmacol 158:1196–1209. https://doi.org/10.1111/j.1476-5381.2009.00387.x
Chen C, Loe F, Blocki A, Peng Y, Raghunath M (2011) Applying macromolecular crowding to enhance extracellular matrix deposition and its remodeling in vitro for tissue engineering and cell-based therapies. Adv Drug Deliv Rev 63:277–290. https://doi.org/10.1016/j.addr.2011.03.003
Benny P, Raghunath M (2017) Making microenvironments: a look into incorporating macromolecular crowding into in vitro experiments, to generate biomimetic microenvironments which are capable of directing cell function for tissue engineering applications. J Tissue Eng 8:2041731417730467. https://doi.org/10.1177/2041731417730467
Good RB, Eley JD, Gower E, Butt G, Blanchard AD, Fisher AJ, Nanthakumar CB (2019) A high content, phenotypic ‘scar-in-a-jar’ assay for rapid quantification of collagen fibrillogenesis using disease-derived pulmonary fibroblasts. BMC Biomed Eng 1:14. https://doi.org/10.1186/s42490-019-0014-z
Rønnow SR, Dabbagh RQ, Genovese F, Nanthakumar CB, Barrett VJ, Good RB, Brockbank S, Cruwys S, Jessen H, Sorensen GL, Karsdal MA, Leeming DJ, Sand JMB (2020) Prolonged Scar-in-a-Jar: an in vitro screening tool for anti-fibrotic therapies using biomarkers of extracellular matrix synthesis. Respir Res 21:108. https://doi.org/10.1186/s12931-020-01369-1
Organ LA, Duggan A-MR, Oballa E, Taggart SC, Simpson JK, Kang’ombe AR, Braybrooke R, Molyneaux PL, North B, Karkera Y, Leeming DJ, Karsdal MA, Nanthakumar CB, Fahy WA, Marshall RP, Jenkins RG, Maher TM (2019) Biomarkers of collagen synthesis predict progression in the PROFILE idiopathic pulmonary fibrosis cohort. Respir Res 20:148. https://doi.org/10.1186/s12931-019-1118-7
Holm Nielsen S, Willumsen N, Leeming DJ, Daniels SJ, Brix S, Karsdal MA, Genovese F, Nielsen MJ (2019) Serological assessment of activated fibroblasts by alpha-smooth muscle actin (α-SMA): a noninvasive biomarker of activated fibroblasts in lung disorders. Transl Oncol 12:368–374. https://doi.org/10.1016/j.tranon.2018.11.004
Hata R-I, Senoo H (1989) L-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissuelike substance by skin fibroblasts. J Cell Physiol 138:8–16. https://doi.org/10.1002/jcp.1041380103
Puerta Cavanzo N, Bigaeva E, Boersema M, Olinga P, Bank RA (2021) Macromolecular crowding as a tool to screen anti-fibrotic drugs: the Scar-in-a-Jar system revisited. Front Med (Lausanne) 7:615774. https://doi.org/10.3389/fmed.2020.615774. PMID: 33521022; PMCID: PMC7841046
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Stebler, S., Raghunath, M. (2021). The Scar-in-a-Jar: In Vitro Fibrosis Model for Anti-Fibrotic Drug Testing. In: Hinz, B., Lagares, D. (eds) Myofibroblasts. Methods in Molecular Biology, vol 2299. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-1382-5_11
Download citation
DOI: https://doi.org/10.1007/978-1-0716-1382-5_11
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-1381-8
Online ISBN: 978-1-0716-1382-5
eBook Packages: Springer Protocols