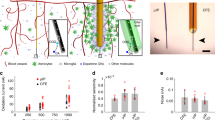
Abstract
Dopamine governs key behavioral processes including motivation, learning, and habit formation. Neurochemical monitoring of dopamine is necessary to identify its role in normal and pathologic conditions and in order to identify targets for treatment and to improve diagnosis. Recent advances have made it possible to record subsecond dopamine release over extended time frames (>months), opening up the possibility to evaluate dopamine’s role over behavioral adaptation, learning, neurodegeneration, and other behavioral processes that take longer than a few hours. Key innovations that we have introduced involve miniaturizing implanted probe dimensions to the size of individual neurons in order to avert inflammatory responses that can restrict chronic viability and sensitivity as well as limit the feasibility of introducing multiple probes into the brain. The purpose of this chapter is to describe methods to fabricate these sensors and to implement them in rodents for the recording of dopamine release over extended periods of time.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Rice ME, Cragg SJ (2008) Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain Res Rev 58:303–313
Gonon F et al (1981) Voltammetry in the striatum of chronic freely moving rats: Detection of catechols and ascorbic acid. Brain Res 223:69–80
Stamford JA, Kruk ZL, Millar J (1988) Stimulated limbic and striatal dopamine release measured by fast cyclic voltammetry: anatomical, electrochemical and pharmacological characterisation. Brain Res 454:282–288
Clark JJ et al (2010) Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat Methods 7:126–129
Garris PA et al (2002) Real-time measurement of electrically evoked extracellular dopamine in the striatum of freely moving rats. J Neurochem 68:152–161
Patriarchi T et al (2018) Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 360(6396):eaat4422. https://doi.org/10.1126/science.aat4422
Sun F et al (2018) A genetically encoded fluorescent sensor enables rapid and specific detection of dopamine in flies, fish, and mice. Cell 174:481–496.e19
McCreery RL (2008) Advanced carbon electrode materials for molecular electrochemistry. Chem Rev 108:2646–2687
Burrell MH et al (2015) A novel electrochemical approach for prolonged measurement of absolute levels of extracellular dopamine in brain slices. ACS Chem Neurosci 6:1802–1812
Kishida KT et al (2016) Subsecond dopamine fluctuations in human striatum encode superposed error signals about actual and counterfactual reward. Proc Natl Acad Sci U S A 113:200–205
Oh Y et al (2018) Tracking tonic dopamine levels in vivo using multiple cyclic square wave voltammetry. Biosens Bioelectron 121:174–182
Wightman RM et al (2007) Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. Eur J Neurosci 26:2046–2054
Threlfell S et al (2012) Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 75:58–64
Howe MW et al (2013) Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. Nature 500:575–579
Hernandez LF et al (2013) Selective effects of dopamine depletion and L-DOPA therapy on learning-related firing dynamics of striatal neurons. J Neurosci 33:4782–4795
Robinson DL, Wightman RM (2004) Nomifensine amplifies subsecond dopamine signals in the ventral striatum of freely-moving rats. J Neurochem 90:894–903
Williams GV, Millar J (1990) Concentration-dependent actions of stimulated dopamine release on neuronal activity in rat striatum. Neuroscience 39:1–16
Schwerdt HN et al (2017) Subcellular probes for neurochemical recording from multiple brain sites. Lab Chip 17:1104–1115
Fox ME et al (2016) Cross-hemispheric dopamine projections have functional significance. Proc Natl Acad Sci U S A 113:6985–6990
Cragg SJ, Hille CJ, Greenfield SA (2000) Dopamine release and uptake dynamics within nonhuman primate striatum in vitro. J Neurosci 20:8209–8217
Taylor IM et al (2015) Kinetic diversity of dopamine transmission in the dorsal striatum. J Neurochem 133:522–531
Schwerdt HN et al (2018) Cellular-scale probes enable stable chronic subsecond monitoring of dopamine neurochemicals in a rodent model. Commun Biol 144:1–11
Schwerdt HN et al (2017) Long-term dopamine neurochemical monitoring in primates. Proc Natl Acad Sci 114:13260–13265
Zachek MK et al (2010) Microfabricated FSCV-compatible microelectrode array for real-time monitoring of heterogeneous dopamine release. Analyst 135:1556–1563
Kozai TDY et al (2015) Brain tissue responses to neural implants impact signal sensitivity and intervention strategies. ACS Chem Neurosci 6:48–67
Spencer KC (2017) A three dimensional in vitro glial scar model to investigate the local strain effects from micromotion around neural implants. Lab Chip 17:795–804
Spencer KC et al (2017) Characterization of mechanically matched hydrogel coatings to improve the biocompatibility of neural implants. Sci Rep 7:1952
Lee RS (2019) Reward prediction error does not explain movement selectivity in DMS-projecting dopamine neurons. elife 8:e42992. https://doi.org/10.7554/eLife.42992
Engelhard B et al (2019) Specialized coding of sensory, motor and cognitive variables in VTA dopamine neurons. Nature 570:509–513
Schmidt AC et al (2014) Multiple scan rate voltammetry for selective quantification of real-time enkephalin dynamics. Anal Chem 86:7806–7812
Hashemi P et al (2012) Brain dopamine and serotonin differ in regulation and its consequences. Proc Natl Acad Sci U S A 109:11510–11515
Asri R et al (2016) Detection of evoked acetylcholine release in mouse brain slices. Analyst 141:6416–6421
Takmakov P et al (2011) Instrumentation for fast-scan cyclic voltammetry combined with electrophysiology for behavioral experiments in freely moving animals. Rev Sci Instrum 82:074302
Glantz JC, Woods JR (1994) Cocaine LD50 in long-evans rats is not altered by pregnancy or progesterone. Neurotoxicol Teratol 16:297–301
Heien MLAV, Johnson MA, Wightman RM (2004) Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Anal Chem 76:5697–5704
Keithley RB, Wightman RM (2011) Assessing principal component regression prediction of neurochemicals detected with fast-scan cyclic voltammetry. ACS Chem Neurosci 2:514–525
Fortin SM et al (2015) Sampling phasic dopamine signaling with fast-scan cyclic voltammetry in awake behaving rats. Curr Protoc Neurosci 70:7.25.1–7.25.20
Acknowledgments
The authors thank Dr. D. Hu (Massachusetts Institute of Technology) for help with surgical procedures. This work is supported by the National Institute of Biomedical Imaging and Bioengineering (R01 EB016101 to A.M.G. and M.J.C.), the National Institute of Neurological Disorders and Stroke (R01 NS025529 to A.M.G., F32 NS093897 and K99 NS107639 to H.N.S), the Army Research Office (W911NF-16-1-0474), the Saks Kavanaugh Foundation, the Nancy Lurie Marks Family Foundation, and Dr. Tenley Albright (to A.M.G.).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Schwerdt, H.N., Graybiel, A.M., Cima, M.J. (2021). Carbon Fiber Probes for Real-Time Monitoring of Dopamine. In: Fakhoury, M. (eds) The Brain Reward System. Neuromethods, vol 165. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-1146-3_6
Download citation
DOI: https://doi.org/10.1007/978-1-0716-1146-3_6
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-1145-6
Online ISBN: 978-1-0716-1146-3
eBook Packages: Springer Protocols