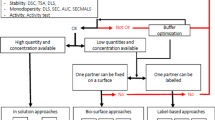
Abstract
Interactions between protein complexes and DNA are central regulators of the cell life. They control the activation and inactivation of a large set of nuclear processes including transcription, replication, recombination, repair, and chromosome structures. In the literature, protein–DNA interactions are characterized by highly complementary approaches including large-scale studies and analyses in cells. Biophysical approaches with purified materials help to evaluate if these interactions are direct or not. They provide quantitative information on the strength and specificity of the interactions between proteins or protein complexes and their DNA substrates. Isothermal titration calorimetry (ITC) and microscale thermophoresis (MST) are widely used and are complementary methods to characterize nucleo-protein complexes and quantitatively measure protein–DNA interactions. We present here protocols to analyze the interactions between a DNA repair complex, Ku70–Ku80 (Ku) (154 kDa), and DNA substrates. ITC is a label-free method performed with both partners in solution. It serves to determine the dissociation constant (Kd), the enthalpy (ΔH), and the stoichiometry N of an interaction. MST is used to measure the Kd between the protein or the DNA labeled with a fluorescent probe. We report the data obtained on Ku–DNA interactions with ITC and MST and discuss advantages and drawbacks of both the methods.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Gavin AC, Bosche M, Krause R et al (2002) Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415(6868):141–147
Uetz P, Giot L, Cagney G et al (2000) A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature 403(6770):623–627
Walker JR, Corpina RA, Goldberg J (2001) Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature 412(6847):607–614
Blier PR, Griffith AJ, Craft J, Hardin JA (1993) Binding of Ku protein to DNA. Measurement of affinity for ends and demonstration of binding to nicks. J Biol Chem 268(10):7594–7601
Arosio D, Costantini S, Kong Y, Vindigni A (2004) Fluorescence anisotropy studies on the Ku-DNA interaction: anion and cation effects. J Biol Chem 279(41):42826–42835
Tadi SK, Tellier-Lebegue C, Nemoz C et al (2016) PAXX is an accessory c-NHEJ factor that associates with Ku70 and has overlapping functions with XLF. Cell Rep 17(2):541–555
Britton S, Coates J, Jackson SP (2013) A new method for high-resolution imaging of Ku foci to decipher mechanisms of DNA double-strand break repair. J Cell Biol 202(3):579–595
Chang HHY, Pannunzio NR, Adachi N, Lieber MR (2017) Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat Rev Mol Cell Biol 18(8):495–506
Frit P, Ropars V, Modesti M, Charbonnier JB, Calsou P (2019) Plugged into the Ku-DNA hub: the NHEJ network. Prog Biophys Mol Biol 147:62–76
Nemoz C, Ropars V, Frit P et al (2018) XLF and APLF bind Ku80 at two remote sites to ensure DNA repair by non-homologous end joining. Nat Struct Mol Biol 25(10):971–980
Ropars V, Drevet P, Legrand P et al (2011) Structural characterization of filaments formed by human Xrcc4-Cernunnos/XLF complex involved in nonhomologous DNA end-joining. Proc Natl Acad Sci U S A 108(31):12663–12668
Malivert L, Ropars V, Nunez M et al (2010) Delineation of the Xrcc4-interacting region in the globular head domain of cernunnos/XLF. J Biol Chem 285(34):26475–26483
Bacquin A, Pouvelle C, Siaud N et al (2013) The helicase FBH1 is tightly regulated by PCNA via CRL4(Cdt2)-mediated proteolysis in human cells. Nucleic Acids Res 41(13):6501–6513
Dherin C, Gueneau E, Francin M et al (2009) Characterization of a highly conserved binding site of Mlh1 required for exonuclease I-dependent mismatch repair. Mol Cell Biol 29(3):907–918
Liberti SE, Andersen SD, Wang J et al (2011) Bi-directional routing of DNA mismatch repair protein human exonuclease 1 to replication foci and DNA double strand breaks. DNA Repair (Amst) 10(1):73–86
Holdgate GA (2001) Making cool drugs hot: isothermal titration calorimetry as a tool to study binding energetics. BioTechniques 31(1):164–170
Krell T (2008) Microcalorimetry: a response to challenges in modern biotechnology. Microb Biotechnol 1(2):126–136
Velazquez-Campoy A, Freire E (2006) Isothermal titration calorimetry to determine association constants for high-affinity ligands. Nat Protoc 1(1):186–191
Asmari M, Ratih R, Alhazmi HA, El Deeb S (2018) Thermophoresis for characterizing biomolecular interaction. Methods 146:107–119
Jerabek-Willemsen M, Wienken CJ, Braun D, Baaske P, Duhr S (2011) Molecular interaction studies using microscale thermophoresis. Assay Drug Dev Technol 9(4):342–353
Raynal B, Lenormand P, Baron B, Hoos S, England P (2014) Quality assessment and optimization of purified protein samples: why and how? Microb Cell Factories 13:180
Myszka DG, Abdiche YN, Arisaka F et al (2003) The ABRF-MIRG'02 study: assembly state, thermodynamic, and kinetic analysis of an enzyme/inhibitor interaction. J Biomol Tech 14(4):247–269
Czarny B, Stura EA, Devel L et al (2013) Molecular determinants of a selective matrix metalloprotease-12 inhibitor: insights from crystallography and thermodynamic studies. J Med Chem 56(3):1149–1159
Dumas P, Ennifar E, Da Veiga C et al (2016) Extending ITC to kinetics with kinITC. Methods Enzymol 567:157–180
Acknowledgments
J.B.C. is supported by ARC program (SLS220120605310), ANR (ANR-12-SVSE8-012), INCA DomRep (PLBIO 2012-280), and CEFIPRA grant 5203C and by the French Infrastructure for Integrated Structural Biology (FRISBI) (ANR-10-INBS-05). A.G. is supported by a CIFRE PhD fellowship with Sanofi. We thank Pierre Soule from Nanotemper Technologies for his availability and all the fruitful discussion. The experiments were performed on the Platform PIM (Platform for measurements of Interactions of Macromolecules) (https://www.i2bc.paris-saclay.fr/spip.php?article280).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Gontier, A. et al. (2021). Measurements of Protein–DNA Complexes Interactions by Isothermal Titration Calorimetry (ITC) and Microscale Thermophoresis (MST). In: Poterszman, A. (eds) Multiprotein Complexes. Methods in Molecular Biology, vol 2247. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-1126-5_7
Download citation
DOI: https://doi.org/10.1007/978-1-0716-1126-5_7
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-1125-8
Online ISBN: 978-1-0716-1126-5
eBook Packages: Springer Protocols