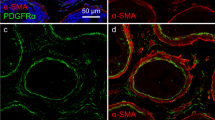
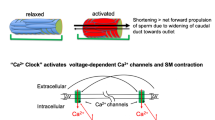
Abstract
Contraction of cauda epididymal duct (CE) smooth muscle is one of the very first events of the seminal emission phase of ejaculation. The contraction of CE smooth muscle is governed by a complex interaction of hormones, autacoids, and by the neurotransmitters released from the epididymal intramural nerve endings, and any impairment in the CE smooth muscle contraction has the potential to impair male fertility. Apart the obvious pathophysiological and toxicological importance of CE smooth muscle contraction, modulation of CE contraction has pharmaceutical interest offering a druggable target to development of drugs to improve/impair male fertility. The in vitro contraction experiments constitute a valuable approach to an in-depth evaluation of functional and molecular changes resulting from pathologies or drug exposure. Therefore, this chapter consists in a description of in vitro pharmacological reactivity contractility of the epididymal duct in a controlled medium, maintained at 30 °C of temperature and continuously bubbled with 95% O2 and 5% CO2 to obtain cumulative concentration–response curves that has been fundamental to some of our investigations on epididymal physiology, toxicology, and pharmacology.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Robaire BHB, Orgebin-Crist MC (2006) The epididymis. In: Neill JD (ed) Knobil and Neill's physiology of reproduction. Elsevier, Amsterdam, pp 1071–1148
Hinton BT, Dott HM, Setchell BP (1979) Measurement of the motility of rat spermatozoa collected by micropuncture from the testis and from different regions along the epididymis. J Reprod Fertil 55(1):167–172
Clement P, Giuliano F (2016) Physiology and pharmacology of ejaculation. Basic Clin Pharmacol Toxicol 119(Suppl 3):18–25. https://doi.org/10.1111/bcpt.12546
Falck B, Owman C, Sjostrand NO (1965) Peripherally located adrenergic neurons innervating the vas deferens and the seminal vesicle of the Guinea-pig. Experientia 21:98–100
Sjostrand NO (1965) The adrenergic innervation of the vas deferens and the accessory male genital glands. Acta Physiol Scand 65(257):7–82
Purves D, Augustine GJ, Fitzpatrick D et al (2001) Autonomic regulation of sexual function. In: Purves D, Augustine GJ, Fitzpatrick D et al (eds) Neuroscience. Sinauer Associates, Sunderland (MA)
Baumgarten HG, Holstein AF, Rosengren E (1971) Arrangement, ultrastructure, and adrenergic innervation of smooth musculature of the ductuli efferentes, ductus epididymidis and ductus deferens of man. Z Zellforsch Mikrosk Anat 120(1):37–79
Silva AM, Queiroz DB, Castro Neto EF, Naffah-Mazzacoratti Mda G, Godinho RO, Porto CS, Gutierrez-Ospina G, Avellar MC (2002) Segment-specific decrease of both catecholamine concentration and acetylcholinesterase activity are accompanied by nerve refinement in the rat cauda epididymis during sexual maturation. J Androl 23(3):374–383
El-Badawi A, Schenk EA (1967) The distribution of cholinergic and adrenergic nerves in the mammalian epididymis: a comparative histochemical study. Am J Anat 121(1):1–14. https://doi.org/10.1002/aja.1001210102
Eliasson R, Risley PL (1968) Adrenergic innervation of the male reproductive ducts of some mammals. 3. Distributions of noradrenaline and adrenaline. Acta Physiol Scand 73(3):311–319. https://doi.org/10.1111/j.1748-1716.1968.tb04109.x
Billups KL, Tillman S, Chang TS (1990) Ablation of the inferior mesenteric plexus in the rat: alteration of sperm storage in the epididymis and vas deferens. J Urol 143(3):625–629
Ricker DD, Chamness SL, Hinton BT, Chang TS (1996) Changes in luminal fluid protein composition in the rat cauda epididymidis following partial sympathetic denervation. J Androl 17(2):117–126
Kempinas WD, Suarez JD, Roberts NL, Strader L, Ferrell J, Goldman JM, Klinefelter GR (1998) Rat epididymal sperm quantity, quality, and transit time after guanethidine-induced sympathectomy. Biol Reprod 59(4):890–896
Kempinas WD, Suarez JD, Roberts NL, Strader LF, Ferrell J, Goldman JM, Narotsky MG, Perreault SD, Evenson DP, Ricker DD, Klinefelter GR (1998) Fertility of rat epididymal sperm after chemically and surgically induced sympathectomy. Biol Reprod 59(4):897–904
Solomon HM, Wier PJ, Ippolito DL, Toscano TV (1997) Effect of prazosin on sperm transport in male rats. Reprod Toxicol 11(4):627–631
Sanbe A, Tanaka Y, Fujiwara Y, Tsumura H, Yamauchi J, Cotecchia S, Koike K, Tsujimoto G, Tanoue A (2007) Alpha1-adrenoceptors are required for normal male sexual function. Br J Pharmacol 152(3):332–340. https://doi.org/10.1038/sj.bjp.0707366
Hellstrom WJ, Sikka SC (2006) Effects of acute treatment with tamsulosin versus alfuzosin on ejaculatory function in normal volunteers. J Urol 176(4 Pt 1):1529–1533. https://doi.org/10.1016/j.juro.2006.06.004
Hisasue S, Furuya R, Itoh N, Kobayashi K, Furuya S, Tsukamoto T (2006) Ejaculatory disorder caused by alpha-1 adrenoceptor antagonists is not retrograde ejaculation but a loss of seminal emission. Int J Urol 13(10):1311–1316. https://doi.org/10.1111/j.1442-2042.2006.01535.x
Kobayashi K, Masumori N, Hisasue S, Kato R, Hashimoto K, Itoh N, Tsukamoto T (2008) Inhibition of seminal emission is the main cause of anejaculation induced by a new highly selective alpha1A-blocker in normal volunteers. J Sex Med 5(9):2185–2190. https://doi.org/10.1111/j.1743-6109.2008.00779.x
Hellstrom WJ, Sikka SC (2009) Effects of alfuzosin and tamsulosin on sperm parameters in healthy men: results of a short-term, randomized, double-blind, placebo-controlled, crossover study. J Androl 30(4):469–474. https://doi.org/10.2164/jandrol.108.006874
Westfall TD, Westfall DP (2001) Pharmacological techniques for the in vitro study of the vas deferens. J Pharmacol Toxicol Methods 45(2):109–122
Bellentani FF, Fernandes GS, Perobelli JE, Pacini ES, Kiguti LR, Pupo AS, Kempinas WD (2011) Acceleration of sperm transit time and reduction of sperm reserves in the epididymis of rats exposed to sibutramine. J Androl 32(6):718–724. https://doi.org/10.2164/jandrol.111.013466
Borges CS, Missassi G, Pacini ES, Kiguti LR, Sanabria M, Silva RF, Banzato TP, Perobelli JE, Pupo AS, Kempinas WG (2013) Slimmer or fertile? Pharmacological mechanisms involved in reduced sperm quality and fertility in rats exposed to the anorexigen sibutramine. PLoS One 8(6):e66091. https://doi.org/10.1371/journal.pone.0066091
Cavariani MM, Kiguti LRD, Rosa JD, Leite GAD, Silva PVE, Pupo AS, Kempinas WD (2015) Bupropion treatment increases epididymal contractility and impairs sperm quality with no effects on the epididymal sperm transit time of male rats. J Appl Toxicol 35(9):1007–1016. https://doi.org/10.1002/jat.3089
Brooks DE (1973) Epididymal and testicular temperature in the unrestrained conscious rat. J Reprod Fertil 35(1):157–160
Jara M, Esponda P, Carballada R (2002) Abdominal temperature induces region-specific p53-independent apoptosis in the cauda epididymidis of the mouse. Biol Reprod 67(4):1189–1196
Wong PY, Au CL, Bedford JM (1982) Biology of the scrotum. II. Suppression by abdominal temperature of transepithelial ion and water transport in the cauda epididymidis. Biol Reprod 26(4):683–689
Foldesy RG, Bedford JM (1982) Biology of the scrotum. I. Temperature and androgen as determinants of the sperm storage capacity of the rat cauda epididymidis. Biol Reprod 26(4):673–682
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Borges, C.S., Kiguti, L.R.A., Pupo, A.S., Kempinas, W.G. (2021). In Vitro Contraction of Isolated Cauda Epididymal Duct Smooth Muscle as a Complimentary Approach to Physiological, Pathological, Toxicological, and Pharmacological Studies on Epididymal Function. In: Palmeira, C.M.M., de Oliveira, D.P., Dorta, D.J. (eds) Toxicity Assessment. Methods in Molecular Biology, vol 2240. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-1091-6_6
Download citation
DOI: https://doi.org/10.1007/978-1-0716-1091-6_6
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-1090-9
Online ISBN: 978-1-0716-1091-6
eBook Packages: Springer Protocols