Abstract
Epitope mapping is the process of experimentally identifying the binding sites, or “epitopes,” of antibodies on their target antigens. Understanding the antibody–epitope interaction provides a basis for the rational design of potential preventative vaccines. Islet autoantibodies are currently the best available biomarkers for predicting future type 1 diabetes. These include autoantibodies to the islet beta cell proteins, insulin and the tyrosine phosphatase islet antigen-2 (IA-2) which selectively bind to a small number of dominant epitopes associated with increased risk of disease progression. The major epitope regions of insulin and IA-2 autoantibodies have been identified, but need to be mapped more precisely. In order to characterize these epitopes more accurately, this article describes the methods of cloning and mutagenesis of insulin and IA-2 and subsequent purification of the proteins that can be tested in displacement analysis and used to monitor immune responses, in vivo, to native and mutated proteins in a humanized mouse model carrying the high-risk HLA class II susceptibility haplotype DRB1*04-DQ8.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Keywords:
- Epitope analysis
- Mutagenesis
- Protein expression
- FPLC
- Reverse phase HPLC
- Protein purification
- Mass spectrometry
- Circular dichroism
1 Introduction
An understanding of the molecular basis of immune recognition of autoantigens in human autoimmune disease is essential for the development of antigen-specific immunotherapies. Antibodies to native protein antigens are often conformationally specific. Epitopes are parts of an antigen that are involved in its recognition by antibodies, B- or T-cells. The conformational epitope recognized is composed of amino acids that are separate from each other in the primary structure of the polypeptide chain but are brought together on the surface during folding. A structural epitope is a three-dimensional structure defining an antigen–antibody complex and includes all atoms of the antigen that are within predefined interatomic distances of antibody atoms. Epitope mapping is the process of locating the binding sites.
Islet autoantibodies are currently the best available biomarkers to predict future type 1 diabetes. These include antibodies to the islet beta cell proteins insulin and the tyrosine phosphatase IA-2 [1, 2]. Defining the epitopes for these antibodies could inform future therapies for type 1 diabetes.
Competitive displacement studies with animal and commercially available insulin preparations have shown that insulin autoantibodies only recognize the correctly folded molecule and are directed predominantly to residues in the A-chain loop of insulin (aa 8-13) and the N terminal of the B chain (aa 1-3) [3–5], but other amino acids may be important [6]. IAA in children who do not progress to multiple autoantibodies were shown to recognize epitopes in the C terminal of the B chain and do not recognize proinsulin [3].
Studies of truncated and chimeric protein constructs have shown that the major diabetes-associated epitope regions of IA-2A are located in the intracellular (ic) portion of the protein, with two in the juxtamembrane (JM) domain and two in the protein tyrosine phosphatase (PTP) domain [7–9]. The majority of autoantibodies to the PTP domain have been shown to recognize two epitope regions; approximately 90 % of binding to these regions in patients with T1D is abolished by modifying just two amino acids [10].
Several techniques have been developed to identify autoantibody epitope regions, including the construction of protein chimeras or deletion constructs [7, 11], competitive inhibition using peptides, animal or mutant proteins [3, 4, 9], phage display [6], and inhibition using monoclonal antibodies or Fab fragments [12, 13].
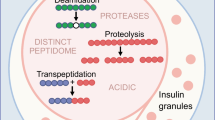
Epitopes can also be mapped using a mutagenesis approach utilizing a set of clones, each containing a unique amino acid mutation suitable for enzyme cleavage. These are then expressed in E. coli or the yeast Pichia pastoris prior to purification of the mutated proteins. For insulin, this could involve mutating various amino acids to lysine which after cleavage with Endoproteinase Lys-C, form various truncated insulins (Fig. 1).
A common approach is to study the effect of changing specific amino acids by using animal insulins or by sequential mutation of single residues [14–16]; methods that have been employed for mapping insulin [3, 4] and IA-2 (Fig. 2; 10, 12, 16) autoantibody epitopes.
Effects of the changes can be assessed by competitive radiobinding assays [3, 4] and/or immunization into mice to evaluate T- and B-cell immune responses elicited by the mutant human insulin and IA-2s in mice expressing the human high risk HLA class II susceptibility haplotype DRB1*04-DQ8 [17].
This chapter describes the methods of cloning and mutagenesis of insulin and IA-2 and subsequent purification of the proteins by various fast protein liquid chromatography (FPLC) and high performance liquid chromatography (HPLC) methods to facilitate identification of key antibody epitopes.
2 Materials
-
1.
LB (Luria–Bertani) broth and agar: Dissolve 10 g tryptone, 10 g NaCl, and 5 g yeast extract in 950 ml H2O and shake until the solutes have dissolved. Adjust the final volume of the solution to 1 l with water. Sterilize by autoclaving for 20 min at 15 psi (1.05 kg/cm2) on liquid cycle. For plates add 15 g/l agar and then autoclave. Before pouring the plates, cool agar to 55 °C before adding appropriate antibiotics, and mix the medium by swirling to avoid producing air bubbles.
For making the low salt LB to be used with the pPICZα vector (Invitrogen) reduce the salt to 5 g/l and autoclave as above.
LB broth for glycerol stocks contains 15 % (v/v) glycerol, i.e., 15 ml glycerol in total 100 ml dH2O.
-
2.
Chloramphenicol 34 mg/ml stock solution: Dissolve 0.34 g of chloramphenicol into 10 ml 100 % ethanol. Filter through a 0.22 μm filter to sterilize. Aliquot and store at −20 °C. Use at 1:1000 dilution in LB broth or agar.
-
3.
Kanamycin 50 mg/ml solution: Dissolve 0.5 g of kanamycin into 10 ml of ddH2O. Filter through a 0.22 μm filter to sterilize. Aliquot and store at −20 °C. Use at 1:1000 dilution in LB broth or agar. For use with Rosetta cells make a 15 mg/ml stock (0.15 g in 10 ml) and use at 1:1000.
-
4.
TAE buffer (50×): To make a concentrated (50×) stock solution of TAE weigh out 212 g Tris base and dissolve in approximately 375 ml deionized H2O. Carefully add 28.55 ml glacial acid and 50 ml of 0.5 M EDTA (pH 8.0) and adjust the solution to a final volume of 500 ml. Sterilize by autoclaving. The pH of this buffer is not adjusted and should be approximately 8.5. To make the working solution of 1× TAE buffer simply dilute the stock solution by 50× in deionized H2O.
-
5.
Yeast Extract Peptone Dextrose Medium (YPD): Dissolve 10 g yeast extract and 20 g of peptone in 900 ml of H2O. Add 20 g of agar if making YPD plates. Autoclave for 20 min on a liquid cycle. Add 100 ml of 10× D (see point 6).
-
6.
20 % Dextrose (10×D): Dissolve 200 g of d-glucose in 1 l of H2O. Autoclave or filter-sterilize.
-
7.
BMMY and BMGY (Buffered Glycerol Complex Medium and Buffered Methanol Complex Medium): Dissolve 5 g yeast extract and 10 g peptone in 350 ml H2O for BMGY (400 ml for BMMY). Autoclave for 20 min on the liquid cycle. Cool to room temperature, then add the following: 50 ml 1 M potassium phosphate buffer pH 6.0, 50 ml 10× YNB, 1 ml 500× B, and 50 ml 10× GY (for BMMY, no 10× GY is added, but 3 ml 100 % methanol instead).
-
8.
Yeast Nitrogen Base with Ammonium Sulfate without amino acids (10× YNB): Dissolve 17 g of YNB without ammonium sulfate and amino acids and 50 g ammonium sulfate in 500 ml of H2O and filter-sterilize. Store at 4 °C.
-
9.
0.02 % Biotin (500× B): Dissolve 20 mg biotin in 100 ml of water and filter-sterilize. Store at 4 °C.
-
10.
10 % Glycerol (10× GY): Mix 50 ml glycerol in 450 ml of water. Sterilize by autoclaving.
-
11.
1 M Potassium Phosphate Buffer pH 6.0: Combine 11.5 g of K2HPO4 and 59.1 g KH2PO4 in 500 ml of H2O and adjust the pH to 6.0 with potassium hydroxide.
-
12.
0.5 M EDTA solution pH 8.0: Stir 93.05 g of disodium ethylenediamine tetraacetate 2H2O into 400 ml of distilled water. Add NaOH solution to adjust the pH to 8.0 or use solid NaOH pellets. Add the NaOH solution or pellets slowly. The EDTA will slowly go into solution as the pH of the solution nears 8.0. Make up the volume to 500 ml with distilled water. Sterilize by autoclaving.
-
13.
Phenylmethylsulfonyl fluoride (PMSF) stock solution: PMSF is used as an irreversible inhibitor of serine protease activity for example in protein purification. To prepare a 100 mM stock solution safely, dissolve 0.0175 g/ml PMSF in isopropanol (see Note 1 ). It is stable for months at 4 °C, but is hydrolyzed rapidly by water. Use at a final concentration of 1 mM.
-
14.
Binding buffer A for GSTrap and equilibration wash buffer for anti-His affinity: 50 mM Tris–HCl pH 7.5, 100 mM NaCl, 1 mM DTT. Dissolve 3.765 g Trizma® Pre-set crystals (Sigma) into 500 ml Endotoxin free water (Fisher). Add 4.383 g of NaCl and 0.077 g DTT.
-
15.
High Salt wash buffer B for GSTrap: 50 mM Tris–HCl pH 7.5, 300 mM NaCl, 1 mM DTT. Dissolve 3.765 g Trizma® Pre-set crystals (Sigma) into 500 ml Endotoxin free water (Fisher). Add 8.766 g of NaCl and 0.077 g DTT.
-
16.
Elution buffer C for GST tag: 50 mM Tris–HCl pH 8, 10 mM reduced glutathione. Dissolve 0.606 g Tris and 0.307 g reduced glutathione in 100 ml Endotoxin free water and adjust to pH 8.
-
17.
Cleavage buffer for PreScission protease: 50 mM Tris–HCl pH 7.5, 100 mM NaCl, 1 mM DTT. Dissolve 3.765 g Trizma® Pre-set crystals (Sigma) into 500 ml Endotoxin free water (Fisher). Add 4.383 g of NaCl, 0.077 g DTT, and 1 ml 0.5 M EDTA.
-
18.
Alkaline elution buffer for anti-His affinity: 0.1 M Tris, 0.5 M NaCl, pH 12.0. Dissolve 1.212 g Tris and 7.305 g NaCl in 100 ml Endotoxin free water (Fisher) and adjust to pH 12.0 with NaOH.
-
19.
Alkaline neutralization buffer: 1 M HCl.
-
20.
2× Sample loading buffer (reducing): Add 0.5 ml 1 M Tris–HCl pH 7, 2.5 ml 20 % SDS, 2 ml glycerol, and 2 mg bromophenol blue. Make up to 10 ml with dH2O. Mix to dissolve. To 950 μl of this add 50 μl β-mercaptoethanol.
-
21.
Resolving gel buffer 1.5 M Tris–HCl, pH 8.8: For 500 ml, dissolve 90.8 g Tris base in 400 ml ddH2O, adjust pH to 8.8 with concentrated HCl and bring the volume to 500 ml with ddH2O.
-
22.
Stacking gel buffer 0.5 M Tris–HCl, pH 6.8: For 500 ml, dissolve 30.3 g Tris base in 400 ml ddH2O, adjust pH to 6.8 with concentrated HCl and bring the volume to 500 ml with ddH2O.
-
23.
10× Tris–glycine running buffer: For 1 l, dissolve 30.2 g Tris base, 144 g glycine in 800 ml ddH2O, add 10 g SDS and bring the volume to 1 l. Dilute to 1× working buffer.
-
24.
10× Transfer buffer (Western): For 500 ml, dissolve 15.1 g Tris base, 72 g glycine in 400 ml ddH2O and bring up to 500 ml. For 1 l of working buffer, add 100 ml 10× transfer buffer, 100 ml methanol, and 800 ml ddH2O.
-
25.
10× TBST (Western wash and block buffer): For 500 ml, dissolve 12.1 g Tris and 40 g NaCl in ddH2O and adjust to pH 7.6. For 500 ml of working buffer, add 50 ml 10× transfer buffer to 450 ml ddH2O and add 0.5 ml Tween 20.
-
26.
Destain solution (for Coomassie): For 500 ml, mix 150 ml methanol, 50 ml glacial acetic acid, and 300 ml ddH2O.
-
27.
Zeocin: Commercially available. Final concentration at 25–50 μg/ml for selection of clones in LB or YPD agar.
-
28.
Isopropyl-beta- d -thiogalactoside (IPTG)—1 M (1000×) Stock Solution: Weigh 2.38 g of IPTG and make up to 10 ml with sterile H2O. Dissolve completely. Filter-sterilize through a 0.22 μm syringe filter. Store in 1 ml aliquots at −20 °C for up to 1 year.
3 Methods
3.1 Cloning the Gene of Interest into an Expression Vector
Choose an appropriate expression vector system (see Note 2 ). Design primers (see Note 3 ) in order that the open reading frame (ORF) of the gene of interest is cloned in frame and downstream of the promoter signal sequence and in frame with the C-terminal tag using appropriate restriction enzymes within the multiple cloning sites. If the tag is not required ensure there is a stop codon (see Note 4 ).
3.1.1 For Expression of Insulin in the Yeast Pichia pastoris
Proinsulin expresses poorly in yeast, but an insulin precursor (IP) containing a mini C-peptide is secreted at high levels into the supernatant [18]. Human IPs were therefore cloned into pPICZα (Invitrogen) and expressed in P. pastoris GS115.
3.1.2 For Expression of Islet Autoantigen-2 (IA-2) in E. coli
The intracellular portion of IA2 (647-979) was cloned from pSP64 (kind gift of Dr Vito Lampasona) into pET49b(+) (Novagen, Merck Millipore) and expressed in Rosetta™(DE3)pLysS (Novagen).
3.1.3 For Both
In intermediary cloning steps for both genes, E. coli library efficiency DH5α™ (competent cells) can be used.
3.1.4 General Cloning Methods
-
1.
Amplify the gene of interest by PCR with appropriately designed primers. Prepare the PCR reaction mix on ice with 10 ng of the template DNA, 1.25 μl each of the 10 μM stock solution of the forward and reverse primers, 5 μl of 5× PCR buffer, 1 μl of a dNTP mixture (10 mM each), 1U of Phusion® High-Fidelity DNA polymerase (NEB UK or alternative), and sterile water to a final volume of 25 μl.
-
2.
Run the PCR reaction in a thermocycler with a standard 35-cycle protocol alternating 30 s at 98 °C, 30 s at 55 °C and 1 min at 72 °C.
-
3.
Use gel electrophoresis to determine whether the PCR reaction has been successful and whether the resulting product is of the expected size. Load 3 μl of the PCR reaction into the wells of a 1 % agarose gel in 1× TAE containing Midori Green (NIPPON Genetics Europe) with a 1 kb DNA ladder (NEB UK). Run the gel at 110 V for 35 min. The amplified product after migration can be analyzed using a Bio-Rad gel imaging system with Image Lab 5.0 software or equivalent system (Fig. 3).
-
4.
Remove primers, nucleotides, enzymes, salts, and other impurities from the PCR product, ready for downstream processing using a QIAquick PCR Purification Kit (Qiagen UK) or alternative kit.
-
5.
Digest 1 μg of PCR product and the expression vector with the appropriate enzymes. After incubation at 37 °C, run the digested DNA on a 1 % agarose gel and extract using the QIAquick Gel Extraction Kit or equivalent.
-
6.
The vector and digested PCR product are ligated using a 3:1 ratio of insert–vector. Typically, 100 ng of linearized plasmid is used with the corresponding amount of insert and 1 U of T4 DNA ligase (LigaFast™ Rapid DNA Ligation System, Promega) together with the ligase buffer to make the final volume to 10 μl with sterile H2O
-
7.
Mix 2 μl of the ligated vector and insert gently with 50 μl of thawed chemically competent E. coli DH5α, and incubate on ice for 30 min. Heat-shock the cells for 30 s in a 42 °C water bath and placed on ice for a further 2 min. Add SOC medium at room temperature (250 μl) to the cells and incubate at 37 °C with shaking at 225 rpm for 1 h.
-
8.
Plate the cells on selective media; for IP and pPICZα, low salt LB with 25 μg/ml Zeocin (Invitrogen) and for IA-2 and pET49b(+), LB and 50 μg/ml kanamycin and incubate overnight at 37 °C.
-
9.
Screen resulting colonies for insert and plasmid DNA by PCR.
3.1.5 Screening E. coli Colonies for Insert by PCR
This is a convenient high-throughput method for determining the presence or absence of insert DNA in plasmid constructs. Individual transformants are lysed in water with a short heating step and added directly to the PCR reaction.
-
1.
Pick a few cells with sterile tip and swirl into 50 μl of dH2O in 0.5 ml microfuge tube.
-
2.
Heat in heating block for 10 min at 95 °C. Vortex briefly and centrifuge for 2 min at 13,000 rpm.
-
3.
Use 1 μl as the template in a 15 μl PCR reaction.
-
4.
Prepare the PCR reaction mix on ice: 0.75 μl each of the 10 μM stock solution of the forward and reverse primers, 1.5 μl of 10× CoralLoad PCR buffer, 0.3 μl of a dNTP mixture (10 mM each), 0.1 μl of Qiagen Taq DNA polymerase, and sterile water to a final volume of 15 μl.
-
5.
Run the PCR reaction in a thermocycler with a standard 35-cycle protocol alternating 30 s at 94 °C, 30 s at 55 °C and 1 min at 72 °C.
-
6.
Positive clones are determined by the presence of a PCR band of the correct size by agarose gel electrophoresis as described above (see Note 5 ). These clones can be inoculated into LB medium for an overnight culture and vector prepared using QIAprep Spin Miniprep Kit. Confirm the DNA sequence by automated sequencing using a Sequencing Service with appropriate primers.
3.2 Site Directed Mutagenesis
In vitro site-directed mutagenesis is an invaluable technique for studying protein structure–function relationships and gene expression, and for carrying out vector modification.
Make single amino acid substitutions using site-directed mutagenesis of the DNA vector (pPICZα IP and pET49b(+) IA-2, Fig. 4) using the QuikChange Site-Directed Mutagenesis kit (Agilent) or equivalent according to manufacturer’s instructions. In this way a diverse collection of engineered mutant clones can be constructed to allow detailed protein structure–function analysis and help to identify specific epitopes in IP and IA-2. All mutations must be confirmed by automated DNA sequencing.
3.2.1 Method
-
1.
Design mutagenic primers using the QuikChange Primer Design Program available online.
-
2.
Have the primers synthesized commercially (Sigma Genosys/Aldrich or equivalent) with PAGE purification. The amount of primer to add to each reaction was calculated using the following equation.
$$ X\ \mathrm{pmoles}\ \mathrm{of}\ \mathrm{oligo}=\frac{\mathrm{ng}\ \mathrm{of}\ \mathrm{oligo}}{330 \times \#\ \mathrm{of}\ \mathrm{bases}\ \mathrm{of}\ \mathrm{oligo}}\times 1000 $$ -
3.
Prepare the DNA template using a QIAprep Spin Miniprep Kit or equivalent (as above).
-
4.
The mutant strand synthesis reactions for thermal cycling were prepared as follows; 5 μl 10× reaction buffer, ddH2O water up to 50 μl, 1 μl (~300 ng DNA template), 125 ng of each primer, 1 μl dNTP (10 mM), and 1 μl PfuUltra DNA polymerase.
-
5.
Carry out the mutagenesis reaction in a thermocycler with the following parameters, 1 cycle 95 °C for 30 s; 18 cycles alternating 30 s at 95 °C, 1 min at 55 °C, and 5 min at 68 °C.
-
6.
Place the reaction on ice to cool to ≤37 °C. Add 1 μl of DpnI restriction enzyme (10 U/μl) directly to the amplification reaction and mix gently. Incubate at 37 °C for 1–2 h.
-
7.
Precipitate the digested DNA in 2 volumes of 100 % ethanol and 1/10 volume 3 M sodium acetate. Centrifuge the solution at 13,000 rpm for 20 min to pellet the DNA, wash once with 80 % ethanol and air-dry. Resuspend the pellet in 10 μl of sterile distilled water.
-
8.
Mix 2 μl of the mutagenesis reaction gently with 50 μl of thawed, chemically competent E. coli DH5α, and incubate on ice for 30 min. Heat-shock the cells for 45 s in a 42 °C water bath and place on ice for a further 2 min. Add room temperature SOC medium (500 μl) to the cells and incubate at 37 °C at 225 rpm for 1 h.
-
9.
Plate the cells on selective media (250 μl per plate). For IP and pPICZα, low salt LB with 25 μg/ml Zeocin and for IA-2 and pET49b(+), LB and 50 μg/ml kanamycin. Incubate overnight at 37 °C.
-
10.
Pick several colonies for plasmid DNA preparation using QIAprep Spin Miniprep Kit. Check these for the mutation by sequencing using appropriate primers.
3.2.2 Transformation into E. coli Rosetta Strains
The plasmid DNA from a clone confirmed by sequencing can be transformed into Rosetta™(DE3)pLysS (Novagen). Rosetta host strains are BL21 derivatives designed to enhance the expression of eukaryotic proteins that contain codons rarely used in E. coli. Gently mix 1 μl of plasmid DNA with 20 μl of cells and incubate on ice for 30 min. Heat-shock the cells for 30 s in a 42 °C water bath and placed on ice for 2 min. Add room temperature SOC medium (250 μl) to the cells and incubate at 37 °C at 225 rpm for 1 h. After incubation the cells are plated on LB with 34 μg/ml chloramphenicol and 15 μg/ml kanamycin. Individual colonies are grown up to make glycerol stocks.
3.2.3 Glycerol Stocks for Long-Term Storage of Plasmids
Bacterial glycerol stocks are important for long-term storage of plasmids. The addition of glycerol prevents damage to the cell membranes and keeps the cells viable. A glycerol stock of bacteria can be stored stably at −80 °C for many years.
-
1.
Inoculate an overnight liquid culture in 5 ml LB and incubate at 37 °C.
-
2.
Centrifuge to pellet the cells and resuspend in LB with 15 % glycerol. Mix gently and transfer to a screw top tube. Freeze the glycerol stock tube at −80 °C. The stock is now stable for years at −80 °C.
-
3.
To recover bacteria from the glycerol stock, use a sterile pipette tip to scrape some of the frozen bacteria off of the top (see Note 6 ). Streak the bacteria onto an LB agar plate and incubate overnight. Single colonies can then be used to inoculate liquid cultures.
3.2.4 Transformation of IP into Pichia pastoris by Electroporation
3.2.4.1 Linearizing the pPICZα Construct
-
1.
Linearize a plasmid DNA pPICZα + IP construct (~5–10 μg) by digestion within the 5′ AOX1 region using SacI. Precipitate the digested DNA using 2 volumes of 100 % ethanol and 1/10 volume 3 M sodium acetate.
-
2.
Centrifuge the solution at 13,000 rpm for 20 min to pellet the DNA, wash once with 80 % ethanol and air-dry. Resuspend the pellet in 10 μl of sterile distilled water. Use 1 μl of the resuspended pellet to check by agarose gel electrophoresis for complete linearization.
3.2.4.2 Preparation of Pichia for Electroporation
-
1.
Inoculate P. pastoris strain GS115 into 50 ml of YPD medium and incubate this preculture overnight at 30 °C in an incubator shaken at 250 rpm.
-
2.
Use this culture (0.5 ml) to inoculate 500 ml of fresh YPD medium and grow overnight again at 30 °C.
-
3.
Harvest two 50 ml aliquots of cells by centrifugation in sterile tubes at 2000 × g at 4 °C for 5 min.
-
4.
Resuspend each of the cell pellets in 50 ml ice-cold sterile water and centrifuge again.
-
5.
Discard the supernatant and resuspend the cell pellets again in 50 ml ice-cold sterile water and centrifuge again.
-
6.
Pool the cells and resuspend in 20 ml ice-cold 1 M sorbitol.
-
7.
After a final centrifugation resuspend the pellet in 1 ml ice-cold sorbitol.
3.2.4.3 Transformation by Electroporation
-
1.
Place a 2 mm gap electroporation cuvette (Bio-Rad) on ice at least 10–15 min before performing the transformation.
-
2.
Gently mix 80 μl of competent cells (as above) with 9 μl of the linearized DNA in the microfuge tube and transfer to the chilled cuvette and incubate for 5 min on ice.
-
3.
Pulse the cells at 1.5 V, 25 μF capacitance, and 200 Ω resistance (Bio-Rad Micropulsar). Add 1 ml of ice -cold 1 M sorbitol to the cuvette and transfer the contents to a sterile 15 ml tube.
-
4.
Incubate the tube statically at 30 °C for 2 h to allow the cells to recover.
-
5.
Spread 50–200 μl of cells on labeled YPD plates with 50 μg/ml Zeocin
-
6.
Incubate plates for 2–3 days at 30 °C until colonies form.
-
7.
Pick 10 colonies for PCR screening.
3.2.4.4 Screening Yeast Colonies for Insert by PCR
This is a convenient high-throughput method for determining the presence or absence of insert DNA in plasmid constructs. Individual transformants are lysed with a heating step and added directly to the PCR reaction.
-
1.
Pick a few cells with sterile tip and swirl into 50 μl of 0.02 M NaOH in 0.5 ml microfuge tube.
-
2.
Heat in heating block for 10 min at 95 °C. Vortex briefly.
-
3.
Use 1 μl as template in 15 μl PCR reaction.
-
4.
Prepare the PCR reaction mix on ice: 0. 5 μl each of the 10 μM stock solution of the forward and reverse primers, 1 μl of 10× CoralLoad PCR buffer, 2 μl 5× Q-solution (see Note 7 ), 0.4 μl of a dNTP mixture (10 mM each), 0.1 μl of Qiagen Taq DNA polymerase, and sterile water to a final volume of 10 μl.
-
5.
Run the PCR reaction in a thermocycler with a standard 35-cycle protocol alternating 30 s at 94 °C, 30 s at 55 °C and 1 min at 72 °C.
-
6.
Determine positive clones by the presence of a PCR band of the correct size by agarose gel electrophoresis as described above. Positive clones at this stage are selected for large scale expression.
-
7.
The method for glycerol stocks of yeast is similar to the method above, except the yeast are spread on YPD plates and incubated at 30 °C for 2 days. The resulting growth is resuspended in 1 ml YPD broth with 15 % glycerol pipetted and stored at −80 °C in sterile screw cap vials.
3.3 Large Scale Expression of IA-2 in E. coli Rosetta™(DE3)pLysS
-
1.
Inoculate a single colony of Rosetta containing the pET49b(+) IA-2 wild type into 50 ml LB containing with 34 μg/ml chloramphenicol and 15 μg/ml kanamycin. Incubate this for 16 h at 37 °C at 225 rpm.
-
2.
Use the overnight culture to inoculate 1 l LB with chlormaphenicol and kanamycin to an OD600 nm of 0.1. Incubate this culture at 37 °C at 225 rpm until the OD600 nm reads ~0.8 (about 4 h), at this point, induce expression by addition of IPTG (1 M stock) to a final concentration of 1 mM. Incubate the culture for a further 4 h with another addition of IPTG after 2 h. Take 1 ml samples at baseline and each hour for cell pellets and OD600 nm measurement. Obtain cell pellets by centrifugation at 13,000 rpm for 1 min and store at −20 °C for analysis of expression by SDS PAGE (Fig. 5).
-
3.
Centrifuge the whole culture after 4 h expression at 8000 rpm for 20 min at 4 °C and resuspend the cell pellet in 15 ml of 15 % glycerol and transfer to a 50 ml centrifuge tube and freeze inverted at −80 °C.
3.4 Large Scale Expression of IP in P. pastoris
-
1.
Inoculate a single colony of P. pastoris containing the integrated IP into 50 ml BMGY and incubate at 28 °C at 250 rpm over the weekend.
-
2.
Inoculate 1 ml of this culture into 150 ml BMGY and incubate at 28 °C at 250 rpm overnight.
-
3.
Pellet cells from two 50 ml aliquots from the overnight culture and resuspend both pellets in a total of 5 ml BMMY.
-
4.
Use this cell suspension to inoculate 500 ml BMMY to OD600 nm equal to 1.0. Add one drop of sterile polypropylene glycol (C3H8O2) to prevent foaming (see Note 8 ). Incubate the culture for 72 h at 28 °C at 250 rpm, feeding with 2 ml 100 % methanol at 24, 32, 48, and 56 h. Remove two 1 ml samples every 24 h, 1 ml for an OD600 nm reading and pellet 1 ml keeping the supernatant at −20 °C for analysis of expression by insulin radioimmunoassay.
-
5.
Centrifuge the whole culture after 72 h at 8000 rpm for 20 min at 4 °C. Discard the cell pellets and filter the supernatant through a 0.45 μM filter, and add 5 ml 0.5 M EDTA and 0.5 ml 100 mM phenylmethanesulfonylfluoride (PMSF). Adjust the pH of the supernatant to 3 with 5 N HCl (~10–12 ml). Store this at 4 °C prior to SP sepharose FPLC.
3.5 Method I Purifying Expressed Protein-IA-2 from E.coli
3.5.1 French Press to Lyse Cells
A French press consists of a piston that is used to apply high pressure to a sample volume of 40–250 ml, forcing it through a tiny hole in the press. Efficient lysis occurs due to the high pressures and shearing used within this process.
-
1.
Load the frozen cell pellet into the French Press apparatus that is prechilled to −80 °C. Pass the pellet twice through the press.
-
2.
After lysis of the pellet resuspend in 120 ml of the following: 50 mM Tris–HCl pH 7.5, 150 mM NaCl, 1 mM DTT. Centrifuge at 8000 rpm for 20 min at 4 °C. Transfer the supernatant to fresh tubes and centrifuge again. Finally store the supernatant in 30 ml aliquots at −20 °C for purification by FPLC.
3.6 Purification of IA-2 Using FPLC
Fast protein liquid chromatography (FPLC), was used to purify the soluble IA-2 protein from the E. coli cell lysates from the French Press. Separation by this method relies on the different components of the lysates having different affinities for the mobile (aqueous buffer) and stationary phase (cross-linked agarose). As the IA-2 was expressed as a fusion protein with the glutathione S-transferase (GST) tag, a GSTrap™ FF 5 ml (GE Life Sciences) column was used as the first purification step. These columns consist of a glutathione ligand coupled via a 10-carbon linker to highly cross-linked 4 % agarose, which gives high binding capacity for GST-tagged proteins. Purification using the GSTrap column was carried out on the AKTA Prime System (GE Life Sciences). This comprises an automated programmable control system, pump, fraction collector, and PrimeView software for data analysis, together with valves for buffer selection, sample injection, gradient formation, and flow diversion. The following protocol is used.
3.6.1 Loading the Cleared Cell Lysate onto the Column
-
1.
Prepare appropriate buffers (see Materials). For loading and washing the cell lysate on the column; attach buffer A (binding buffer) and B (300 mM NaCl wash buffer) to the appropriate lines and fill the tubing and pump with these buffers using the System Wash Method.
-
2.
Connect the GSTrap column to the system and equilibrate with binding buffer.
-
3.
Place the tubing from buffer A into the cell lysate and load the sample onto the GSTrap column at a flow rate of 0.8 ml/min. When all the lysate is loaded, pause the system and replace the tubing into buffer A and then continue to load the lysate remaining in the tubing. Wash the column with buffer B at the same flow rate for 10 column volumes until the absorbance returns to baseline. The effluent passes through two detectors which measure salt concentration (by conductivity) and protein concentration (by absorption of ultraviolet light at a wavelength of 280 nm). This process is monitored on PrimeView software and connecting PC (Fig. 6). Collect the effluent for later analysis by SDS PAGE.
-
4.
Remove the column from the machine. At this stage the IA-2 GST tag fusion protein is bound to the GSTrap column. It is necessary to cleave the tag from the IA-2 protein.
3.6.2 PreScission Protease on Column Cleavage of GST-Tagged Protein Bound to GSTrap FF
-
1.
Prepare 5 ml of PreScission cleavage buffer by adding 0.5 M EDTA to buffer A at a final concentration of 1 mM.
-
2.
Prepare a PreScission protease mix by adding 50 μl of PreScission Protease to 4.6 ml of cleavage buffer prepared in step 1.
-
3.
Load this slowly onto the column using a syringe and Luer connector.
-
4.
Seal the top and bottom of the column with stop plugs and incubate at 4 °C overnight.
3.6.3 Elution of the Cleaved Protein
-
1.
Fill the machine lines with buffer A (binding buffer) and buffer C (elution buffer 50 mM Tris–HCl, 10 mM reduced glutathione, pH 8.0).
-
2.
Use a manual run, with a flow rate of 1 ml/min and fraction collector set to 1 ml fractions.
-
3.
Connect the column to the system and start the run.
-
4.
The IA-2 protein is eluted first as it is no longer bound to the glutathione and this appears as a “peak” in protein concentration on the UV trace marked off in fractions (Fig. 7).
-
5.
When the UV trace returns to baseline after eluting the first peak, manually change the system to 100 % buffer C and fraction size 2 ml and continue elution (Fig. 7).
-
6.
A second peak is the cleaved GST-tag.
-
7.
The fractions, load and effluent were analyzed by SDS PAGE (Fig. 8).
Fig. 8 SDS PAGE analysis of the fractions eluted from the GSTrap column after cleavage. FT1, effluent from column as protein is loaded. FT2, effluent whilst adding the PreScission protease. Fractions 1–10 correspond to first FPLC peak and contain cleaved IA-2. Fractions 14–17 correspond to the second FPLC peak and contain the GST tag
3.6.4 Second Purification by Anti-His Affinity Resin
As a result from the first purification, some of the GST-tag and uncleaved protein co-elutes with the IA-2 protein. In order to further purify the IA-2 protein, the IA-2 containing fractions are pooled and passed through an anti-His affinity resin (GenScript). This is possible as the site of tag cleavage also includes a His tag. The anti-His affinity resin consists of anti-His monoclonal antibody conjugated to agarose resin.
3.6.5 Affinity Resin Preparation
-
1.
Rinse an empty column (Pierce ThermoScientific) with equilibration wash buffer.
-
2.
Thoroughly resuspend 1 ml of resin to form a slurry and add to the column, allowing it to settle under gravity.
-
3.
Wash the resin with 3 resin volumes of equilibration wash buffer. The column is now ready for use.
-
4.
The column can be stored in wash buffer containing 0.02 % sodium azide at 4 °C.
3.6.6 Binding the His Tag/GST Tag Protein and Alkaline Elution
-
1.
Load the pooled protein fractions onto the prepared column and collect the flow-through into a 15 ml tube, this contains the protein.
-
2.
Reload the flow-through for a second time to reach maximal binding of the His tag/GST tag and maximum yield of protein.
-
3.
Wash the resin with two 1 ml aliquots of wash buffer.
-
4.
Use an alkaline elution procedure to elute the bound His tag/GST tag. Label six tubes and add 50 μl of 1 M HCl for neutralization to each tube.
-
5.
Add six 1 ml aliquots of alkaline elution buffer (0.1 M Tris, 0.5 M NaCl, pH 12.0), allowing each aliquot to drain from the column into the separately labeled tubes.
-
6.
Finally wash the resin with wash buffer before storage (as step 4 above).
-
7.
Analyze the various fractions and flow-throughs by SDS PAGE (Fig. 9).
3.7 Endotoxin Removal
Endotoxins are lipopolysaccharides (LPS) derived from cell membranes of gram-negative bacteria and are responsible for its organization and stability. Although endotoxins are linked within the bacterial cell wall, they are continuously released during cell growth and division as well as at cell lysis during protein purification. Removal of endotoxin is one of the most difficult downstream processes during protein purification. The maximum level of endotoxin for intravenous applications in humans of pharmaceutical and biologic product is set to 5 endotoxin units (EU) per kg of body weight per hour [19]. For mice (30 g body weight) this translates to 6 EU/mg at a dose of 0.025 mg/h [20]. Many commercial products are available to remove endotoxin from recombinant proteins. The Detoxi-Gel endotoxin removing columns (Thermo Scientific) use immobilized polymyxin B to bind and remove endotoxins from proteins. Use these according to the manufacturer’s protocol. The level of endotoxin in the final protein can be quantified using the Thermo Scientific Pierce LAL Chromogenic Endotoxin Quantification Kit with a limit of detection of 0.1 EU/ml. Use this according to the manufacturer’s protocol.
3.8 SDS PAGE, Coomassie Blue Staining, and Western Analysis
As SDS polyacrylamide gel electrophoresis (PAGE) and Western blot analysis are widely used techniques, the methods are described briefly. For IA-2, these methods are used to show time course of protein expression, elution of protein from the GSTrap, and the removal of the GST-tag from the protein after anti-HIS affinity purification.
3.8.1 Protein Separation by Gel Electrophoresis
-
1.
Mix the protein samples with sample loading buffer (reducing).
-
2.
Prepare resolving and stacking gels at an adequate percentage of acrylamide based on the molecular mass of the protein of interest. For IA-2 this is a 12 % resolving gel and 6 % stacking gel (Table 1).
Table 1 Components of gels for SDS PAGE -
3.
Cast the resolving gel and gently overlay with isopropanol to accelerate polymerization. After polymerization, remove the isopropanol and pour the stacking gel, gently inserting the gel comb without introducing air bubbles.
-
4.
When the gel is set, load the samples and protein ladder.
-
5.
Run the electrophoresis at 110 V for approx. 100 min until the dye front reaches the bottom of the gel in 1× Tris–glycine running buffer.
-
6.
Following the electrophoresis, remove the gel carefully and either transfer to PhastGel Blue R stain (Sigma) or continue with Western blotting. After staining for 1 h remove from the stain and destain.
3.8.2 Transferring the Protein from the Gel to Membrane
-
1.
Transfer the proteins from the gel to supported nitrocellulose membrane by electrophoretic transfer using a Mini Trans-Blot Cell (Bio-Rad) according to the instructions provided by the manufacturer. The gel is run at 250 mA for 60 min.
-
2.
After electrophoretic transfer, remove the membrane from the transfer unit and block with 5 % BSA in 1× TBST for 1 h.
-
3.
Incubate the membrane overnight with primary antibody (GST (B-14), Monoclonal Antibody 1:3000 dilution, Santa Cruz Biotechnology) in 1× TBST.
-
4.
Following overnight incubation, remove the primary antibody and wash the membrane six times with 1× TBST (~5 min per wash).
-
5.
Incubate the membrane with secondary antibody (anti-mouse IgG peroxidase, Sigma Aldrich) for 1 h and then wash six times with 1× TBST (~5 min per wash).
-
6.
Develop the membrane by incubating with Clarity Western ECL chemiluminescent substrate (Bio-Rad). Detect immunoreactive bands using a Bio-Rad Chemidoc MP with Image Lab 5.0 software (Fig. 10).
3.9 Method II Purifying Expressed Protein: IP from Pichia
3.9.1 FPLC Using SP Sepharose Fast Flow Strong Cation Exchanger
Ion exchange chromatography is based on the reversible interaction between a charged protein and an oppositely charged ion exchange medium. The net surface charge of a protein varies according to the surrounding pH. If the pH is below the isoelectric point (pI), a protein will bind to a negatively charged cation exchanger. For purification of IP, the pH was adjusted below the pI 4.74, therefore SP Sepharose FF (GE LifeSciences) was used. This consists of 6 % agarose cross linked to a sulfopropyl (–CH2CH2CH2SO3–) group. The protein binds as it is loaded, becoming concentrated on the column. Uncharged proteins, or those with the same charge as the ionic groups, elute in the flow-through. Increasing ionic strength (using a gradient e.g., increase in pH) displaces bound proteins as ions in the buffer compete for binding sites and thus the bound substances are eluted at different times.
-
1.
Mix the SP Sepharose slurry (50–60 ml) thoroughly and pack into a XK26/20 column (GE Life Sciences) connected to an AKTA Prime system (GE Life Sciences) according to manufacturer’s protocol.
-
2.
Prepare appropriate buffers (see Materials). For loading and washing the culture supernatant on the column, attach buffer A (50 mM acetic acid) and B (50 mM ammonium acetate adjusted to pH 7.5 with ammonium hydroxide) to the appropriate lines and fill the tubing and pump with these buffers using the System Wash Method.
-
3.
Before each chromatographic run, equilibrate the packed column by washing with at least 150 ml of 50 mM acetic acid pH 3 (buffer A) at a flow rate of 5 ml/min or until the effluent shows stable conductivity and UV trace.
-
4.
Place the line to buffer A in the culture supernatant that is kept on ice and load onto the column at 3 ml/min. Once loaded the line is transferred back to buffer A and continued to load and wash until the UV trace returns to baseline.
-
5.
Elute the protein at 2 ml/min with a pH gradient (0–100 % buffer B) over 75 min starting fraction collection (2 ml aliquots) after 90 min (Fig. 11). Assay the peaks corresponding to eluted protein and fractions using radioimmunoassay.
-
6.
Pool the fractions containing IP and adjust the pH back to 3.0. These pooled fractions are loaded onto 2× 1 ml SP Sepharose columns (Fig. 12).
-
7.
The method for loading and elution from the smaller SP columns is essentially as described in steps 1–6 above. Gradient elution at a flow rate of 1 ml/min is from 0 to 100 % buffer B over 75 min with 2 ml fraction collection (Fig. 12). Assay the fractions corresponding to the peaks by radioimmunoassay. Pool the final fractions that contain IP and freeze-dry.
3.10 Dialysis of Culture Supernatant Before Ion Exchange
Dialysis is a separation technique that removes small, unwanted compounds in solution by selective and passive diffusion through a semipermeable membrane. For IP that is secreted into the culture supernatant dialysis cleans up the solution, allowing better binding to the SP column. However, it does also precipitate a proportion of the protein which must be processed downstream by further methods. The culture supernatant (sample) is poured into a suitable dialysis membrane and sealed. It is placed in a buffer solution (dialysate). The protein that is larger than the membrane-pores is retained within the membrane, but small molecules and buffer salts pass freely through the membrane, reducing the concentration of those molecules in the sample. Changing the dialysate buffer allows more contaminants to diffuse into the dialysate. In this way, the concentration of small contaminants within the sample can be decreased to acceptable levels.
3.10.1 Preparation of Dialysis Tubing
Cut the Visking dialysis membrane (7000MWCO, Medicell Membranes), to size for 250 ml volume. Heat the membrane in 1.5 l of 2 % sodium bicarbonate and 1 mM EDTA at 80 °C for 30 min. Rinse the tubing in pyrogen-free distilled water.
-
1.
Fill the dialysis tubing with culture supernatant at pH 3 and dialyze overnight in cold room in a 2 l measuring cylinder with 50 mM acetic acid pH 3 with mixing.
-
2.
Change the acetic acid buffer before dialyzing for another 1 h at room temperature.
-
3.
Centrifuge the supernatant at 8500 rpm for 20 min to remove any precipitated protein or particulates. Load the dialyzed supernatant onto the SP column as described above.
3.11 Insulin Radioimmunoassay
This assay was used to test which fractions from FPLC contained the IP and has been adapted from a method described in detail elsewhere [21]. Briefly, it is as follows:
-
1.
Pipette 5 μl of HUI-018 anti-human insulin monoclonal antibody (Dako) diluted 1/2000 with normal human serum (negative pool) into the wells of a deep-well plate (except for the wells containing high and low controls)
-
2.
Then pipette 5 μl of human insulin standards (e.g., Actrapid, diluted in TBT) spanning the range 1 U/ml to 0.2 mU/ml (and 0, TBT alone) in duplicate into the wells containing HUI-018 antibody.
-
3.
Pipette 5 μl of supernatant undiluted, diluted 1:10 and/or 1:100 with TBT from the expression time course and the undiluted FPLC fractions into the remaining wells containing HUI-018 antibody.
-
4.
Finally pipette 5 μl of the control sera into additional empty wells.
-
5.
12,000 cpm 125I human insulin (>2000 Ci/mmol, Perkin Elmer, NEX420050UC) in 25 μl TBT is pipetted into each well of the plate containing antibody or control sera.
-
6.
The plate is spun down briefly, mixed and incubated overnight in the fridge.
-
7.
Fifty microliters of washed PGS suspension in TBT (10 μl/well) is added to each well containing reaction mixture, and the plate is spun briefly and incubated with shaking in the fridge for 1 h 45 min.
-
8.
Wash the plate five times with 800 μl/well TBT by centrifugation at 500 × g using 8-well dispenser (Nunc) and aspirator (Sigma) manifolds. After the final wash, transfer the pellet to microtubes using an 8-way multichannel pipette and count on a gamma counter for 15 min.
3.12 Reverse Phase HPLC
After the fractions has been freeze-dried further purification is carried out by reverse phase high performance liquid chromatography (HPLC). HPLC relies on protein hydrophobicity to separate proteins. The stationary phase is hydrophilic and has a strong affinity for hydrophilic molecules in the mobile phase, and thus, they bind to the column. Hydrophobic molecules pass through the column and are eluted first. The hydrophilic molecules can then be eluted from the column by increasing the polarity of the solution in the mobile phase. The length of time a protein takes to pass through the column depends on how much it interacts with the stationary phase and is termed its retention time.
3.12.1 For Purification of IP
Equipment: The HPLC system is composed of a quaternary delivery pump, degasser, thermostatic column compartment, autosample, VWD and fraction collector (Agilent 1260 Infinity). The HPLC system is equipped with two different columns: an analytical Eclipse Plus C18, 3.5 μm particle size, 4.6 mm internal diameter × 100 mm length (Agilent) and a semi-preparative Eclipse XDB C18, 5 μm particle size, 9.4 mm × 250 mm (Agilent). Equipment control, data acquisition and integration were controlled by PC with OpenLAB CDS ChemStation data handling system.
Chromatographic conditions: The mobile phase A consists of water with 0.1 % TFA and mobile phase B acetonitrile (ACN) and 0.1 % TFA. The gradient elution profile for each column is described in Table 2.
The column temperature is maintained at 20 °C. Peak responses are measured at 215 nm using a variable wavelength detector (VWD).
3.12.2 Method
-
1.
Resuspend freeze-dried IP in 100 μl 1 mM HCl and 100 μl water/0.1% TFA/20% ACN before injection into the HPLC using the Eclipse XDB 9.4 mm column and associated conditions (Fig. 13).
-
2.
Collect the peaks with retention time corresponding to uncleaved IP and pool for freeze drying.
3.12.3 Endoproteinase Lys-C Digestion
The HPLC purified IP requires cleavage at the lysine to remove the leader sequence and the short C-peptide joining the A and B chains. Cleavage is also necessary for truncated insulins where amino acids were mutated to additional lysines. This is achieved by Endoproteinase Lys-C digestion. This enzyme from Lysobacter enzymogenes is a serine endoprotease, which specifically cleaves peptide bonds at the carboxyl side of lysine.
-
1.
Dissolve the IP in 100 μl 100 mM Tris–HCl, pH 8.5.
-
2.
Add resuspended Endoproteinase Lys-C (0.4 μg) to the protein and mix gently.
-
3.
Incubate this for 3–5 h at 37 °C. Monitor the digestion by HPLC using the Eclipse Plus 4.6 mm at baseline, 1, 3, and/or 5 h. Remove a 3 μl aliquot from the digest reaction and mix with 100 μl water/0.1 % TFA/10 % ACN before injection into the HPLC (Fig. 14).
-
4.
The digest reaches completion, when the peak analysis at each time point remains unchanged. The peaks are then collected by HPLC and freeze dried.
3.13 Mass Spectrometry
Confirmation of the proteins mass was carried out by the EPSRC UK National Mass Spectrometry Facility at Swansea University. The facility performs MALDI-TOF spectrometry on an Applied Biosystems Voyager DE-STR. The procedure involves mixing solutions of sample protein in acetonitrile/0.1%TFA and matrix, e.g., sinapinic acid in a 1:1 ratio, and pipetting 0.5–1 μl onto the target well of a sample plate. The sample spot is dried, allowing co-crystallization of the mixture, then irradiated with a pulsed N2 laser (337 nm, f = 3 or 20 Hz). The sample is desorbed and ionized, then accelerated into a flight tube (typically 20 kV). The instrument may be run in any combination of positive or negative and linear or reflector modes. The resulting chromatogram gives the molecular weight of the protein (Fig. 15).
3.14 Circular Dichroism
Circular dichroism (CD) spectroscopy was carried out in University of Bristol Chemistry Department. It is a rapid method that determines the secondary structure and folding properties of proteins that have been expressed and purified and also determines whether a mutation affects its conformation or stability. Circular dichroism is the difference in the absorption of left‐handed circularly polarized light (L‐CPL) and right‐handed circularly polarized light (R‐CPL) and occurs when proteins contain one or more light‐absorbing groups. CD spectra are collected in high transparency rectangular quartz cuvettes (cells) with 1 mm pathlength. The buffers for CD spectroscopy must not contain any materials that are optically active and should be as transparent as possible, e.g., 100 mM sodium phosphate pH 7.5. Samples for CD spectroscopy must be at least 95 % pure and at a concentration of at least 0.1 mg/ml. The protein concentration was determined using a NanoPhotometer P360 (Implen).
-
1.
Select an appropriate wavelength range and step size, e.g., “far-UV” 260–180 nm at 0.5 nm step.
-
2.
Select an appropriate bandwidth and time per point, e.g., 1 nm BW and 0.5 s per point.
-
3.
Perform a baseline measurement of buffer only.
-
4.
Replace the buffer with sample and put the cell in the cell holder taking care to maintain its orientation.
-
5.
Acquire a spectrum of the sample. Repeat with further samples making sure the cuvette is cleaned thoroughly between samples (see Note 9 ).
-
6.
Analyze the data using DichroWeb online analysis for protein Circular Dichroism spectra (Fig. 16).
4 Notes
-
1.
PMSF is extremely toxic, and preparation of solutions should be done very carefully and in the fume hood.
-
2.
E. coli or yeast are most suited to large scale production of proteins. Both can be cultured to high cell densities with minimum and low cost complexity of growth media. High levels of expression can be achieved, in E. coli the protein is secreted into the periplasm whilst in yeast it is secreted into the medium. Yeast has advantages in that it is eukaryotic and proteins are therefore post translationally modified and folded. As for expression vector consider the size of the insert, copy number of the plasmid (high copy number offers greater yields), restriction sites within the multiple cloning site and the antibiotic resistance marker.
-
3.
Considerations for primers: The 3′-end of the primer molecule is critical for the specificity and sensitivity of PCR. It is recommended not to have 3 or more G or C bases at this position. Primer pairs should be checked for complementarity at the 3′-end. This often leads to primer-dimer formation. Bases at the 5′-end of the primer are less critical for primer annealing. Therefore, it is possible to add restriction sites, to the 5′-end of the primer molecule to aid cloning adding a few bases overhang to allow efficient cleavage. Primer length of 18–30 bases is optimal for most PCR applications. The melting temperature of both primers should be similar.
-
4.
To increase the efficiency of termination it is possible to use 2 or 3 stop codons in series.
-
5.
The PCR product can be loaded onto agarose gels directly without addition of loading buffer because coral load was used.
-
6.
Do not let the glycerol stock unthaw. Subsequent freeze and thaw cycles reduce shelf life and viability of the stored bacteria.
-
7.
Q-solution is critical for this protocol; the main ingredient is betaine. It improves the amplification of DNA by reducing the formation of secondary structures.
-
8.
When up-scaling to large shake-flasks a particular problem is foaming, which is commonly prevented by the addition of antifoam, e.g., sterile polypropylene glycol. Foaming can lead to reduced yields since bursting bubbles can damage proteins and can also result in a loss of sterility if the foam escapes.
-
9.
Clean cells are the foundation of any spectrophotometric analysis. The residue from previous analysis will cause inaccuracies, low sensitivity and lack of precision. Rinse with ethanol and copious amounts of distilled water. Also inspect the condition of the cells. If they are cracked, chipped or scratched it is important to replace the cells with new ones.
References
Palmer JP (1987) Insulin autoantibodies—their role in the pathogenesis of IDDM. Diabetes Metabol Rev 3(4):1005–1015
Notkins AL, Lan MS, Leslie RDG (1998) IA-2 and IA-2 beta: the immune response in IDDM. Diabetes Metabol Rev 14(1):85–93
Achenbach P, Koczwara K, Knopff A, Naserke H, Ziegler AG, Bonifacio E (2004) Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J Clin Invest 114:589–597
Castano L, Ziegler AG, Ziegler R, Shoelson S, Eisenbarth GS (1993) Characterization of insulin autoantibodies in relatives of patients with type I diabetes. Diabetes 42:1202–1209
Koczwara K, Muller D, Achenbach P, Ziegler AG, Bonifacio E (2007) Identification of insulin autoantibodies of IgA isotype that preferentially target non-human insulin. Clin Immunol 124:77–82
Devendra D, Galloway TS, Horton SJ, Evenden A, Keller U, Wilkin TJ (2003) The use of phage display to distinguish insulin autoantibody (IAA) from insulin antibody (IA) idiotypes. Diabetologia 46:802–809
Bearzatto M, Naserke H, Piquer S, Koczwara K, Lampasona V, Williams A, Christie MR, Bingley PJ, Ziegler AG, Bonifacio E (2002) Two distinctly HLA-associated contiguous linear epitopes uniquely expressed within the islet antigen 2 molecule are major autoantibody epitopes of the diabetes-specific tyrosine phosphatase-like protein autoantigens. J Immunol 168:4202–4208
Zhang B, Lan MS, Notkins AL (1997) Autoantibodies to IA-2 in IDDM: location of major antigenic determinants. Diabetes 46:40–43
Seissler J, Schott M, Morgenthaler NG, Scherbaum WA (2000) Mapping of novel autoreactive epitopes of the diabetes-associated autoantigen IA-2. Clin Exp Immunol 122:157–163
Elvers KT, Geoghegan I, Shoemark DK, Lampasona V, Bingley PJ, Williams AJ (2013) The core cysteines, (C909) of islet antigen-2 and (C945) of islet antigen-2β, are crucial to autoantibody binding in type 1 diabetes. Diabetes 62:214–222
Bonifacio E, Lampasona V, Bernasconi L, Ziegler A-G (2000) Maturation of the humoral autoimmune response to epitopes of GAD in preclinical childhood type 1 diabetes. Diabetes 49:202–208
Weenink SM, Lo J, Stephenson CR, McKinney PA, Ananieva-Jordanova R, Rees Smith B, Furmaniak J, Tremble JM, Bodansky HJ, Christie MR (2009) Autoantibodies and associated T-cell responses to determinants within the 831-860 region of the autoantigen IA-2 in Type 1 diabetes. J Autoimmun 33:147–154
Padoa CJ, Banga JP, Madec A-M, Ziegler M, Schlosser M, Ortqvist E, Kockum I, Palmer J, Rolandsson O, Binder KA, Foote J, Luo D, Hampe CS (2003) Recombinant Fabs of human monoclonal antibodies specific to the middle epitope of GAD65 inhibit type 1 diabetes-specific GAD65Abs. Diabetes 52:2689–2695
Kristensen C, Kjeldsen T, Wiberg FC, Schäffer L, Hach M, Havelund S, Bass J, Steiner DF, Andersen AS (1997) Alanine scanning mutagenesis of insulin. J Biol Chem 272:12978–12983
Chen H, Shi M, Guo ZY, Tang YH, Qiao ZS, Liang ZH, Feng YM (2000) Four new monomeric insulins obtained by alanine scanning the dimer-forming surface of the insulin molecule. Protein Eng 13:779–782
McLaughlin KA, Richardson CC, Williams S, Bonifacio E, Morgan D, Feltbower RG, Powell M, Rees Smith B, Furmaniak J, Christie MR (2015) Relationships between major epitopes of the IA-2 autoantigen in type 1 diabetes: implications for determinant spreading. Clin Immunol 160:226–236
Wen L, Chen NY, Tang J, Sherwin R, Wong FS (2001) The regulatory role of DR4 in a spontaneous diabetes DQ8 transgenic model. J Clin Invest 107:871–880
Kjeldsen T, Pettersson AF, Hach M (1999) Secretory expression and characterization of insulin in Pichia pastoris. Biotechnol Appl Biochem 29(Pt 1):79–86
Daneshian M, Guenther A, Wendel A, Hartung T, von Aulock S (2006) In vitro pyrogen test for toxic or immunomodulatory drugs. J Immunol Methods 313(1-2):169–175
Malyala P, Singh M (2008) Endotoxin limits in formulations for preclinical research. J Pharm Sci 97(6):2041–2044
Wyatt R, Williams AJ (2015) Islet autoantibody analysis: radioimmunoassays. Methods Mol Biol. [Epub ahead of print] PMID: 26659803
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Elvers, K.T., Williams, A.J.K. (2016). Molecular Methods and Protein Synthesis for Definition of Autoantibody Epitopes. In: Gillespie, K. (eds) Type-1 Diabetes. Methods in Molecular Biology, vol 1433. Humana Press, New York, NY. https://doi.org/10.1007/7651_2016_339
Download citation
DOI: https://doi.org/10.1007/7651_2016_339
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3641-0
Online ISBN: 978-1-4939-3643-4
eBook Packages: Springer Protocols