Abstract
MHCI-peptide tetramer staining is an important technique in order to identify antigen-specific T cells within a heterogeneous cell population. The reagents may be used to isolate antigen-specific T cells and can help identify their role in disease. Here, we describe how to make tetramer from peptide:MHC monomers together with a protocol for staining antigen-specific cell populations with advice on generating a complementary antibody phenotyping panel.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Keywords:
1 Introduction
Identification of antigen-specific T cell populations by MHCI-peptide tetramer staining has revolutionized studies in infection, cancer, and autoimmunity [1]. Antigen specificity is dictated by the MHC class I folded with the chosen peptide, although there may be some promiscuity with some T cell receptors (TCR) recognizing multiple peptides [2]. There are many existing protocols available for generation of MHCI-peptide-tetramer complexes. Here, we describe how biotinylated peptide:MHC monomers are used to bind to a streptavidin conjugated to a fluorochrome, thus forming a tetramer. This enables detection of low frequency antigen-specific cells in the context of autoimmunity, using flow cytometry. Cell populations containing antigen-specific CD8 T cells are preincubated with Dasatinib (a tyrosine kinase inhibitor), preventing T cell receptor (TCR) recycling from the cell surface which subsequently provides better tetramer staining [3]. Post-tetramer staining, an antibody panel is used to further phenotype the cells. The technique to be discussed has been optimized for detection of low frequency antigen-specific CD8 T cells and includes staining for murine CD8+ T cells specific for insulin B15–23 [4] in the Non Obese Diabetic (NOD) mouse model of type 1 diabetes. However this technique is also applicable to other antigen-specific CD8+ T cells in the NOD mouse such as those specific for Islet-specific glucose-6-phosphatase catalytic-subunit related protein amino acids 206-214 (IGRP206–214) [5].
2 Materials
2.1 Tetramer Synthesis Components
-
Sterile 1× PBS without calcium and magnesium
-
70 % Ethanol
-
Peptide:MHC class I monomers
-
Protease Inhibitor Cocktail Set I (Merck Millipore 539131) made to 100× using sterile water.
-
High quality Streptavidin bound to a fluorochrome (see Note 1 )—here Streptavidin BV421 (BioLegend 405225)
-
1.5 ml microfuge tubes
2.2 Tetramer Staining Components
-
Tetramer
-
Freshly isolated cells
-
Tetramer Wash Buffer (TWB): 2 % FCS in 1× PBS and store at 4 °C
-
FACS tubes
-
10 mg Dasatinib (Axon Medchem, 1392) diluted in DMSO to 1 mM, aliquot into 5 μl aliquots and store at −20 °C until required
-
Fcx block (Trustain)
-
Antibody Panel (see Note 2 ) including viability dye eFluor 780 (eBioscience 65-0865-14), CD4 PeCy7 (eBioscience 25-0042-52), CD19 PerCpCy5.5 (eBioscience 65-0865-14), CD8 FITC (BD 553030), CD11b APC (BD 553312).
2.3 T Cell Receptor Transgenic CD8 T Cells
-
CD8 T cells from the G9Cα−/− TCR transgenic mice [6] that recognize H-2Kd-insulin B15–23 peptide
3 Methods
3.1 Tetramer Synthesis Protocol
-
1.
Clean bench and anything that will be handled such as pipettes and styrofoam boxes with 70 % ethanol.
-
2.
Thaw the monomer on ice in a box with a lid on as this will prevent monomer degradation prior to making tetramer and avoid refreezing.
-
3.
A suitable amount should be sufficient for 50 tests (i.e., 50 μg monomer) assuming 1 μg of tetramer will be the optimal amount for staining the sample. When making tetramer, it is important to add the monomer and streptavidin-fluorochrome at a ratio of 4:1 (i.e., for MHC class I monomers 50 μg is approximately equivalent to 1 nmol and therefore a total of 0.25 nmol of streptavidin-fluorochrome will be added). To calculate the amount of monomer required, divide the total amount (in μg) by the concentration of the monomer in μg/μl, i.e., 50 μg/X μg/μl = Volume in μl and add this amount to a sterile microfuge tube on ice.
-
4.
To this aliquot of monomer on ice, add 1 μl of the diluted protease inhibitor and mix well.
-
5.
To calculate the amount of streptavidin-fluorochrome conjugate required, multiply the weight in KDa (for both the streptavidin and fluorochrome) by the nmol required (0.25 nmol), i.e., (52.8 kDa (streptavidin) + X kDa (fluorochrome)) × 0.25 nmol = Volume in μl. This assumes a streptavidin-fluorochrome concentration of 1 mg/ml (see Note 3 ).
-
6.
Add 1/5 of the total streptavidin volume required to the monomer and protease inhibitor solution, mix well and leave on ice in the dark for 20 min (see Note 4 ).
-
7.
Repeat the previous step until all of the required streptavidin-fluorochrome solution has been added.
-
8.
Once all the streptavidin has been added, store the tetramer in the dark in a refrigerator at 4 °C.
3.2 Tetramer Staining Protocol
Carry out all procedures at room temperature unless otherwise specified.
-
1.
Acquire single cell suspension of cells and count. Aliquot the cells at 0.5–1 million cells per FACS tube (i.e., per sample including a positive and negative tetramer control sample—see Note 5 ).
-
2.
Add 1 ml of TWB to wash the cells and pellet them in a centrifuge at 400 × g for 5 min at 4 °C.
-
3.
While the cells are pelleting, defrost a 5 μl (1 mM) aliquot of Dasatinib and add to 50 ml of TWB (1:10,000 dilution), giving a concentration of 100 nM.
-
4.
Pour off the supernatant and remove any additional supernatant using a pipette ensuring that there is no disruption of the cell pellet.
-
5.
Once aspirated, resuspend the cell pellet in 50 μl of TWB, then add 50 μl of the previously prepared 100 nM Dasatinib solution (step 3), giving a final concentration of 50 nM of Dasatinib.
-
6.
After the Dasatinib has been added, incubate the cells at 37 °C in 5 % CO2 for 30 min.
-
7.
While the cells are incubating, change the temperature on the centrifuge to room temperature (~22 °C), which prevents any sudden temperature shock to the cells and improves viability. This incubation time also enables the preparation of the tetramer to be added (see Note 6 ). Add 1 μg of tetramer to maintain a total staining volume of 50 μl. Keep the tetramer on ice and in the dark when not in use, as this prevents tetramer degradation.
-
8.
Post-incubation, pellet the cells (400 × g, 5 min, 22 °C) and discard the supernatant by pouring it off. Then resuspend each tube in 25 μl wash buffer containing 2 μl Trustain and incubate at room temperature for 5 min.
-
9.
Post-incubation add 100 μl of TWB to wash the samples and pellet the cells (400 × g, 5 min, 22 °C).
-
10.
Post-pelleting, discard the supernatant by tipping the solution off and add 50 μl of tetramer to each of the samples. Incubate the samples at 37 °C in 5 % CO2 for 15 min (see Note 7 ).
-
11.
During this incubation, make up the antibody mastermix to stain the cells in 100 μl in TWB (see Note 8 ) and keep the antibody solution on ice, in the dark, until ready to be used. During this time, the compensation controls should be set up for the flurochromes/antibodies to be used, including a control for the fluorochrome used in the tetramer and an unstained control.
-
12.
Post-incubation, wash the cells in 1 ml TWB and pellet the cells at 400 × g for 5 min at 22 °C. Then pour off the supernatant and resuspend the pellet in 100 μl of the antibody mastermix. Incubate the cells in the refrigerator for 30 min at 4 °C.
-
13.
Lower the temperature of the centrifuge to 4 °C to pellet the cells after the incubation at 400 × g for 5 min at 4 °C
-
14.
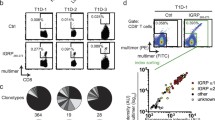
Pour off the supernatant and resuspend in 100 μl of TWB. Place the samples at 4 °C in the dark until analysis on a flow cytometer, gating on live single CD8+CD19−CD11b−CD4−Tetramer+ T cells (see Note 9 ). Figure 1 shows the staining using H-2Kd-insulin B15–23 Tetramer and T cell receptor transgenic T cells specific for Insulin B15–23 peptide.
4 Notes
-
1.
A high quality streptavidin is required for tetramer staining. Ideally a higher concentration of streptavidin should be used to ensure that all the monomers are bound, as free floating monomers will bind to the TCR and make the tetramer staining less effective. In our work, we identified the Life technologies premium grade APC-streptavidin (S-32362) at 1 mg/ml and also BV421 streptavidin (BioLegend) at 0.5 mg/ml (405225) as good to use for tetramers due to the brightness of the fluorochromes and lower background staining (i.e., less nonspecific binding).
-
2.
The antibody mastermix chosen for use will vary depending on the tissue source and additional phenotype information required. In our work, we remove CD19+ B cells, CD4+ T cells, CD11b+ monocytes and macrophages and dead cells. It is important, when devising the phenotyping panel using antibodies to exclude cells that may contribute to nonspecific binding, that possible spectral overlap between the tetramer and any exclusion antibody (i.e., FITC and PE) is considered. Where possible, try to use antibodies on channels that are excited by other lasers to ensure maximum specificity in the staining.
-
3.
If there is a lower concentration of streptavidin-fluorochrome complex, i.e., 0.5 mg/ml, the final volume is multiplied by 2 as twice as much is required to assemble the tetramer.
-
4.
If the volume of streptavidin-fluorochrome solution is too low to add over five incubations, the streptavidin-fluorochrome solution may be diluted in sterile 1× PBS and 1/5 of the total volume (including PBS) to the monomer is added each time.
-
5.
When carrying out tetramer staining it is important to have a positive control, which consists of cells known to bind the tetramer specifically. Cloned T cells or in the case of work with murine models, T cell receptor transgenic cells are particularly useful. A negative control is important, as in any experiment. The positive control will indicate that the tetramer is staining well. The negative control is important for estimating background staining. This background level can then be deducted from the positive staining to give a more accurate result. In this example shown TCR transgenic T cells have been used for the positive control T cells, which recognize the tetramer (H-2Kd presenting Insulin B15–23) and the negative control is H-2Kd presenting a minimal binding peptide AYAAAAAAV. This minimal peptide has two amino acids, in this case for H-2Kd (one at position 2 and the other at position 9), that are needed to bind to the MHC.
-
6.
Once the tetramer has been made, it can have a short life so it is best to use the tetramer within 3–4 weeks. Prior to tetramer staining, where possible, a titration should be performed to identify the best concentration of tetramer to use to ensure all the positive control is stained, without increasing nonspecific staining. When titrating tetramer the concentrations we use are 0.25, 0.5, 1, 1.5, and 2 μg.
-
7.
The protocol presented here is a warm tetramer staining method at 37 °C but it is possible to conduct tetramer staining at 4 °C or at room temperature but this will also need to be tested with varying incubation times to ensure optimal tetramer staining.
-
8.
The individual antibodies in the mastermix in this method should be pre-titrated and is used at 100 μl per sample and consists of anti-CD8 FITC, anti-CD4 PeCy7, anti-CD19 PerCpCy5.5, anti-CD11b APC, and Viability dye eFluor 780 made up to 100 μl with TWB per sample.
-
9.
When gating on specific cell populations, any nonspecific binding—dead cells and doublets (formed by cells adhering together) should be gated out, as well as other cell populations, i.e., B cells, monocytes, and macrophages and CD4+ T cells, as shown in the example.
References
Altman JD, Moss PA, Goulder PJ et al (1996) Phenotypic analysis of antigen-specific T lymphocytes. Science 274(5284):94–96
Wooldridge L, Ekeruche-Makinde J, van den Berg HA et al (2012) A single autoimmune T cell receptor recognizes more than a million different peptides. J Biol Chem 287(2):1168–1177
Weichsel R, Dix C, Wooldridge L et al (2008) Profound inhibition of antigen-specific T-cell effector functions by dasatinib. Clin Cancer Res 14(8):2484–2491
Wong FS, Karttunen J, Dumont C et al (1999) Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library. Nat Med 5(9):1026–1031
Lieberman SM, Evans AM, Han B et al (2003) Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci U S A 100(14):8384–8388
Wong FS, Siew LK, Scott G et al (2009) Activation of insulin-reactive CD8 T-cells for development of autoimmune diabetes. Diabetes 58(5):1156–1164
Acknowledgement
This work was supported by a Medical Research Council (grant number G0901155) to FSW and a Diabetes UK PhD studentship to JAP. We acknowledge the NIH Tetramer Core Facility (contract HHSN272201300006C) for provision of MHC | peptide tetramers.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this protocol
Cite this protocol
Pearson, J.A., Wong, F.S. (2015). Identification of Islet Antigen-Specific CD8 T Cells Using MHCI-Peptide Tetramer Reagents in the Non Obese Diabetic (NOD) Mouse Model of Type 1 Diabetes. In: Gillespie, K. (eds) Type-1 Diabetes. Methods in Molecular Biology, vol 1433. Humana Press, New York, NY. https://doi.org/10.1007/7651_2015_295
Download citation
DOI: https://doi.org/10.1007/7651_2015_295
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3641-0
Online ISBN: 978-1-4939-3643-4
eBook Packages: Springer Protocols