Abstract
Background
The purpose of the present study was to evaluate layered of satranidazole powder using natural polysaccharides as coating materials for colon targeting that were inexpensive and natural with a non-toxic nature using a composite response design of 3 levels and 2 factors for each of the four responses in the quadratic model. The independent variables were the ratio of coating consistency % (X1) and coating level % (X2) in the pellet. The dependent factors were % release of drug at 2 h. (Y1), % release of drug at 6 h. (Y2), % release of drug difference in presence & absence of colonic enzyme (Y3) and mean dissolution time (Y4). The various models were fitted for the responses with an explanation of suitable statistical methods. Variance analysis and different factor levels of responses were constructed by response surface plots.
Results
Satranidazole pellets were efficiently prepared by the variable amount of ingredients that showed compatibility with possible pellet characterization and drug dissolution profiles to optimize the formulation.
Conclusions
The strategy of response surface can be a successful tool for improving the prepared satranidazole pellets which can be an appropriate replacement of regular one.
Similar content being viewed by others
Background
Satranidazole is a 5-nitroimidazole compound substituted in position number two and chemically designated by 1-methylsulfonyl-3-(1-methyl-5-nitro-2-imidazolyl)-2-imidazolidinone [1] with possible activity against bacterial and protozoal diseases for the management of amebiasis [2]. Satranidazole (STZ) is reported to show higher plasma absorption than metronidazole [3] and when introduced directly to the colonic region, the highest drug concentration reaches with increasing drug residence time. Therefore, it was found to be useful against infection by protozoa, particularly Entamoeba histolytica [4]. Also, satranidazole has better patient compliance and nonappearance of neurological responses which favored in patients with susceptible neurological side effects [5].
Statistical experimental design technique is a classical tool useful for the optimization and characterization of pharmaceutical processes by studying the effects of variables with their interaction [6]. The exploratory design gives an economical method to get the best measure of data by expending the least amount of experimental effort which can produce a high-quality and low-cost pharmaceutical product. Quality by design [7] has been implemented by the US FDA authorities who maintain drug quality management. The experimental models can be optimized by considering independent and dependent variables to keep the process in a systemic and rapid mode [8]. Pellets in the form of tablets are affected by various parameters of their formulation. Therefore, a suitable method design can be useful for the optimization of pellets instead of the conventional optimization method where unpredictable records and inappropriate waste of chemicals are required to run the process. Thus, optimization by statistical design could be a suitable way to address the aforementioned situation [9].
The central composite design is a valuable framework for the response surface methodology that has factorial, central and axial focuses [10]. The response surface technique dependent on a measurable and numerical design with a polynomial connection to the research data [11]. The statistical design generally leads to a regression linear model or quadratic composite design. This allows prediction of the properties of the system using the response surface method within a variable space. In the experimental procedure, one or more of the process factors may be changed to study the impact of alterations on the selected responses. The purpose is to get statistical information that can analyze a valid conclusion to find the ideal configuration of all factors and responses. Therefore, the response surface method [12] is an exceptionally valuable model for designing the core compound to influence formulation through the use of variables.
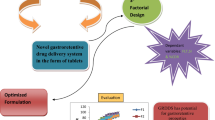
The present research work was based on the use of natural polysaccharides as a coating material provided with microbial degradation and enzymatically degradable for the release directed to the colon, triggered by physiological characteristics of the colonic environment that were inexpensive and of natural and non-toxic origin. The pellets were efficiently organized by single unit that were associated with uniform distribution and drug release throughout the gastrointestinal tract, including minimal variability. The powder coating technology was also given a heterogeneous pellet shape with uniform particle size. The purpose of the present study was to apply a central composite model followed by a response surface technique for the advancement of satranidazole pellets. As an element of the improvement method, the independent effects with dependable factors of the formulation were investigated by the central quadratic composite model. The consistency of the coating was evaluated with its percentage of coating levels to find its result in the dissolution profile at various time intervals in different media. This allows dissolution studies to be validated as an important tool for characterizing pellet formulations through a minimal number of experiments within the statistical design. Therefore, the technique for response surface can optimize the pellets formulation as a substitute for conventional products.
Materials
Satranidazole was gifted by Alkem Laboratories Ltd., Sikkim, India. Locust bean gum, xanthan gum and chitosan gum were obtained from Merck Specialities Pvt. Ltd., Mumbai, India. Non-pareil sugar seeds (25/30 #) were gifted by Anthem Biosciences Pvt. Ltd., Ramanagara District, Karnataka, India. Hydroxypropyl methylcellulose (HPMC-K10) and polyvinyl pyrrolidone K 30 (PVP-K30) were obtained from Loba Chemie Pvt. Ltd., Mumbai, India. Any remaining solvents and compounds were of analytical grade.
Methods
Statistical design of experiments
A 3-level, 2-factor composite response surface design among four responses in the quadratic model was used for optimization [13]. The experiments were operated by the software of Design-Expert (Version 8.0.3 Stat Ease Inc., Minneapolis, MN). The study was done through the two independent factors during their adequate range as shown in Table 1, together among the dependent factors. The resulting formulations are explained in Table 2.
Preparation of satranidazole pellets
Preparation of drug containing pellets by powder layering technique
Satranidazole layered pellets were prepared using coating pan equipment maintaining all instrument parameters. The optimal characteristics of the pellets were considered by a number of parameters, such as primary core load, powder application rate, plate speed, type, position of atomizers, atomization pressure, the type of air cap and the bed temperature. Non-pareil sugar seeds were placed into the coating pan and then treated with a nebulizer binder solution which is prepared by polyvinyl pyrrolidone (K 30) as a binder with isopropyl alcohol and demineralized water mixed in 70:30 proportions using a mechanical stirrer at 200 rpm. It was applied by spray guns and with a dispersed powdered drug applied by a particularly designed powder feed unit [14]. The sphere bed was dried to completely remove the solvent and finally, drug-containing pellets were formed.
Coating of layered pellets
Satranidazole layered pellets were coated using coating pan equipment maintaining all instrument parameters. The coating solution was applied by spraying through spray nozzle, and the process parameters were optimized. The level of coating was determined from the increase in weight after drying using the following equation:
-
1.
$$\% \,{\text{of}}\,{\text{the}}\,{\text{coating}}\,{\text{level}} = \left( {{\text{weight}}\,{\text{of}}\,{\text{coated}}\,{\text{pellets}} - {\text{weight}}\,{\text{of}}\,{\text{uncoated}}\,{\text{pellets}}} \right)/{\text{weight}}\,{\text{of}}\,{\text{coated}}\,{\text{pellets}}$$(1)
-
2.
$$\begin{aligned} & {\text{The}}\,{\text{efficiency}}\,{\text{of}}\,{\text{the}}\,{\text{coating}}\,{\text{process}}\left( \% \right) \\ & \quad = \left( {{\text{Mass}}\,{\text{of}}\,{\text{the}}\,{\text{coated}}\,{\text{particle}} - {\text{Mass}}\,{\text{of}}\,{\text{the}}\,{\text{uncoated}}\,{\text{particle}}} \right)/\Sigma \,{\text{Mass}}\,{\text{of}}\,{\text{drug,}}\,{\text{binder}}\,{\text{and}}\,{\text{excipients}} \\ \end{aligned}$$(2)
Various mixtures of locust bean gum (LBG) and xanthan gum (XG) were applied in a ratio of 2: 1 and a mixture of locust bean gum (LBG) and chitosan gum (CG) biopolymers in a ratio of 1: 2 by different levels of coating.
Before film coating, a seal coating was performed to restrict the migration of the drug layer in drug form before and after functional coating, respectively [15]. The seal coat was performed using HPMC as a film former in a concentration of 5–15%. A total of 18 batches of coating were developed according to the response surface software model described in Table 2. The composition of the coating formulation was revealed with the corresponding batch in Table 3.
Dissolution studies of formulations
In vitro dissolution studies
Dissolution methods were determined by paddle-type USP XXIII dissolution apparatus using 900 ml of dissolution fluid with 50 rpm speed at 37 °C ± 0.5 °C. The perfect sink conditions prevailed during the drug dissolution study period. The release of the drug simulating to the gastrointestinal transit time was accomplished by varying the pH of the liquid at various time intervals. The pellets (300 mg) were weighed accurately and gently spread over the surface of 900 mL of dissolution medium. The dissolution fluid pH was kept at pH 1.2 during 2 h in gastric simulated liquid using 0.1 (N) HCl, pH 4.5 during the third to fourth hour in a mix of gastric and simulated intestinal liquid using NaH2PO4 (1.7 g) and Na2HPO4.3H2O (2.35 g) were added to the dissolution medium, adjusting the pH to 4.5 with NaOH 1.0 M and pH 7.4 in a ratio of 39:61 throughout fifth to sixth hour in simulated intestinal liquid (SIF) and pH 7 for the seventh hour onwards in simulated colonic liquid [16].
Each sample (1 ml) was periodically withdrawn and replaced with an equal amount of fresh dissolution medium (for simulated colonic fluid (SCF), bubbled with carbon dioxide). The volume was made up to 10 ml and centrifuged. The supernatant was filtered through Whatman filter paper, and the drug content was determined spectrophotometrically at 320 nm (UV 1700, Shimadzu, Japan). Each experiment was performed in at least triplicate. All test batches and optimized granules were used for dissolution study for more than 12 h.
Preparation of rat cecal content medium
The simulated colonic fluid for rat cecal content was formed using 20 mM Na2HPO4. 12 H2O, 10 mM NaHCO3, 8 mM NaCl, 0.4 mM MgCl2.6 H2O and 0.5 mM CaCl2.2H2O in 1000 ml of water and stored in a refrigerator [17]. Here, albino rats weighing around 250 ± 20 g were utilized for the current assessment. Four albino rats each for male and female, weighing 200 g to 250 g, were administered by 2% pectin solution 1 ml per day; microbial enzymes were initiated in-vivo during the procedure. The rats were killed by an extreme amount of sodium pentobarbital injected a week later. The abdomens of the rats were opened and the cecum content was isolated and transferred to simulated colonic fluid continuously with a carbon dioxide bubble to uphold anaerobic conditions. Finally, colonic cecum contents were collected and dissolved with 0.1 M isotonic acetate buffer of pH 5 to make a final cecal content (5% w/v) [18, 19].
Release kinetics profile
The drug release kinetics were controlled by the model-independent approach in which the dissolution efficiency (DE) was calculated by the area under the dissolution curve up to a certain time period (t). It was calculated by the dissolution efficiency from the dissolution curve using the trapezoidal technique, and it was determined by the percentage of the area of the rectangle through the dissolution time, the average dissolution time and the dissolution efficiency [20], and the mean dissolution time (MDT) was used to interpret the profile differences in a single value. The mean dissolution rate (MDT) was measured as the dissolution rate where the greater the mean dissolution time (MDT), the slower the release rate.
It was determined by the following equations:
-
1.
$${\text{Dissolution efficiency}} = \left\{ {0\smallint t\left( y \right){\text{d}}t/y*100} \right\}*t*100$$(3)
-
2.
$${\text{Mean dissolution time}} = \sum i = {\text{ni}} = {\text{l}}\,{\text{tmid}}*\Delta M/ \sum i = {\text{ni}} = {\text{l}}\,\Delta M$$(4)
where i = test number of the dissolution study, n = test time number of the dissolution study, tmid = time of midpoint among i and i−1, and M is the quantity of dissolved drug among i and i−l.
The similarities between two dissolution profiles were evaluated using an independent procedure of the pairwise model, similarity factor (f2) [21].
where n = pull points points, Rt = time point for reference profile t, and Tt = test profile at the similar time end.
The assessment of the similarity factor (f2) should be between 50 and 100. The similarity factor value (f2) of 100 suggests that the test and reference profiles were identical, and as the value becomes smaller, the dissimilarity between the releases profiles increases.
Scanning electron microscopy (SEM)
Scanning electron analysis was arranged by spraying the pellets on a single side of a piece of adhesive, and pellets were plated with gold (20 Å). The surface morphology of the pellet was observed by a scanning electron microscope (FEI Quanta-200 MK2) [22].
Numerical optimization for response surface method
The optimization study was determined by expert design software. Polynomial models were used for all response variables that included quadratic terms and interaction through multiple regression analysis technique [23]. The multiple regression analysis models are represented as the following equation:
-
1.
$$y = \beta_{0} + \beta_{1} X_{1} + \beta_{2} X_{2} + \beta_{3} X_{1} X_{2} + \beta_{4} X_{1}^{2} + \beta_{5} X_{2}^{2} + \beta_{6} X_{1} X_{2}^{2} + \beta_{7} X_{1}^{2} X_{2}$$(6)
β0 is the intersection that represents the arithmetic average value of every quantitative results of 18 formulations, β1−β7 are the coefficients of dependent response factors of Y and X1 and X2 are the independent factors. The quadratic condition and interaction are represented by X1X2 and xi2, where, i = 1–2. The polynomials strength was recognized based on the statistical ANOVA study. Contour plots were determined by the polynomial functions of the model. These graphs were extremely valuable to see the interaction results on the factors of responses. Formulations were organized by different response variables. Consequently, the experimental data resulting from the variable responses were compared with their predicted values.
Results
Statistical design of experiments and preparation of satranidazole pellets
The surface response design was based on the variable factor allocated in the response surface [24] shown in Table 1 and 18 formulations were arranged based on the formulation components within the response surface design shown in Table 2. The design was prepared by using two independent factors with four responses in the quadratic model combined with an efficient role for the pellets [25].
Dissolution studies of formulations
Dissolution studies were determined for each formulation. From the measurement of the dissolution studies, it was hard to conclude, so we chose the implication of the design of the response surface for the dissolution profile [26]. The initial release of satranidazole at 2 h 0–2% was expected and up to the 6th hour below 20% and the maximum drug release at 12 h for optimized batch.
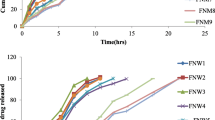
The dissolution profile of the satranidazole [27] pellets (Batch F1–F9) is described in Fig. 1 in simulated gastrointestinal fluid (pH 1.2, pH 4.5 and pH 7) (Fig. 1a) and simulated colonic liquid (pH 7) (Fig. 1b) by locust bean gum with xanthan gum blend as a coating material up to 10 to 30% coating level. The dissolution profile of the satranidazole pellets (Batch F10–F18) is described in Fig. 2 in simulated gastrointestinal fluid (pH 1.2, pH 4.5 and pH 7) (Fig. 2a) and simulated colonic liquid (pH 7) (Fig. 2b) [28] by chitosan with locust bean gum blend as a coating substance up to 10 to 30% coating level.
Dissolution profiles of satranidazole pellets (Batch F1–F9) (Table 2) at a simulated gastrointestinal fluid (pH 1.2, pH 4.5 & pH 7.4) and b simulated colonic fluid (pH 7)
Dissolution profiles of satranidazole pellets (Batch F10–F18) (Table 2) at a simulated gastrointestinal fluid (pH 1.2, pH 4.5 & pH 7.4) and b simulated colonic fluid (pH 7)
Scanning electron microscopy (SEM)
An electron microscope study shows that the pellets had a very spherical shape among a smooth surface. Figure 3 shows the scanning electron morphology [29] for optimized batches during the test before dissolution (Fig. 3a, b) and after dissolution (Fig. 3c). At higher resolution, the surface coating layer was visible. The pellets were clearly specified to be of the reservoir type (Fig. 3d).
Numerical optimization for response surface method
A responsive surface design was applied, with the coating consistency (X1) and the coating level (X2) as independent factors, to optimize the formulation with drug release at 2 h. (Y1), drug release at 6 h. (Y2), the release of the drug difference in the presence and absence of colonic content (Y3) and the mean dissolution time (MDT (Y4) as dependent variables shown in Table 1.
Overall 18 experimental formulations based on the response surface design [30], revealed in Table 2 along with the experimental responses. The mathematical relationship was revealed by the subsequent equations generated by the software, shown in Table 4, and all formulations had to be within the satisfactory limit that contains the details of the batch generated by the software (Table 2) with experimental values of the formulations with percentage of error in the forecast. The observed values of each optimized parameter were found to be very close to the target and the predicted values with less than 5% error. Therefore, the release was finally optimized by a similarity factor value (f2) and the highest similarity factor value was 98.97 to optimize the batch (F9) with a desirability value of 0.478 achieved by the software of design with specified independent variables. The statistical analyses of the models were performed by ANOVA (analysis of variance) [31] shown in Table 5. To estimate the significance of the model, a significance level of 5% was considered for the statistical analysis and the p values [32] are represented in Table 5.
Response surface method analysis
The surface response plots with contour plots were graphed with the regression equation [33] and expressed by two independent factors at the same time against each response (Figs. 4, 5, 6, 7). Therefore, the statistical networks between the independent and dependent factors were further interpreted using the response surface method [34].
Response surface method analysis for (Table 2) Y1 [a response surface plot & b response contour plot]
Response surface method analysis for (Table 2) Y2 [a response surface plot & b response contour plot]
Response surface method analysis for (Table 2) Y3 [a response surface plot & b response contour plot]
Response surface method analysis for (Table 2) Y4 [a response surface plot & b response contour plot]
Figure 4a, b shows the response surface and contour plots for drug release at 2 h (Y1) with a linear shape and a similar pattern of drug release at 6 h (Y2) in Fig. 5a, b continued for other responses for significant p values less than 0.05 which are depicted in Table 5. These were linear for all four responses.
From Fig. 6a, b, we can observe that the drug release difference in presence and absence of colonic enzyme (Y3) at 10 h from the responses by the predicted R-squared value (0.270) was not close to the R-value adjusted square (0.523) as normally expected. This indicated a large block effect.
Figure 7a, b shows the surface response and contour graphs were used for the mean dissolution time by an F value of 2.75 and an F value less than 0.05 indicated that the pattern is significant. In this case, there were no significant model terms. p Values greater than 0.100 indicate that the models were not significant and a negative predicted R-squared value of the mean dissolution time [35] implies that the overall mean is a superior interpreter of the current model response. The formulations were found during the satisfactory limit in terms of physicochemical evaluations with everything that is represented in Table 2 with experimental and observed results where all the dependent variables result, that was the release of the drug at 2 h. (%), drug release at 6 h. (%), difference in drug release at 10 h. (%) and the mean dissolution time (MDT) were shown. The similarity factor (f2) of the optimized batch F9 was 98.87%. Drug release was also finally optimized by determining the similarity factor (f2) and the similarity value of the optimized batch F9 was 98.87%, achieved by the design software with specified independent variables.
Discussion
The objective of applying the response surface design was to optimize the satranidazole powder layered pellets [36] and the amounts of locust bean gum, xanthan gum, chitosan gum combination in the pellet for drug release at 2 h., drug release at 6 h., difference in drug release at 10 h. and the mean dissolution time (MDT) showing compliance using possible results as a suitable substitute for the conventional product.
Using the response surface design model, batches F2, F1 and F7 were found to release the drug 42.70%, 33.11% and 14.6% at 14 h, respectively. These batches were maintained with a minimal lag period (> 2% drug release) in simulated gastric fluid. F2 batch was kept in a sustained release at a constant rate and F1, F7 batches were found the release pattern in parallel. Batches F4, F8, and F3 were found to release the drug by 23.32%, 18.34%, and 14.03% at 14 h, respectively, with a minimal amount in gastric fluid and constant drug release throughout the in vitro dissolution study. F5, F9, and F6 did not show much variation in drug release. All formulations were kept up with minimal drug release in the gastric medium and maximal delivery in the colonic part. Locust bean gum [37] with a mixture of xanthan gum was the most effective polymer in controlling the release property in that the increased quantity of xanthan gum diminishes the drug discharge rate by decreasing the amount of locust bean gum.
It was found that the release profile of satranidazole pellets mixed by locust bean gum with chitosan with a ratio of 1: 1 (Batch F13, F10 and F14) and a ratio of 1: 2 (Batch F15, F11 and F16) of medication release was within 43.35% and 14.69%, respectively. The release rate was found to decrease in a linear fashion while the amount of chitosan gum [38] was found to increase and the release pattern within batches of fixed mix ratio was found slow as the coating level increased as expected. The release profile of formulations F17, F12 and F18 was found to deliver the medication by 62.3%, 38.6% and 28.65% at 14 h, respectively, and all batches were released by bursts up to the 5th hour followed by maintaining a constant slow release rate.
The response surface study using two central composite design blocks of four responses in the quadratic representation is revealed in a linear model. In these restrictions, the release of drug in the simulated gastric liquid was a minimum quantity and the release triggered in the colon that maximizes the release rate to achieve local application of the medication inside the colonic region [39]. It could be done by the four responses with quadratic models which were significant. The p values were < 0.05 with polynomial equations for the intersection coefficients, first-order effects, interaction conditions, and higher-order effects. The indication and the quantity were the comparative influence of all factors on the response, and it was found that the release of drugs at 2 h and 10 h was more significant, while the mean dissolution time was less important in all the responses.
It could be done by the reponse surface method with quadratic models which are significant. The p values were < 0.05 with polynomial equations for the intersection coefficients, first-order effects, interaction conditions, and higher-order effects. The indication and the quantity were the comparative influence of all factors on the response and it was found that the release of drugs at 2 h and 10 h was more significant [40], while the mean dissolution time was less important in all the responses.
It was established that the observed of each parameter was extremely close to the objective and the predicted values with less than 5% error. Drug release was also finally optimized by determining the similarity factor (f2). Here, the highest f2 (98.87% for batch F9) achieved by the design software with specified independent variables. Therefore, the optimized batch was F9 with a 2: 1 ratio of locust bean gum-xanthan gum blends using 20% coating level.
Conclusions
It was revealed that the proper response surface design can be used effectively in enhancing satranidazole pellets to meet colonic release. The surface response graphs and optimization allowed the satranidazole pellets to be layered by a combination of natural polysaccharides with the required release profile. The results suggested that the formulation showed proper evaluations with dissolution profiles directed to the colon under different pH conditions. The coating formulation was shown to consist of locust bean gum-xanthan gum mixtures in a 2:1 (20%) coating ratio to be effective for colonic release of satranidazole pellets. The optimized batch showed release and response profiles close to the predicted response. Therefore, the response surface method can be an effective tool to optimize the pellet as a suitable substitute for conventional products.
Availability of data and materials
All necessary data generated or analyzed during this study are included in this published article. Any additional data could be available from the corresponding author upon request.
Abbreviations
- STZ:
-
Satranidazole
- RSM:
-
Response surface method
- HPMC:
-
Hydroxypropyl methylcellulose
- SEM:
-
Scanning electron microscopy
- f2:
-
Similarity factor
References
Mazumder R, Mahanti B, Majumdar S, Pal R, Chowdhury AD (2020) Improved comprehensive analytical method for assessment of satranidazole in drug and product. Future J Pharm Sci 6(1):1–11
Bansal K, Rawat MK, Jain A, Rajput A, Chaturvedi TP, Singh S (2009) Development of satranidazole mucoadhesive gel for the treatment of. AAPS Pharm Sci Tech 10:716–723
Pawar HA, Joshi PR (2014) Development and evaluation of taste masked granular formulation of satranidazole by melt granulation technique. J Pharm 1:1–7
Hemphill A, Muller N, Muller J (2019) Comparative pathobiology of the intestinal protozoan parasites giardia lamblia, entamoeba histolytica, and cryptosporidium parvum. Pathogens 8(3):116
Parmar DSM, Jadav SP (2007) The concept of personal drugs in the undergraduate pharmacology practical curriculum. Ind J Pharmacol 39(3):165–167
Paterakis PG, Korakianiti ES, Dallas PP, Rekkas DM (2002) Evaluation and simultaneous optimization of some pellets characteristics using a 33 factorial References 136 design and the desirability function. Int J Pharm 248:51–60
Sangshetti JN, Deshpande M, Zaheer Z, Shinde DB, Arote R (2017) Quality by design approach: regulatory need. Arab J Chem 10:3412–3425
Gupta B, Poudel BK, Tran TH, Pradhan R, Cho HJ, Jeong JH et al (2015) Modulation of pharmacokinetic and cytotoxicity profile of imatinib base by employing optimized nanostructured lipid carriers. Pharm Res 32:2912–2927
Meka VS, Thing LK, Gorajana A, Kolapalli VR (2015) Formulation and optimization of gastric floating drug delivery system using central composite design and its biopharmaceutical evaluation. Pak J Pharm Sci 28(4):1373–1387
Late SG, Banga AK (2010) Response surface methodology to optimize novel fast disintegrating tablets using β cyclodextrin as diluents. AAPS Pharm Sci Tech 11(4):1627–1635
Bushra R, Shoaib MH, Ali H, Zafar F, Naeem MI, Aslam N, Yousuf RI (2014) Formulation design and optimization of aceclofenac tablets (100 mg) using central composite design with response surface methodology. Lat Am J Pharm 33(6):1009–1018
Majumdar S, Dey S, Ganguly D, Mazumder R (2020) Enhanced topical permeability of natural flavonoid baicalein through nano liposomal gel: In-vitro and in-vivo investigation. J Drug Deliv Sci Technol 57:101666
Fukuda IM, Pinto CFF, Moreira CDS, Saviano AM, Lourenco FR (2018) Design of experiments (DoE) applied to pharmaceutical and analytical quality by design (QbD). Braz J Pharm Sci 54(Spl):01006
Kovacevic J, Mladenovic A, Djuris J, Ibric S (2016) Evaluation of powder, solution and suspension layering for the preparation of enteric coated pellets. Eur J Pharm Sci 85:84–93
Albertini B, Melegari C, Bertoni S, Dolci LS, Passerini N (2018) A novel approach for dry powder coating of pellets with ethylcellulose. Part II: evaluation of caffeine release. AAPS Pharm Sci Tech 9(3):1426–1436
Ren Y, Jiang L, Yang S, Gao S, Yu H, Hu J, Hu D, Mao W, Peng H, Zhou Y (2017) Design and preparation of a novel colon-targeted tablet of hydrocortisone. Braz J Pharm Sci 53(1):1–11
Tozaki H, Komoike J, Tada C, Maruyama T, Terabe A, Suzuki T, Yamamoto A, Muranishi S (1997) Chitosan capsules for colon-specific drug delivery: improvement of insulin absorption from the rat colon. J Pharm Sci 86(9):1016–1021
Gliko-Kabir I, Yagen B, Penhasi A, Rubinstein A (2000) Phosphated crosslinked guar for colon-specific drug delivery I. Preparation and physicochemical characterization. J Controll Release 63:121–127
Zhang H, Alsarra IA, Neau SH (2002) An in vitro evaluation of a chitosan-containing multiparticulate system for macromolecule delivery to the colon. Int J Pharm 239:197–205
Zhang Y, Huo M, Zhou J, Zou A, Li W, Yao C, Xie SDD (2010) Solver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J 12(3):263–271
Junior ADFS, Barbosa IS, Santos VLD, Silva RL (2014) Junior EC (2014) Test of dissolution and comparison of in vitro dissolution profiles of coated ranitidine tablets marketed in Bahia, Brazil. Braz J Pharm Sci 5(1):83–89
Hirjau M, Miron DS, Anuta V, Lupuliasa D, Ghica MV, Jinga V, Dinu-Pirvu CE (2020) Evaluation of experimental multi-particulate polymer-coated drug delivery systems with meloxicam. Coatings 10(5):490
Deshmukh RK, Naik JB (2013) Diclofenac sodium-loaded eudragit® microspheres: Optimization using statistical experimental design. J Pharm Innov 8:276–287
Ghelicha R, Jahannamab MR, Abdizadeha H, Torknikc FS, Vaezic MR (2019) Central composite design (CCD)-Response surface methodology (RSM) of effective electrospinning parameters on PVP-B-Hf hybrid nanofibrous composites for synthesis of HfB2-based composite nanofibers. Composites B 166:527–541
Patela S, Patelb N, Misraa M, Joshic A (2018) Controlled-release domperidone pellets compressed into fast disintegrating tablets forming a multiple-unit pellet system (MUPS). J Drug Del Sci Technol 45:220–229
Kassem MA, Shaboury KME, Mohamed AI (2019) Application of central composite design for the development and evaluation of chitosan-based colon targeted microspheres and in- vitro characterization. Ind J Pharm Sci 81(2):354–364
Pawar HA, Joshi PR (2014) Development and validation of a discriminating in vitro dissolution method for oral formulations containing satranidazole. Int J Spectrosc 3:1–7
Singh SK, Yadav AK, Prudhviraj G, Gulati M, Kaur P, Vaidya Y (2015) A novel dissolution method for evaluation of polysaccharide based colon specific delivery systems: a suitable alternative to animal sacrifice. Eur J Pharm Sci 73:72–80
Joshi R, Raje S, Akram W, Garud N (2019) Particle engineering of fenofibrate for advanced drug delivery system. Future J Pharm Sci 5:1–11
Nayak AK, Pal D (2011) Development of pH-sensitive tamarind seed polysaccharide–alginate composite beads for controlled diclofenac sodium delivery using response surface methodology. Int J Biol Macromol 49:784–793
Sawyer S (2009) Analysis of variance: the fundamental concepts. J Man Manip Ther 17(2):27E-38E
Greenland S, Senn SJ, Rothman KJ, Carlin JB, Poole C, Goodman SN, Altman DG (2016) Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol 31:337–350
Peng X, Yang G, Shi Y, Zhou Y, Zhang M, Li S (2020) Box-Behnken design based statistical modeling for the extraction and physicochemical properties of pectin from sunflower heads and the comparison with commercial low-methoxyl pectin. Sci Rep 10:1–10
Shahzad MK, Ubaid M, Murtaza G (2012) Formulation and optimization of celecoxib-loaded microspheres using response surface methodology. Trop J Pharm Res 11(5):695–702
Tesfay D, Abrha S, Yilma Z, Woldu G, Molla F (2020) Preparation, optimization, and evaluation of epichlorohydrin cross-linked enset (ensete ventricosum (welw.) cheeseman) starch as drug release sustaining excipient in microsphere formulation. BioMed Res Int 1:1–19
Kibria G, Akhter A, Islam KMA (2010) Formulation and evaluation of domperidone pellets prepared by powder layering technology. Asian J Pharm 4(1):41–47
Grenha A, Dionísio M (2012) Locust bean gum: Exploring its potential for biopharmaceutical applications. J Pharm Bioallied Sci 4(3):175
Shi Y, Wan A, Shi Y, Zhang Y, Chen Y (2014) Experimental and mathematical studies on the drug release properties of aspirin loaded chitosan nanoparticles. BioMed Res Int 1:1–8
Vertzonia M, Augustijns P, Grimm M, Koziolek M, Lemmens G, Parrott N, Pentafragka C, Reppas C, Rubbens J, Αbeele JVD, Vanuytsel T, Weitschies W, Wilson CG (2019) Impact of regional differences along the gastrointestinal tract of healthy adults on oral drug absorption: an UNGAP review. Eur J Pham Sci 15:153–175
Markl D, Zeitler JA (2017) A review of disintegration mechanisms and measurement techniques. Pharm Res 34(5):890–917
Acknowledgements
Authors wish to give thanks to School of Pharmacy, Techno India University, EM 4, Sector-V, Kolkata-700091, West Bengal, India, Calcutta Institute of Pharmaceutical Technology and Gitanjali College of Pharmacy for constant support and given research laboratory to carry out this project work and also thanks to help and encourage by our fellow colleagues in completion of the research project work.
Funding
The authors have no funding to report.
Author information
Authors and Affiliations
Contributions
We declare that this work was done by the authors named in this article: RM, BM and RNP conceived and designed the study. RM and ADC carried out the laboratory work, collected and analyzed the data and SM drafted the manuscript. BM supervised the work and RM assisted in the data analysis. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mazumder, R., Mahanti, B., Majumdar, S. et al. Response surface method for optimization of prepared satranidazole powder layered pellets. Futur J Pharm Sci 7, 190 (2021). https://doi.org/10.1186/s43094-021-00337-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-021-00337-w