Abstract
Background
Being the most widely used construction material, concrete health is considered a very important aspect from the structural point of view. Microcracks in concrete cause water and chlorine ions to enter the structure, causing the concrete to degrade and the reinforcement to corrode, posing an unacceptable level of structural risk. Hence repair of these cracks in an eco-friendly and cost-effective way is in the interest of various researchers. Microbially induced calcite precipitation (MICP) is an effective way considered by various researchers to heal those concrete cracks along with an important environmental contribution of CO2 (carbon dioxide) sequestration in the process.
Main content
As the current concentration of CO2 in the earth’s atmosphere is about 412 ppm, it possesses a deadly threat to the environmental issue of global warming. The use of bacteria for MICP can not only be a viable solution to repairing concrete cracks but also can play an important role of CO2 arrestation in carbonate form. This will help in carbon level management to lessen the adverse effects of this greenhouse gas on the atmospheric environment, particularly on the climate. To overcome the insufficiency of studies concentrating on this aspect, this review article focuses on the metabolic pathways and mechanisms of MICP and highlights the value of MICP for CO2 arrestation/sequestration from the atmosphere during the process of self-healing of concrete cracks, which is also the novelty of this work. An overview of recent studies on the implementation of MICP in concrete crack repair is used to discuss and analyse the factors influencing the effectiveness of MICP in the process, including various approaches used for CO2 sequestration. Furthermore, this investigation concentrates on finding the scope of work in the same field for the most effective ways of CO2 sequestration in the process of self-healing cracks of concrete.
Conclusion
In a prospective study, MICP can be an effective technology for CO2 sequestration in concrete crack repair, as it can reduce adverse environmental impacts and provide greener environment. This critical study concludes that MICP can bear a significant role in arrestation/sequestration of CO2, under proper atmospheric conditions with a cautious selection of microorganisms and its nutrient for the MICP procedure.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.1 Background
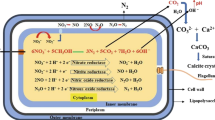
The construction industry directly aids in the development of economic activities whereas, simultaneously, it also exploits natural and physical resources. The construction and building sector accounts for roughly a quarter of all carbon dioxide (CO2) emissions [1]. Concrete has been the most commonly used building material in the world since the nineteenth century. The formation of microcracks in concrete structures reduces their service life by allowing water and chlorine ions to enter the structure [2], causing concrete deterioration and reinforcement of corrosion, resulting in an excessive degree of structural risk. As a result of this phenomenon, expensive repairs and maintenance are needed on a regular basis. This structural degradation, coupled with the weakening of concrete structures, leads to an economic burden by increasing repairing cost. In general, for crack remedy, materials like epoxies are used. However, they have their own drawbacks, such as they are expensive, change the appearance of the structure to a certain level, and need consistent maintenance. In traditional, but high-binder mixture concrete, microcracks with a width smaller than 0.2 mm can be seen to be autogenously self-healed [3,4,5,6,7]. But simultaneously, the application of high-binder concrete mix will allow the extra use of cement in concrete which in return encourages an enhanced cement production that leads to higher global anthropogenic CO2 emission. To minimize the risk in a cost-effective manner, researchers have focused on developing a safe and self-healing process that could revolutionize the construction of long-lasting concrete structures. This self-healing method is established by bacteria-induced calcium carbonate (calcite) precipitation in the concrete cracks. This phenomenon is known as microbially induced calcite precipitation (MICP), and the concrete with this phenomenon is known as ‘Bacterial concrete’ or ‘Self-healing concrete’. This phenomenon focuses on the application of microbes as a healing agent for cracks in existing concrete structures. The basic strategy (Fig. 1) is to use microorganisms, preferably those which can form stable and dormant spores, and apply them in the concrete structure as microcapsules/pellets that also contain specific nutrients (such as calcium lactate). When the concrete slabs are intact and solid, these spores remain dormant, but when cracks are formed in the concrete, water seeps in, and the bacteria start growing. The bacteria utilize the nutrient such as calcium lactate for its growth. The microbial urease can hydrolyse urea to produce ammonia and CO2. The released ammonia increases the pH of the surroundings, which helps in the accretion of insoluble CaCO3. It is the most used and studied pathway in the line of self-healing microbial concrete (Fig. 1). In the process, another severe environmental problem in the current scenario, i.e., emission of greenhouse gas, CO2 can also be checked. In fact, the current concentration of about 412 ppm CO2 in the earth’s atmosphere [8], already possesses the risk of global warming. The use of bacteria for MICP can not only be a feasible solution to repair concrete cracks but also can arrest CO2 as carbonate. This will help us to achieve the goal of carbon level management and reduce the potential environmental threat of global warming. The present review article highlights the role of a different group of microorganisms and the pathways operative in them, which help in CO2 sequestration from the atmosphere and in the process can be utilized for self-healing of concrete cracks.
2 Main text
2.1 Research significance
It is well known that carbon dioxide is the leading and the primary greenhouse gas with the highest percentage among other greenhouse gases. According to the National Ready Mixed Concrete Association, the production of 1 kg of concrete releases 0.9 kg of CO2 [9]. Concrete is the most commonly used material on the planet, accounting for 8% of overall global carbon emissions [10]. It is intrinsically fragile, necessitating regular repair or replacement, which are costly and emit enormous amounts of CO2. Hence, this CO2 sequestration via the healing/repair of concrete will reduce the amount of CO2 in the environment, specifically, it will balance some of the quantity of emitted CO2, from the construction industry. The utilization of the MICP process can not only be a viable approach to repairing concrete cracks but it can also intercept CO2 as carbonate from escaping into the environment. Though there are several individual review articles present on MICP and concrete healing, there is a scarcity in the field addressing the research gap that signifies both the aspect of concrete healing and its utilization in CO2 sequestration. Therefore, this review addresses the novel research aspect of the utilization of concrete crack healing by MICP keeping the target of CO2 sequestration from the environment. This laborious study included the efforts of a vast field of expertise including individual sectors such as Civil Engineering, Chemical Engineering, Microbiology, Biotechnology, Chemistry, etc. as well as their interdisciplinary exertion. Hence, this study will be advantageous for a vast readership, ultimately benefiting and contributing in the goal of carbon level management and reduction of the possible environmental impact of global warming.
2.2 Importance of CO2 sequestration and its role in self-healing concrete
The concentration of the principal greenhouse gas, CO2 has been on the rise due to massive urbanization, industrialization, and various anthropogenic activities that are dependent on fossil fuels, resulting in global warming and thus, climate change. The development in the construction industry also resulted in the enhancement of cement production leading to higher CO2 emissions in the environment. Therefore, there is an urgent requirement for the effective management of carbon footprint. CO2 sequestration, also known as "Carbon trap" or "CO2 arrestation," is a method for storing CO2 or its associated forms from the atmosphere for extended periods of time in order to reduce the amount of carbon in the atmosphere, resulting in a healthier environment. In this scenario, the utilization of microorganisms for CO2 sequestration is a feasible and economical approach that is also beneficial for a sustainable environment as well. This is known as biological CO2 sequestration. From the structural point of view, the self-healing method of concrete cracks, established by bacteria-induced calcium carbonate (calcite) precipitation i.e., MICP, in the concrete cracks is a very effective method of CO2 sequestration. The microorganisms mainly conduct the sequestration of atmospheric CO2 and fix it into different carbonate minerals such as calcite, magnesite, and dolomite [11,12,13]. A wide range of microorganisms can induce carbonate precipitation through biological activities such as photosynthesis, ureolysis, denitrification, and sulphate reduction, or by acting as a template for crystal nucleation [14]. A significant amount of research has been conducted on MICP technology concentrating on ureolytic bacteria for crack repair in concrete structures and on cyanobacteria for carbon sequestration.
2.3 Organisms and pathways
Biomineralization or to be specific, MICP has a contribution to the crack healing of concrete. In this process, metabolites generated by the induced microorganisms, react either among themselves or with components in the environment to produce and precipitate biominerals in carbonate forms that help in healing the concrete cracks (Fig. 1). Biomineralization yields a variety of minerals [15,16,17] especially bacteria are capable of producing a wide variety of them in the form of sulphides, carbonates, phosphates, and silicates [18, 19]. Among them, CaCO3 precipitation is of interest to researchers due to its efficient compatibility and bonding capacity with concrete compositions. Microorganisms, in the presence of a Ca-source, can produce CaCO3 extracellularly by means of an autotropic or heterotrophic metabolic pathway [19] (Table 1).
2.3.1 Heterotrophic pathway
Heterotrophic growth on organic acid salts (citrate, acetate, succinate, lactate, oxalate, glyoxylate, and malate) of different bacterial genera such as Bacillus, Arthrobacter, and Rhodococcus species, produces CO32− minerals. Based on the presence of salts and C-sources in the medium, these bacteria can produce various crystals such as CaCO3 and MgCO3, using organic compounds as a source of energy. Arthrobacter and Bacillus species are capable of CaCO3-precipitation [18] under an alkaline carbonate medium which is a very important aspect for being applicable in the healing of concrete cracks. Thus, future explorations must be made from different alkaline environments to find the most suitable microorganism that can realize the dream of self-healing concretes.
The summary of chemical reactions to form CaCO3 in the presence of Ca(CH3COO)2 acting as the source of low molecular weight acid and Ca2+ are shown in the equations below [Eqs. (1)–(3)] [20].
The other two mechanisms of calcium carbonate production are sulphur cycle and nitrogen cycle. The dissimilatory sulphate reduction is followed in sulphur cycle mechanism. In the presence of organic matter, calcium source, and sulphate in the medium, calcium carbonate is produced. Due to the degasification of hydrogen sulphide, pH is increased which leads to the reaction toward calcium carbonate precipitation [21]. The entire reaction of producing calcium carbonate by the reduction of calcium sulphate (CaSO4) to calcium sulphide (CaS) using sulphate reducing bacteria, is shown below [Eqs. (4)–(7)] [22].
The Nitrogen cycle includes three different types of pathways, (i) urea or uric acid degradation (ureolysis), (ii) ammonification of amino acids, and (iii) dissimilatory nitrate reduction to produce carbonate or bicarbonate [18]. When adequate calcium ions are present in the medium, bacteria can go through these nitrogen metabolisms leading to calcium carbonate precipitation [23].
In ureolysis pathway, the microbial urease produces ammonia and CO2 from the hydrolysis of urea [24,25,26]. The released ammonia subsequently increases the pH of the surroundings [27,28,29], leading to the accretion of insoluble CaCO3 [Eqs. (4) to (7)] [30] and in the process will prevent CO2 from being released into the atmosphere. These metabolic chemical translations help in calcium carbonate precipitation, generally in the form of calcite which is stable and also abundant in nature. This calcium carbonate precipitation plays the important role of a barrier and blocks the ingress of corrosive chemicals into concrete cracks [31], and thus, saving the concrete structure. It is the most used and studied pathway in the line of self-healing microbial concrete.
Apart from its several positives, this pathway has a few drawbacks such as the emission of nitrogen oxide in the atmosphere, and increased risk of salt damage by conversion to nitric acid in concrete due to the production of an excessive amount of ammonia in the matrix [18]. To deal with this drawback of excessive ammonium ion production, few researchers [6, 25, 26, 32] have proposed the idea of metabolic conversion of organic compound (organic acid salt) to calcium carbonate. When organic acids (such as calcium lactate) are aerobically oxidized, carbon dioxide is generated in an alkaline atmosphere, which is then converted to CaCO3 [Eq. (24)] in the presence of Ca+2 [25, 33,34,35]. Compared to ureolysis pathway, this metabolic conversion is more suitable with respect to compatibility with concrete matrix composition, protection of reinforcement bars, and most importantly high production of CaCO3 but no ammonium.
Few aerobic Gram-negative bacterial strains can use amino acids as their sole energy source during the ammonification of amino acids. Myxococcus has been shown to be an effective biosynthesis bacterium for a variety of minerals, including carbonates, sulphates, phosphates, oxalates, chlorides, and silicates [36]. Myxococcus xanthus can induce calcium carbonate precipitation in a nutrient medium of calcium acetate by active alkalinization, with ammonia and carbon dioxide as by-products. At higher pH levels, CO2 appears to dissolve and turn into either HCO3− or CO32− [36].
Under the nitrogen cycle, another subclass is denitrification, which works as a dissimilatory nitrate reduction. In this pathway, minerals are precipitated via the respiratory process of denitrifying bacteria, through oxidation of organic compounds, and by the reduction of nitrate (NO3−) to nitrite (NO2−), nitric oxide (NO), nitrous oxide (N2O), and ultimately to nitrogen gas (N2). Mainly facultative anaerobes, such as Denitrobacillus, Micrococcus, Alcaligenes, Spirillum, Thiobacillus, Pseudomonas and Achromobacter species [18], are capable of denitrification. Hence, such processes are effective for application in anaerobic zones. But similar to ammonification, this process also produces some by-products in the process such as carbon dioxide, water, and nitrogen. Due to the consumption of H+, an increase in pH takes place during the denitrification process resulting in CO32− or HCO3− production [37] which further reacts with the calcium source leading to the precipitation of calcium carbonate [Eqs. (16)–(18)]. Production of calcium carbonate using the denitrification process in concrete is still under research and development stage. Moreover, ureolysis is a much faster process to biomineralize CaCO3 compared to this denitrification pathway [38].
2.3.2 Autotrophic pathway
In the autotrophic pathway, microbes convert CO2 to carbonate, mainly in three different ways: (i) oxygenic photosynthesis (by cyanobacteria and algae), (ii) anoxygenic photosynthesis (by purple bacteria), and (iii) non-methylotrophic methanogenesis (by methanogenic archaea) [21].
Both oxygenic and anoxygenic photosynthetic bacteria produce CaCO3 in the presence of calcium ions. The difference between these two photosynthetic metabolic processes is the use of electron donors which is H2O in the former and H2S in the latter [39, 40]. As no oxygen is generated in the later process it is called anoxygenic photosynthesis. During microbial photosynthesis, due to the removal of CO2 from the bicarbonate solutions, a localized increment in pH occurs, which leads to CaCO3-precipitation in the presence of Ca2+ ions using either reaction 1 or 2 [Eqs. (23) and (24)] [41]. As the main requirement of this process is the occurrence of CO2 in the environment, utilization of this photosynthesis pathway for concrete crack repair is possible only in environments where the concrete is exposed to CO2 and light.
The summary of photosynthetic chemical reactions for the production of calcium carbonate is shown below in Eqs. (19) to (24):
Thereafter in the occurrence of calcium ions,
In the non-methylotrophic methanogenesis pathway, CO2 and H2 are converted to CH4. HCO3− is generated by the anaerobic oxidation of methane with electron acceptors such as sulphate [23]. When calcium ions are present, the carbonate formed produces calcium carbonate, which then precipitates [Eqs. (25)–(27)]. This pathway is more applicable to the marine ecosystem. CO2-type hydrogenotrophic methanogens (e.g., Methanobrevibacter aboriphilus, Methanosarcina barkeri, Methanothermobacter thermautotrophicus, and Methanothermobacter marburgensis) [19] are a few examples of methanogens that employ this pathway. These types of methanogenic archaea under anaerobic conditions can produce methane by using CO2 and H2 as carbon and energy sources, respectively.
2.4 Merits and demerits of different MICP cycles used in concrete
The use of oxygenic photosynthesis by different cyanobacteria such as Dichothrix, Lyngbya, Gloeocapsa, and Synechococcus species, might be a very important process for concrete crack healing, as these cyanobacteria are capable of surviving in alkaline environments similar to concrete that have pH of 11–13. By the use of methane oxidation, a few researchers [42] also suggested that, in this pathway, bacteria can utilize the environmentally harmful gas such as CH4, as well as H2S, which is harmful to concrete, in their metabolism process and thereafter, ultimately ending in the calcite precipitation. Whereas, the sulphur cycle hampers the health of concrete by producing harmful corrosive agent H2S that leads to the rapid deterioration of the concrete structure. The most frequently used MICP mechanism for MICP in concrete is the utilization of ureolysis pathway, where the microbial urease produces ammonia and CO2 from hydrolysis of urea combined with different calcium compounds as nutrients. Although this process enhances the concrete properties such as compressive strength, concrete crack healing, decreasing the material permeability, etc., it has a few major drawbacks like, it is a temperature-dependent mechanism, with emission of environmentally harmful gas nitrogen oxide in the atmosphere, along with the increased risk of salt damage by conversion to nitric acid in concrete due to production of an excessive amount of ammonia in the matrix. To overcome the drawback of excessive ammonium ion production of ureolysis, the idea of metabolic conversion of organic compound (organic acid salt) to calcium carbonate came into the scenario, where the bacteria directly utilize the organic material for their metabolism and CaCO3 is produced as a by-product leading to a higher production of CaCO3 without any ammonium. Another most useful mechanism is Denitrification with these advantages as it can happen in oxygen-deficient subsurface environments with only the presence of nitrate, such as the inner part of concrete cracks. But the by-products of denitrification (i.e., N2 gas, possibly a very little amount of unprecipitated CO2) are mainly non-toxic and chemically inert, whereas the gases produced in other metabolic pathways such as ammonia (from ureolysis), and hydrogen sulfide (from sulfate reduction), that poses threat to the environment, structures as well as human health. Although the final result of denitrification is innocuous nitrogen gas, there is a significant disadvantage to this process in that three toxic intermediates, namely nitrite, nitric oxide, and nitrous oxide, can build if incomplete microbial nitrate reduction takes place. Thus, making this process undergoes further research to overcome the drawbacks.
2.5 CO2 sequestration via carbonate precipitation in microbial concrete and its effect
The application of MICP is not only confined to addressing the environmental issues of sequestering the greenhouse gas CO2 but is also effective in structural engineering issue as self-healing of concrete cracks [36, 43]. Compared to the conventional approaches to crack repair of concrete, calcium carbonate bio-deposition by MICP (Table 2) also blocks the penetration of aggressive substances. In MICP, both the quality and quantity of precipitated crystal, in terms of density, thickness, cohesion, and effective bond with the concrete matrix [36, 44], bear major impacts on the effectiveness of surface crack remediation.
Researchers [21, 25, 36, 44,45,46,47,48,49,50,51] all over the globe have conducted studies on the application of MICP for calcium carbonate precipitation in the healing of concrete cracks along with the effect of the same on different properties of concrete. Most researchers used the alkaliphilic spore-forming bacteria such as S. pasteurii, B. sphaericus, and B. subtilis [52,53,54] (Table 2) for this task for their capability to withstand the high alkalinity of concrete. In most cases, the pathway of ureolysis was the preferred choice for its highest output of CaCO3 deposition compared to other pathways [44, 47, 48]. Stable spore formers that can remain dormant for years are again preferred as they can remain idle in the concrete structure for years (even better if they can remain viable for decades) and will work only when cracks are formed and moisture seeps in. The healing agent consisting of the microorganism can be applied in concrete cracks either directly or in encapsulated form. According to Lee and Park [55], a perfect encapsulation carrier for the bacterial cells that facilitate MICP-mediated self-healing, needs protection from the harsh environment, which can be accomplished by immobilization [56]. Wang et al. [30] utilized B. sphaericus in the form of microencapsulated bacterial spores. Ghosh et al. [57, 58] used anaerobic microorganisms (Shewanella species) directly in cement mortar matrix via mixing water for MICP which showed a positive effect on compressive strength, decreasing its porosity whereas the application of E. coli doesn’t show such increment in mortar strength. However, Schreiberová et al. [59] studied the effect of different nutrients for MICP in cement mortar from past literature and concluded in their study that urea, calcium formate, calcium nitrate, and calcium lactate have the ability to improve compressive strength, while yeast extract resulted in a significant decrease in compressive strength. The utility of concrete surface treatment by calcium carbonate precipitation using a two-step immersion technique was first investigated [36, 44, 47] using pure B. sphaericus strains and ureolytic mixed cultures. The mortar/concrete specimens were submerged in a nutrient solution for 72 h after being soaked in stock culture for 24 h. The results showed that the calcium carbonate deposition on the specimens' exteriors, reduced the capillary water uptake along with the permeability towards gas. With higher CaCO3 precipitation, the rate of reduction in water adsorption of the bacteria-treated specimen also increases [38, 44, 47, 48]. Nosouhian et al. [60] chose a two-step treatment using bacteria in their research, to investigate concrete durability improvement in a sulfate environment. In MICP, the rate of precipitation and the presence of organic substances also affect the density and cohesion [61]. Qian et al. [62] implemented a bacterial treatment consisting of only a single step, by submerging cement stones in the medium containing urea, S. pasteurii cells, and calcium nitrate. The precipitated dense and coherent layer (150–290 μm thick) of carbonate performed very well in an acidic environment and in decreasing the water-absorption rate by at least 50%. Jonkers and Schlangen [63] used alkaliphilic spore-forming bacteria (Sporosarcina pasteurii, B. cohnii, B. halodurans, B. pseudofirmus) with different carbon sources, for MICP in concrete cracks. Studies showed that by incorporating high numbers of bacteria (109 cells cm−3) and using amino acids aspartate and glutamate (0.5% of cement weight) in the concrete matrix, MICP performs satisfactorily in crack healing, sustaining the compressive, tensile and flexural strength of the concrete with the minimal loss [63]. MICP by means of ureolytic bacteria (mainly from the genus Bacillus) has proven useful in various studies for concrete crack healing [28, 64] with regaining strength [25, 45, 46] as well as decreasing the material permeability [47, 48] in the process. Achal et al. [65] utilized the MICP of calcite on reinforced concrete using the bacterial strain of Bacillus sp. in urea hydrolysis utilizing corn steep liquor (CSL) (a high protein-containing industrial by-product) as the source of nutrient in the reinforced concrete. Kaur et al. [13] investigated the effect of utilizing CO2 by Bacillus megaterium as an alternative to urea in ureolysis, by using CO2 influx (20 ml/min) in place of urea. They studied and compared the applicability and effectiveness of using CO2 influx as well as urea in CaCO3 precipitation for concrete crack healing. The precipitated CaCO3 through CO2 influx is equivalent to that produced when 2% urea was used. Carbonation curing improves the strength by forming calcium carbonate crystals when reaction of CO2 with the hydrated and un-hydrated products of cement hydration takes place. These crystals form the microstructure and pore structure of the mortar, eventually improving properties such as moisture transport, mechanical strength, and CO2 diffusivity. Therefore, carbonation makes the concrete more durable because it makes the concrete totally dense, decreases the whole porosity, and increases the sulphate and alkali aggregate resistance [13]. Even, the strength of concrete increased by 117% for accelerated carbonation curing while the increment was only 47% in urea-treated specimens. Apart from these technical benefits, the use of direct CO2 influx also helps in CO2 sequestration actively without compromising the performance of the concrete itself.
Literature [56] showed that different biotic as well as abiotic influences, such as bacterial genotype and concentration, nucleation site, the concentration of nutrients (carbon, nitrogen, and calcium source), pH, and temperature, can influence the biosynthesis of calcium carbonate. Instead of urea hydrolysis, few researchers attempted to study the effect of metabolic conversion of calcium lactate or calcium formate i.e., bacterially mediated calcite precipitation for self-healing of concrete cracks which showed successful outcome. Some researchers [26, 32, 37, 66,67,68] utilized the denitrification pathway for calcite-based mineral precipitation using alkaliphilic bacteria for crack repair in concrete which also resulted in effective frost salt scaling. Few researchers [67, 69] investigated both the efficiency of alkaliphilic bacteria (B. sphaericus) for ureolysis and denitrification bacteria (D. nitroreducers) for denitrification to precipitate carbonate for self-healing of concrete in different nutrients. Although the use of denitrification bacteria has been tested and referred by various researchers for carbonate precipitated self-healing of concrete crack, still it possesses some major drawbacks such as the release of ammonium as a by-product which possesses an unpleasant smell. The use of organic compounds by heterotrophic bacteria releases CO2 in the process. Although the by-product CO2 later takes part in carbonate precipitation, still there is a possibility of release of the by-product CO2 in the atmosphere causing the opposite effect of CO2 arrestation leading to global climate change. Therefore, to avoid this possibility, researchers shift their focus on MICP using photoautotrophic cyanobacteria that obtain energy through photosynthesis, grow in a nutrient-poor medium while using CO2 as a carbon source, and release O2 in the process [70, 71]. Cyanobacteria's calcification activity is affected by environmental conditions [72] such as light intensity and UV pre-treatment. The most used cyanobacteria for this application are of genus Synechococcus [72]. Using these cyanobacteria, a new type of building material known as Living Building Material (LBM) is also proposed in a recent study [73]. Using the photosynthetic cyanobacterium Synechococcus sp. PCC 7002, in an inert structural sand-gelatin scaffold to toughen the hydrogel matrix via CaCO3 precipitation, using controlled temperature and humidity atmosphere, from one parent generation of LBMs, the authors ensured three successive regenerations. This investigation opens a new door to the self-generation of building materials (Frankenstein-type material) that also supports actively CO2 sequestration. UV tolerance of the strain is also an important aspect since these microbes have to stay in the concrete and work there which in most cases are exposed to direct sunlight. Recently a few researchers [74] also investigated the effects of MICP on the properties of recycled aggregates (RA) based on two different methods, i.e., urea decomposition precipitation system and CO2 capture precipitation system. Results showed that the direct immersion treatment of the RAs under urea decomposition precipitation system, had the best modification performance. Although the overall modification performance of urea decomposition precipitation system, was superior than that of the CO2 capture precipitation system, still the latter method offered a greener environmental approach due to its effective contribution in CO2 capture and immobilization. Some researchers [75] also reviewed the current state of utilizing the MICP process in application of soil stabilization. Some other investigators [76] reviewed the works on utilization of MICP on solid waste treatment and soil remediation to understand the present scenario of it. They concluded the importance of MICP technology in application in soil improvement factors. Thus, the process also implements in CO2 sequestration and helps for an environmentally friendly future. Also, in the field of solid waste treatment and soil remediation, application of MICP technology has great potential.
This review study unveils the lacunas in the present canvas for the exact application of the utilization of MICP process in concrete crack-healing and sequestration of environmental CO2 in the process. Although, there are numbers of work done concentrating on concrete crack repair using MICP process with very effective and approachable outcomes, still only a very few investigators have focused their study to utilize MICP in concrete crack repair for direct CO2 sequestration from its gaseous streams [13, 74]. In the same aspect, the selection and applicability of suitable organism as well as the conditions for the favourable growth of the organism and its pathway is yet to be investigated. The studies on the viability of the microorganism in the real field application is very limited. Furthermore, the performance of the repaired concrete has to be assessed properly.
2.6 Gaseous CO2 sequestration
The applications of MICP in the self-healing of concrete cracks have great potential. Mainly ureolysis and bacterially mediated calcite precipitation, as well as oxygenic photosynthesis by cyanobacterial, showed promising results in the scenario. MICP using ureolysis pathway resulted in the production of CO2 as a by-product in the middle of the reaction, later it was used to form the calcium carbonate precipitate, which in turn decreased the contribution of CO2 arrestation of the process. Rather, this phenomenon can negatively affect as the release of CO2 in the atmosphere can affect the greenhouse effect resulting in global warming. Few researchers [77] have noticed that ureolysis by S. pasteurii resulted in no net CO2 sequestration, since the moles of calcite precipitated were equal to or less than the moles of urea-derived carbonate ions, due to CO2 generation during the urea hydrolysis process. But to utilize the same ureolysis in a positive way for CO2 sequestration, Kaur et al. [13] replaced urea with CO2 influx which resulted in a positive outcome for crack healing. This process can therefore be further utilized for CO2 sequestration while self-healing of concrete cracks by the means of ureolysis replacing the urea to sufficient CO2 influx. Moreover, using the oxygenic photosynthesis by cyanobacteria always showed positive results for carbonate precipitation, as the cyanobacteria utilize CO2 as an alternative inorganic source due to the low concentration of the bicarbonate. This brings the environmental advantage of carbon sequestration. The study and research conducted solely on MICP, as well as utilization of MICP in concrete and by the means of cyanobacteria or utilizing gaseous CO2, has been evaluated approximately for the last 7 years and shown in Fig. 2.
Pie chart on approximate research works done on MICP and its utilization on concrete healing for the last 8 years, where a represents the overall papers published on MICP, b represents the percentage of paper published on MICP which is used in Concrete, and c represents paper published specially with cyanobacteria and/or gaseous CO2 used in MICP for Concrete
3 Conclusions
The potential of CO2 sequestration by microbial self-healing concrete via the MICP technique indicated it to be an effective solution to global warming. In addition, it also positively affects the mechanical properties of concrete. In the current scenario, the huge production of CO2 from the cement factory, which imposes a great threat to the environment, can be utilized in these MICP mechanisms for concrete crack healing. This also can act as a means of environmental CO2 sequestration, although the MICP mechanisms having its own merits and demerits. It is already an established truth that the mechanism of ureolysis, as well as oxygenic photosynthesis by cyanobacteria, utilizes the atmospheric CO2 in the MICP process. MICP by means of replacement of urea to CO2 influx can effectively contribute to CO2 arrestation from the atmosphere. Moreover, the mostly used pathway, i.e., using cyanobacteria also bears the potential of repairing concrete cracks alongside the major contribution to CO2 arrestation in the process. However, the applicability of optimum conditions as well as of effective microorganisms and its pathway needs more rigorous study to establish a mechanism of MICP for concrete crack healing which will also act as an effective solution in atmospheric CO2 sequestration. Even so, it can be concluded that MICP can bear a significant role in arrestation as well as sequestration of CO2, under proper atmospheric conditions with a cautious selection of microorganisms and its nutrient for the MICP procedure.
Thus, we believe that future MICP research have a huge potential with emphasis on microbial repairing of concrete and its role in CO2 sequestration. The reasons being:
-
1.
Very few studies have been conducted till now regarding the active utilization of CO2 from the atmosphere/environment leading to its sequestration in MICP, used for concrete crack-repair.
-
2.
For different types of concrete using different materials and for its improved performance as well as its crack-repair process, incorporating the CO2 sequestration via MICP from direct atmosphere is a brilliant opportunity. Bacteria with high effectiveness in solidification as well as with higher environmental adaptability, should be cultured, cultivated, with the related costs taking into the consideration.
-
3.
Researches on utilization of solid waste having huge amount of calcium should be explored for extracting the metal through MICP.
-
4.
To adapt MICP in concrete crack repair with effective CO2 sequestration from the environment itself in the process, investigators have a great area of future work to find the suitable and effective organism, pathway as well as conditions for the favourable growth of the organism.
Although the present review work has been done vigorously to project the exact current scenario for the utilization and contribution of MICP in concrete crack repair, still there is a scope for more investigations on the use of MICP in concrete crack repair, while sequestering atmospheric CO2 simultaneously.
4 Recommendation
The authors strongly recommend detailed experimental investigation on the use of atmospheric CO2 in MICP for concrete crack healing, with consideration and implementation of various factors and parameters required for the same. Thereafter, the performance of the repaired concrete in practical field is also needed to be evaluated. On successful outcome, a viable solution for a greener earth can be advanced. Along with the concrete repair process, in the field of solid waste treatment and soil remediation, application of MICP technology for atmospheric CO2 sequestration has great potential, which can also be studied via proper and detailed experimental process in the same way for a greener and sustainable environment.
Availability of data and materials
Not Applicable.
Abbreviations
- MICP:
-
Microbially induced calcite precipitation
- CSL:
-
Corn steep liquor
- RA:
-
Recycled aggregate
References
Khelifi W, Bencedira S, Azab M, Riaz MS, Abdallah M, Baki ZA, Krauklis AE, Aouissi HA (2022) Conservation environments’ effect on the compressive strength behaviour of wood-concrete composites. Materials 15:3572. https://doi.org/10.3390/ma15103572
Davies R, Teall O, Pilegis M, Kanellopoulos A, Sharma T, Jefferson A, Gardner D, Al-Tabbaa A, Paine K, Lark R (2018) Large scale application of self-healing concrete: design, construction, and testing. Front Mater 5:51. https://doi.org/10.3389/fmats.2018.00051
Edvardsen (1999) Water permeability and autogenous healing of cracks in concrete. ACI Mater J 96(4):448–454
Neville M (2002) Autogenous healing—a concrete miracle? Concr Int 24:76–82
Li VC, Yang E (2007) Self healing in concrete materials. In: van der Zwaag S (ed) Self healing materials—an alternative approach to 20 centuries of materials science. Springer, Dordrecht, pp 161–194
Jonkers HM (2011) Bacteria-based self-healing concrete. Heron 56:5–16
Joshi S, Goyal S, Mukherjee A, Reddy MS (2017) Microbial healing of cracks in concrete: a review. J Ind Microbial Biotechnol 44:1511–1525. https://doi.org/10.1007/s10295-017-1978-0
Buis A (2019) The atmosphere: getting a handle on carbon dioxide, global climate change vital signs of the planet, NASA, https://climate.nasa.gov/news/2915/the-atmosphere-getting-a-handle-on-carbon-dioxide/. Accessed 17 Apr 2021
Fayomi GU, Mini SE, Fayomi OSI, Ayoola AA (2019) Perspectives on environmental CO2 emission and energy factor in Cement Industry. IOP Conf Ser Earth Environ Sci 331:012035. https://doi.org/10.1088/1755-1315/331/1/012035
Ellis LD, Badel AF, Chiang ML, Park RJY, Chiang YM (2020) Toward electrochemical synthesis of cement—an electrolyzer-based process for decarbonating CaCO3 while producing useful gas streams. Proc Natl Acad Sci 117(23):12584–12591. https://doi.org/10.1073/pnas.1821673116
Dhami NK, Mukherjee A, Reddy MS (2014) Synergistic role of bacterial urease and carbonic anhydrase in carbonate mineralization. Appl Biochem Biotechnol 172:2552–2561. https://doi.org/10.1007/s12010-013-0694-0
Bharti RK, Srivastava S, Thankur IS (2014) Isolation, purification, characterization and mass spectroscopic analysis of carbonic anhydrase from Serratia sp. for sequestration of carbon dioxide and formation of calcite. J Environ Chem Eng 2:31–39. https://doi.org/10.1016/j.jece.2017.01.050
Kaur G, Dhami NK, Goyal S, Mukherjee A, Reddy MS (2016) Utilization of carbon dioxide as an alternative to urea in biocementation. Constr Build Mater 123:527–533. https://doi.org/10.1016/j.conbuildmat.2016.07.036
Zhu T, Dittrich M (2016) Carbonate precipitation through microbial activities in natural environment, and their potential in biotechnology: a review. Front Bioeng Biotechnol 4:4. https://doi.org/10.3389/fbioe.2016.00004
Kumar M, Sundaram S, Gnansounou E, Larroche C, Thakur IS (2018) Carbon dioxide capture, storage and production of biofuel and biomaterials by bacteria: a review. Biores Technol 247:1059–1068. https://doi.org/10.1016/j.biortech.2017.09.050
Achal V, Pan X, Fu Q, Zhang D (2012) Biomineralization based remediation of As(III) contaminated soil by Sporosarcina ginsengisoli. J Hazard Mater 201:178–184
Fujita Y, Taylor JL, Wendt LM, Reed DW, Smith RW (2010) Evaluating the potential of native ureolytic microbes to remediate a90Sr contaminated environment. Environ Sci Technol 44:7652–7658. https://doi.org/10.1021/es101752p
Seifan M, Samani AK, Berenjian A (2016) Bioconcrete: next generation of self-healing concrete. Appl Microbiol Biotechnol 100:2591–2602. https://doi.org/10.1007/s00253-016-7316-z
Mistry AN, Ganta U, Chakrabarty J, Dutta S (2018) A review on biological systems for CO2 sequestration: organisms and their pathways. Environ Prog Sustain Energy 38:127–136. https://doi.org/10.1002/ep.12946
Knorre H, Krumbein KE (2000) Bacterial calcification. In: Riding RE, Awramik SM (eds) Microbial sediments. Springer, Berlin, pp 25–31. https://doi.org/10.1007/978-3-662-04036-2_4
Castainer S, Metayer-Levrel GL, Perthuisot JP (1999) Ca-carbonates precipitation and limestone genesis—the microbiogeologist point of view. Sediment Geol 126:9–23
Ehrlich HL (1995) Geomicrobiology. Marcel Dekker, New York. https://doi.org/10.4319/lo.1982.27.5.0984
Castainer S, Metayer-Levrel GL, Perthuisot JP (2000) Bacterial roles in the precipitation of carbonate minerals. In: Riding RE, Awramik SM (eds) Microbial sediments. Springer, Berlin, pp 32–39. https://doi.org/10.1007/978-3-662-04036-2_5
Belie ND, Muynck W (2008) Crack repair in concrete using biodeposition. ICCRRR II, Scape Town, pp 291–292
Jonkers HM, Thijssen A, Muyzer G, Copuroglu O, Schlangen E (2010) Application of bacteria as self-healing agent for the development of sustainable concrete. Ecol Eng 36:230–235. https://doi.org/10.1016/j.ecoleng.2008.12.036
Wiktor V, Jonkers HM (2011) Quantification of crack-healing in novel bacteria-based self-healing concrete. Cem Concr Compos 33:763–770. https://doi.org/10.1016/j.cemconcomp.2011.03.012
Stocks-Fischer S, Galinat JK, Bang SS (1999) Microbiological precipitation of CaCO3. Soil Biol Biochem 31:1563–1571. https://doi.org/10.1016/s0038-0717(99)00082-6
Tittelboom KV, Belie ND, Muynck WD, Verstraete W (2010) Use of bacteria to repair cracks in concrete. Cem Concr Res 40:157–166. https://doi.org/10.1016/j.cemconres.2009.08.025
Pacheco-Torgal F, Labrincha JA (2013) Biotech cementitious materials: some aspects of an innovative approach for concrete with enhanced durability. Constr Build Mater 40:1136–1141. https://doi.org/10.1016/j.conbuildmat.2012.09.080
Wang JY, Soens H, Verstraete W, Belie ND (2014) Self-healing concrete by use of microencapsulated bacterial spores. Cem Concr Res 56:139–152. https://doi.org/10.1016/j.cemconres.2013.11.009
Muynck WD, Belie ND, Verstraete W (2007) Improvement of concrete durability with the aid of bacteria. In: Proceedings of the first international conference on self healing materials. Noordwijk aan zee, The Netherlands
Sierra-Beltran MG, Jonkers HM, Schlangen E (2014) Characterization of sustainable bio-based mortar for concrete repair. Constr Build Mater 67:344–352. https://doi.org/10.1016/j.conbuildmat.2014.01.012
Achal V, Mukherjee A, Reddy MS (2011) Microbial concrete: way to enhance the durability of building structures. J Mater Civ Eng 23:730–734. https://doi.org/10.1061/(ASCE)MT.1943-5533.0000159
Achal V, Pan X (2011) Characterization of urease and carbonic anhydrase producing bacteria and their role in calcite precipitation. Curr Microbiol 62:894–902. https://doi.org/10.1007/s00284-010-9801-4
Tittelboom KV, Belie ND (2013) Self-healing in cementitious materials—a review. Materials 6:2182–2217. https://doi.org/10.3390/ma6062182
Seifan M, Berenjian A (2019) Microbially induced calcium carbonate precipitation: a widespread phenomenon in the biological world. Appl Microbiol Biotechnol 103:4693–4708. https://doi.org/10.1007/s00253-019-09861-5
Erşan YC, Belie ND, Boon N (2015) Microbially induced CaCO3 precipitation through denitrification: an optimization study in minimal nutrient environment. Biochem Eng J 101:108–118. https://doi.org/10.1016/j.bej.2015.05.006
Muynck WD, Belie ND, Verstraete W (2010) Microbial carbonate precipitation in construction materials: a review. Ecol Eng 36:118–136. https://doi.org/10.1016/j.ecoleng.2009.02.006
Munn CB (2004) Marine microbiology: ecology and applications. Bios Scientific Publisher, London
Okafor N (2011) Environmental microbiology of aquatic and waste systems. Springer, Dordrecht
Hammes F, Verstraete W (2002) Key roles of pH and calcium metabolism in microbial carbonate precipitation. Rev Environ Sci Biotechnol 1:3–7. https://doi.org/10.1023/A:1015135629155
Li L, Xue S, Xi J (2019) Anaerobic oxidation of methane coupled to sulfate reduction: consortium characteristics and application in co-removal of H2S and methane. J Environ Sci 76:238–248. https://doi.org/10.1016/j.jes.2018.05.006
Seifan M, Samani AK, Burgess JJ, Berenjian A (2016) The effectiveness of microbial crack treatment in self healing concrete. In: Berenjian A, Jafarizadeh-Malmiri H, Song Y (eds) High value processing technologies. Nova Science Publishers Inc, New York, pp 97–124
Wang J, Ersan YC, Boon N, Belie ND (2016) Application of microorganisms in concrete: a promising sustainable strategy to improve concrete durability. Appl Microbiol Biotechnol 100:2993–3007. https://doi.org/10.1007/s00253-016-7370-6
Ramachandran SK, Ramakrishnan V, Bang SS (2001) Remediation of concrete using micro-organisms. ACI Mater J 98:3–9. https://doi.org/10.14359/10154
Ramakrishnan V (2007) Performance characteristics of bacterial concrete—a smart biomaterial. In: Proceedings of the First International Conference on Recent Advances in Concrete Technology, Washington, DC, pp 67–78.
Muynck WD, Debrouwer D, Belie ND, Verstraete W (2008) Bacterial carbonate precipitation improves the durability of cementitious materials. Cem Concr Res 38:1005–1014. https://doi.org/10.1016/j.cemconres.2008.03.005
Muynck WD, Cox K, Belie ND, Verstraete W (2008) Bacterial carbonate precipitation as an alternative surface treatment for concrete. Constr Build Mater 22:875–885. https://doi.org/10.1016/j.conbuildmat.2006.12.011
Belie ND, Wang J (2015) Bacteria-based repair and self-healing of concrete. J Sustain Cem Based Mater 5:35–56. https://doi.org/10.1080/21650373.2015.1077754
Anbu P, Kang CH, Shin YJ, So JS (2016) Formations of calcium carbonate minerals by bacteria and its multiple applications. Springer Plus 5:250. https://doi.org/10.1186/s40064-016-1869-2
Vijay K, Murmu M, Deo SV (2017) Bacteria based self healing concrete—a review. Constr Build Mater 152:1008–1014. https://doi.org/10.1016/j.conbuildmat.2017.07.040
Luo M, Qian C, Li R, Rong H (2015) Efficiency of Concrete Crack-healing based on Biological Carbonate Precipitation. J Wuhan Univ Technol Mater Sci Ed. https://doi.org/10.1007/s11595-015-1304-5
Krishnapriya S, Venkatesh Babu DL, Arulraj GP (2015) Isolation and identification of bacteria to improve the strength of concrete. Microbiol Res 174:48–55. https://doi.org/10.1016/j.micres.2015.03.009
Puranik SA, Jain S, Sritam G, Sandbhor S (2019) Bacterial concrete—a sustainable solution for concrete maintenance. Int J Innov Technol Explor Eng 8:227–232. https://doi.org/10.35940/ijitee.K1046.09811S19
Lee YS, Park W (2018) Current challenges and future directions for bacterial self-healing concrete. Appl Microbiol Biotechnol. https://doi.org/10.1007/s00253-018-8830-y
Seifan M, Berenjian A (2018) Application of microbially induced calcium carbonate precipitation in designing bio self-healing concrete. World J Microbiol Biotechnol 34:168. https://doi.org/10.1007/s11274-018-2552-2
Ghosh P, Mandal S, Chattopadhyay BD, Pal S (2005) Use of microorganism to improve the strength of cement mortar. Cem Concr Res 35:1980–1983. https://doi.org/10.1016/j.cemconres.2005.03.005
Ghosh S, Biswas M, Chattopadhyay BD, Mandal S (2009) Microbial activity on the microstructure of bacteria modified mortar. Cem Concr Compos 31:93–98. https://doi.org/10.1016/j.cemconcomp.2009.01.001
Schreiberová H, Bílý P, Fládr J, Šeps K, Chylík R, Trtík T (2019) Impact of the self-healing agent composition on material characteristics of bio-based self-healing concrete. Case Stud Constr Mater 11:e00250. https://doi.org/10.1016/j.cscm.2019.e00250
Nosouhian F, Mostofinejad D, Hasheminejad H (2016) Concrete durability improvement in a sulfate environment using bacteria. J Mater Civ Eng 04015064:1–12. https://doi.org/10.1061/(ASCE)MT.1943-5533.0001673
Rodriguez-Navarro C, Rodriguez-Gallego M, Chekroun KB, Gonzalez-Munoz MT (2003) Conservation of ornamental stone by Myxococcus xanthus-induced carbonate biomineralization. Appl Environ Microbiol 69:2182–2193. https://doi.org/10.1128/aem.69.4.2182-2193.2003
Qian CX, Wang JY, Wang RX, Cheng L (2009) Corrosion protection of cement-based building materials by surface deposition of CaCO3 by Bacillus pasteurii. Mater Sci Eng C-Biomim Supramol Syst 29:1273–1280. https://doi.org/10.1016/j.msec.2008.10.025
Jonkers MH, Schlangen E (2007) Crack repair by concrete-immobilized bacteria. In: Proceedings of the first international conference on self healing materials. 18–20 April 2007, Noordwijk aan Zee, The Netherlands
Tziviloglou E, Wiktor V, Jonkers HM, Schlangen E (2016) Bacteria-based self-healing concrete to increase liquid tightness of cracks. Constr Build Mater 122:118–125. https://doi.org/10.1016/j.conbuildmat.2016.06.080
Achal V, Mukherjee A, Goyal S, Reddy MS (2012) Corrosion prevention of reinforced concrete with microbial calcite precipitation. ACI Mater J 109:157–164. https://doi.org/10.14359/51683702
Wiktor V, Jonkers HM (2012) Application of bacteria-based repair system to damaged concrete structures. In: 2nd international workshop on structural life management of underground structures, Daejon, South-Korea, pp 31–34
Wiktor V, Jonkers HM (2015) Field performance of bacteria-based repair system: pilot study in a parking garage. Case Stud Constr Mater 2:11–17. https://doi.org/10.1016/j.cscm.2014.12.004
Luo M, Qian CX (2016) Performance of two bacteria-based additives used for self-healing concrete. J Mater Civ Eng 04016151(2016):1–6. https://doi.org/10.1061/(ASCE)MT.1943-5533.0001673
Erşan YÇ, Da Silva FB, Boon N, Verstraete W, De Belie N (2015) Screening of bacteria and concrete compatible protection materials. Constr Build Mater 88:196–203. https://doi.org/10.1016/j.conbuildmat.2015.04.027
Liang A, Paulo C, Zhu Y, Dittrich M (2013) CaCO3 biomineralization on cyanobacterial surfaces: insights from experiments with three Synechococcus strains. Colloids Surf B 111C:600–608. https://doi.org/10.1016/j.colsurfb.2013.07.012
Zhu T, Paulo C, Merroun ML, Dittrich M (2015) Potential application of biomineralization by Synechococcus PCC8806 for concrete restoration. Ecol Eng 82:459–468. https://doi.org/10.1016/j.ecoleng.2015.05.017
Zhu T, Lin Y, Lu X, Dittrich M (2018) Assessment of cyanobacterial species for carbonate precipitation on mortar surface under different conditions. Ecol Eng 120:154–163. https://doi.org/10.1016/j.ecoleng.2018.05.038
Heveran CM, Williams SL, Qiu J, Artier J, Hubler MH, Cook SM, Cameron JC, Srubar WV III (2020) Biomineralization and successive regeneration of engineered living building materials. Matter 2:481–494. https://doi.org/10.1016/j.matt.2019.11.016
Wang R, Jin P, Ding Z, Zhang W (2021) Surface modification of recycled coarse aggregate based on Microbial Induced Carbonate Precipitation. J Cleaner Prod. 328:129537. https://doi.org/10.1016/j.jclepro.2021.129537
Liu J, Li G, Li X (2021) Geotechnical engineering properties of soils solidified by microbially induced CaCO3 precipitation (MICP). Hindawi Adv Civ Eng 2021:6683930. https://doi.org/10.1155/2021/6683930
Song M, Ju T, Meng Y, Han S, Lin L, Jiang J (2022) A review on the applications of microbially induced calcium carbonate precipitation in solid waste treatment and soil remediation. Chemosphere 290:133229. https://doi.org/10.1016/j.chemosphere.2021.133229
Mitchell AC, Dideriksen K, Spangler LH, Cunningham AB, Gerlach R (2010) Microbially enhanced carbon capture and storage by mineral-trapping and solubility-trapping. Environ Sci Technol 44:5270–5276. https://doi.org/10.1021/es903270w
Acknowledgements
The authors would like to acknowledge the Department of Civil Engineering and Chemical Engineering, National Institute of Technology Durgapur for providing necessary cooperation for this investigation.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
AB performed a major contribution in researching the past work done on this topic and writing the article. AS and DG contributed to studying and preparing the PI Chart for the manuscript. BD helped with the diagram of MICP in the manuscript as well as thoroughly guided the overall manuscript. SD and AKS contributed in the area of all the chemical reaction parts and the concrete part respectively. Overall, all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bandyopadhyay, A., Saha, A., Ghosh, D. et al. Microbial repairing of concrete & its role in CO2 sequestration: a critical review. Beni-Suef Univ J Basic Appl Sci 12, 7 (2023). https://doi.org/10.1186/s43088-023-00344-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-023-00344-1