Abstract
An intense red phosphor, CaYAlO4:Mn4+, was developed by solid state reaction. The photoluminescence excitation and emission spectra, concentration effect, thermal-dependent luminescence quenching properties and decay curves are investigated. The results show Mn4+ ion exhibits a broadband excitation extending from 250 to 550 nm and emits an intense red light at 710 nm with high color purity and stable chromaticity coordinates. These results demonstrate that Mn4+ ion can play a role of activator in narrow red emitting phosphor potentially useful in UV (~370 nm) GaN-base or Blue (~460 nm) InGaN-base LED.
Similar content being viewed by others
Background
Nowadays, phosphor-converted (pc-) white LEDs are attracting significant attention [1]–[3]. They can be classified into two approaches: blue (440 ~ 470 nm) InGaN and near (n)-UV (350 ~ 420 nm) GaN chip combined with phosphors. Recently, the commonly commercial white LED is based on the combination of blue InGaN chip and yellow YAG: Ce3+ phosphor. However, such white LEDs encounter low color-rendering index (Ra < 80) due to the scarcity of red emission [4]–[6].
Red emitting phosphor is one of key tricolor luminescent materials for white LEDs. Up to now, many researchers have been done on Eu3+[7]–[9] or Sm3+[10]–[12] ion doped phosphors. Unfortunately, the red phosphor doped by Eu3+or Sm3+show weak absorption peaks at about 400 nm or 460 nm because the 4f-4f absorption transitions are forbidden by the parity selection rule and its optical oscillator strength is small. Furthermore, the price of rare earth is quite high. Furthermore, some nitride based phosphors have been developed to increase the color index, or create warm-white lighting [13]–[15]. However, the synthesize conditions of nitride phosphors are usually very harsh, such as high temperature (1500–2000°C), oxygen-free environment.
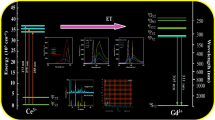
Generally, Mn4+ ion with 3d3 configurations in octahedral site gives a deep red emission and has broad absorption in visible region, such as K2SiF6: Mn4+, K2GeF6: Mn4+, K2TiF6: Mn4+ and CaAl12O19: Mn4+[16]–[18]. CaYAlO4 (CYA) crystallizes in the perovskite phase with tetragonal K2NiF4 structure [19]. Al3+ ion is octahedrally coordinated with six oxygens. Therefore, Mn4+ ion may emit red ligtht when occupied Al3+ ion site in CaYAlO4. In this paper, an intense red phosphor, CaYAlO4:Mn4+, was developed by solid state reaction. The photoluminescence excitation and emission spectra, concentration effect, thermal-dependent luminescence quenching properties and decay curves are investigated.
Methods
Syntheses: All samples CaYAlO4:Mn4+ x (x = 0.001, 0.005, 0.01, 0.03, 0.05) were prepared by a conventional solid-state reaction technique. The starting materials, CaCO3 (A.R.), Al2O3 (A.R.), Y2O3 (99.99%) and MnCO3 (A.R.) were weighed in stoichiometric amounts. Subsequently the powder mixture was thoroughly mixed in an agate mortar by grinding and was transferred into crucibles. Finally, they were sintered at 1250°C for 4 h in air.
Measurements: The phase purity of the prepared phosphors was investigated by a Rigaku D/max-IIIA X-ray Diffractometer with Cu Kα radiation (λ = 1.5406 Å) at 40 kV and 30 mA. The XRD patterns were collected in range of 10° ≤ 2θ ≤ 80°.
The photoluminescence (PL), photoluminescence excitation (PLE) spectra, temperature-dependent PL spectra and the decay curves at room temperature were measured by FSP920 Time Resolved and Steady State Fluorescence Spectrometers (Edinburgh Instruments) equipped with a 450 W Xe lamp, a 100w μF920H lamp with a pulse width of 1 ~ 2 μs, a repetition rate of 50 Hz and the lifetime range of 100 μs ~ 200 s, TM300 excitation monochromator and double TM300 emission monochromators, Red sensitive PMT and R5509-72 NIR-PMT in a liquid nitrogen cooled housing (Hamamatsu Photonics K.K). The spectral resolution is about 0.05 nm in UV–VIS.
For the high temperature PL spectra in 300–500 K, the powder sample was mounted in an Optistat DNV actively cooled optical cryostat with an ITC601 temperature controller.
The powder diffuse reflection spectra (DRS) of these samples were measured on a Cary 5000 UV–vis-NIR spectrophotometer (Varian) equipped with double out-of-plane Littrow monochromator, using polyfluortetraethylene as a standard reference in the measurements.
The room temperature quantum efficiency (QE) of the sample was measured using a barium sulfate coated integrating sphere (150 mm in diameter) attached to the FSP920.
Results and discussion
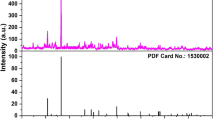
The XRD patterns of CYA: Mn4+ x (x =0, 0.001, 0.005, 0.05) are shown in Figure 1. The results indicate that all the peaks of Mn4+ ion doped CYA can be indexed to a pure CaYAlO4 (JCPDS 81–0742). The dopants have no obvious influence on the crystalline structure of the host. The CaYAlO4 has tetragonal system with a space group of I4/mmm (139) and a = 3.6750(5) Å c = 12.011(2) Å c/a = 3.2683 V = 162.22(4) Å3, Z = 2 [20]. There are two types of cation sites in CaYAlO4. The Ca2+ and Y3+ ions are distributed in the nine-coordinated sites and the Al3+ ions occupy the six-coordinate site. It is reported that the effective ionic radius of Ca2+ ion (CN = 9), Y3+ ion (CN = 9), Al3+ ion (CN = 6) and Mn4+ ion (CN = 6) are 1.18 Å, 1.075 Å, 0.535 Å and 0.53 Å, respectively [21]. It is obvious that the ionic radius of Mn4+ is close to Al3+ and smaller than Ca2+ or Y3+, suggesting that Mn4+ ions prefer to occupy Al3+ site in the present host.
Figure 2 shows the powder DRS of CYA: Mn4+ x (x = 0.001, 0.005, 0.01, 0.03, 0.05). It is clearly observed that the phosphors of CYA: Mn4+ 0.001 shows a platform of high reflection in the wavelength range of 580–1200 nm and then starts to decrease dramatically from 580 to 200 nm. As the increasing of Mn4+ concentration, two broad absorption bands appears at 200–425 nm and 425–580 nm, which is derived from the 4A2 → 4 T1 and 4 T2 transition of Mn4+, respectively.
The PLE and PL spectra of CYA: Mn4+ 0.001 are showed in Figure 3. The PLE spectrum contains two broad bands at 250–420 nm and 420-550 nm, which can be attributed to 4A2 → 4 T1 and 4 T2 transition of Mn4+, respectively. The PL spectra under the excitation at 335 nm, 370 nm and 460 nm exhibit a narrow band between 660 nm and 770 nm with a sharp peak at 710 nm, which is due to 4E → 4A2 transition of Mn4+. It is to say this phosphor can be effectively excited by UV or blue LED chip and emits red light.
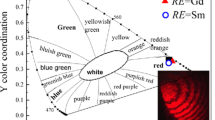
In order to further optimize the red emission of Mn4+ ion, the concentration dependent emission intensity of CYA: Mn4+ x (x = 0.001, 0.005, 0.01, 0.03, 0.05) is studied. It can be seen in Figure 4 that the emission intensity of Mn4+ ion at 710 nm initially increase, then reaches a maximum at x = 0.005 and decrease due to concentration quenching. It is interesting that the chromaticity coordinates of CYA: Mn4+ x are almostly the same with the change of Mn4+ ion dopt content. The inset of Figure 4 gives the chromaticity coordinates of CYA: Mn4+ 0.005 under 370 nm excitation. The color purity of the point in spectrum locus is 100%. So the color purity of phosphor CYA: Mn4+ is near 100%.
The QE of phosphor CaYAlO4: Mn4+ was recorded using an integrating sphere attached to the FSP920. QE is defined as the ratio of the number of emitted photons (I em) to the number of absorbed photons (I abs), and can be calculated by the following equation [22].
where E R, E S are the spectra of the excitation light without and with the sample in the integrating sphere, respectively, and L S is the luminescence emission spectrum of the sample in the integrating sphere. The QE of the CYA: Mn4+ 0.005 was measured and calculated to be about 26% and 28% under 335 nm and 460 nm excitation, respectively.
The luminous efficiency of the radiation (LER) is an important parameter which shows how bright the radiation is perceived by the average human eye. Figure 5 shows the nonalized spectral eye sensitivity cures for photopic vision and spectrum of phosphor CYA: Mn4+ 0.005. It can be caculated from the emission sepctrum as: [23].
Where V (λ) and I (λ) are eye sensitity cure and phosphor emission spectrum respectively. The LER of the CYA: Mn4+ 0.005 is 3 lum/w which indicates the phosphor is too red for general lighting . However, it may be a promising phosphor for other artificial lighting applications, such as in plant photomorphogenesis [24].
For LEDs application, the thermal stability of phosphor is one of the important factors. Figure 6 shows the PL spectral (λex = 370 nm) of CYA: Mn4+ 0.005 at the temperature range of 300–460 K. It illustrates that the position and shape of the emission spectra do not change with increasing temperature. The temperature-dependence of the integrated emission intensity for CYA: Mn4+ 0.005 is presented in the inset of Figure 6. It is clearly observed that the integrated emission intensity of CYA: Mn4+ 0.005 decreases as the temperature increases from 300 K to 460 K. The integrated emission intensity at 100 and 150°C remain about 70% and 50% when compared to room temperature. The above results mean this phoshor has a good thermal stability and is a candidate for pc-LEDs.
Figure 7 shows the decay curves of Mn4+ 4E → 4A2 emission excited by 335 nm. The decay behavior can be expressed asfollows: [25].
where I and I0 are emission intensity, A is constant, t is time and, τ is decay time for exponential component. For x = 0.001, 0.005, 0.01 all samples show a nearly single exponential decay behavior like CYA: Mn4+ 0.001 (the inset of Figure 7) and the life time is estimated to be 1.590 ms, 1.444 ms and 1.306 ms, respectively. When the Mn4+ concentration is further increased, the decay curves decrease more rapidly and become nonexponential. Such a fast decline of Mn4+ 4E is due to the interaction or energy migration between Mn4+ ions. The same phenomenon was found in the decay curves of Mn4+ emission excited by 460 nm.
Conclusion
In summary, a series of CaYAlO4: Mn4+ red phosphors with good thermal stability were investigated. Mn4+ ion gives an intense red light at 710 nm with high color purity and intense broad absorption in UV and blue range. We demonstrate that it can be a useful red phosphor for LEDs, combined with blue (440 ~ 470 nm) InGaN and near (n)-UV (350 ~ 420 nm) GaN chip.
Abbreviations
- LED:
-
Light-emitting diode
- PL:
-
Photoluminescence
- PLE:
-
Photoluminescence excitation
- CYA:
-
CaYAlO4
- CN:
-
Coordination number
- LERm:
-
Luminous efficiency of the radiation
References
Mancic L, Marinkovic K, Marinkovic BA, Dramicanin M, Milosevic O: YAG: Ce3+ nanostructured particles obtained via spray pyrolysis of polymeric precursor solution. J Eur Ceram Soc 2010, 30: 577–582.
Li YX, Min YL, Zhou XZ, You XZ: Coating and stability of YAG:Ce phosphor synthesized using inorganic–organic hybrid gel method. Chin J Inorg Chem 2003, 19: 1169–1174.
Lu CH, Hong HC, Jaganathan R: Sol–gel synthesis and photoluminescent properties of cerium-ion doped yttrium aluminium garnet powders. J Mater Chem 2002, 12: 2525–2530.
Zhang M, Wang J, Zhang QH, Ding WJ, Su Q: Optical properties of Ba2SiO4:Eu2+ phosphor for green light-emitting diode (LED). Mater Res Bull 2007, 42: 33–39.
Geng DL, Shang MM, Zhang Y, Lian HZ, Lin J: Color-tunable and white luminescence properties via energy transfer in single-phase KNaCa2 (PO4)2: A (A = Ce3+, Eu2+, Tb3+, Mn2+, Sm3+) phosphors. Inorg Chem 2013, 52: 13708–13718.
Sun JG, Arunkumar P, Won BI: A new blue-emitting oxohalide phosphor Sr4OCl6: Eu2+ for thermally stable, efficient white-light-emitting devices under near-UV. J Phys Chem C 2014, 118: 2686–2692.
Sohn KS, Park DH, Cho SH, Kwak JS, Kim JS: Computational evolutionary optimization of red phosphor for use in tricolor white LEDs. Chem Mater 2006, 18: 1768–1772.
Shinde KN, Dhoble SJ: A novel reddish-orange phosphor, NaLi2PO4: Eu3+. Luminescence 2013, 28: 93–96.
Zhou Z, Wang NF, Zhou N, He ZX, Liu S, Liu YN, Tian ZW, Mao ZY, Hintzen HT: High colour purity single-phased full colour emitting white LED phosphor Sr2V2O7: Eu3+. J Phys D 2013, 46: 035104–035110.
Mo FW, Zhou LY, Pang Q, Gong FZ, Liang ZJ: Potential red-emitting NaGd (MO4)2: R (M = W, Mo, R = Eu3+, Sm3+, Bi3+) phosphors for white light emitting diodes applications. Ceram Int 2012, 38: 6289–6294.
Xiao Q, Zhou QT, Li M: Synthesis and photoluminescence properties of Sm3+-doped CaWO4 nanoparticles. J Lumin 2010, 130: 1092–1094.
Zhang SM, Zhu B, Zhou SF, Qiu JR: Orange-red upconversion luminescence of Sm3+-doped ZnO-B2O3-SiO2 glass by infrared femtosecond laser irradiation. J Soc Inf Display 2009, 17: 507–510.
Li YQ, Van Steen JEJ, Van Krevel JWH, Botty G, Delsing ACA, Disalvo FJ, De With G, Hintzen HT: Luminescence properties of red-emitting M2Si5N8:Eu2+ (M = Ca, Sr, Ba) LED conversion phosphors. J Alloys Compd 2006, 417: 273–279.
Xie RJ, Hirosaki N: Silicon-based oxynitride and nitride phosphors for white LEDs-A review. Sci Technol Adv Mat 2007, 8: 588–600.
Xie RJ, Hirosaki N, Li YQ, Takeda T: Rare-earth activated nitride phosphors: synthesis, luminescence and applications. Mater 2010, 3: 3777–3793.
Yusuke A, Sadao A: Optical transitions and internal vibronic frequencies of MnF2− 6 Ions in Cs2SiF6 and Cs2GeF6 red phosphors. J Electrochem Soc 2011, 158: J179-J183.
Paulusz AG: Efficient Mn () emission in fluorine coordination. J Electrochem Soc 1973, 120: 942–947.
Yusuke A, Toru T, Sadao A: Photoluminescent properties of K2SnF6H2O: Mn4+ red phosphor. Opt Mater 2010, 32: 1095–1101.
Murata T, Tanoue T, Iwasaki M, Morinaga K, Hase T: Fluorescence properties of Mn4+ in CaAl12O19 compounds as red-emitting phosphor. J Lumin 2005, 114: 207–212.
Lv S, Zhu Z, Wang Y, You Z, Li J, Tu C: Spectroscopic investigations of Ho3+/Er3+: CaYAlO4 and Eu3+/Er3+:CaYAlO4 crystalsfor2.7 μm emission. J Lumin 2013, 144: 117–121.
Shannon RD: Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr 1976, A32: 751–767.
Zhang XJ, Huang L, Pan FJ, Wu MM, Wang J, Chen Y, Su Q: Highly thermally stable single-component white-emitting silicate glass for organic-resin-free white-light-emitting diodes. ACS Appl Mater Interfaces 2014, 6: 2709–2717.
Smet PF, Parmentier AB, Dirk P: Selecting conversion phosphors for white light-emitting diodes. J Electrochem Soc 2011, 158: R37-R54.
Akvilė Z, Skirmantė B, Artūras Z, Pranciškus V, Aivaras K, Pranciškus V, Aivaras K: Sol–gel synthesized far-red chromium-doped garnet phosphors for phosphor-conversion light-emitting need of plants. Appl Optics 2014, 53: 907–914.
Lu J, Huang YL, Tao Y, Sec HJ: Spectroscopic parameters of Ce3+ ion doped Na2CaMg (PO4)2 phosphor. J Alloy Compd 2010, 500: 134–137.
Acknowledgements
This work was financially supported by the “973” programs (2014CB643801), the NSFC (21271191), the Joint Funds of the National Natural Science Foundation of China and Guangdong Province (U1301242), the Doctoral Program of Higher Education of China (20130171130001), Teamwork Projects of Guangdong Natural Science Foundation (S2013030012842), Guangdong Provincial and Guangzhou Science & Technology Project (2012A080106005, 2013Y2-00118) and Guangdong Provincial Department of Science and Technology for Industrial applications of rare earth materials (2012B09000026).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contribution
YC and MW carried out the experimental details and wrote the draft paper. JW, MW and CW designed the study and gave the discussion. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, Y., Wang, M., Wang, J. et al. A high color purity red emitting phosphor CaYAlO4:Mn4+ for LEDs. J Sol State Light 1, 15 (2014). https://doi.org/10.1186/s40539-014-0015-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40539-014-0015-4